Abstract
Aim
The aim of this study was to examine whether the presence of the apolipoprotein E (ApoE) allele APOEε4 is associated with less severe manifestations of cerebral palsy (CP), consistent with the suggested beneficial effect of this allele on neurodevelopment in children.
Method
ApoE genotyping was performed on buccal epithelial cells from 255 children (141 males 114 females; mean age 12y, SD 2y 3mo, range 9–17y) recorded in the Cerebral Palsy Register of Norway. The main outcome measure of CP severity was the Gross Motor Function Classification System (GMFCS). Secondary outcome measures were fine motor function, epilepsy, and the need for gastrostomy tube feeding (GTF).
Results
There was no association between the APOEε4 genotype and GMFCS levels (odds ratio [OR] 1.15; 95% confidence interval [CI] 0.66–1.99). However, the APOEε4 genotype was more often present among children with epilepsy (OR 2.2; 95% CI 1.1–4.2) and/or receiving GTF (OR 2.7; 95% CI 1.1–6.6). Among children with unilateral CP, the presence of APOEε4 was associated with more severe fine motor impairment (OR 2.6; 95% CI 1.3–6.9).
Interpretation
Our main hypothesis that APOEε4 would have a protective effect on neurodevelopment was not supported. Instead, subgroup analyses suggested an adverse effect of the APOEε4 genotype on the developing brain after injury.
Cerebral palsy (CP) is described as a group of non-progressive disorders of the development of movement and posture, caused by an injury or a chain of harmful events occurring in the fetal or infant brain and leading to activity limitation.1 Several studies have suggested that individual susceptibility to such injuries or events may vary, and the role of genes in this regard has therefore recently attracted attention.2,3 Among the most studied is the APOE gene, coding for apolipoprotein E (ApoE).4 This gene has three alleles described as APOEε2, APOEε3, and APOEε4, differing from each other in only one single nucleotide, but resulting in proteins with different biological properties. In adults the allele APOEε4 has been shown to be involved in the development of Alzheimer disease and in less efficient `repair processes' after traumatic central nervous system injury.5 In mice, ApoE isoforms seem to influence a number of functions in the central nervous system differentially, such as modulating inflammation, axonal sprouting, and synapse formation.4 The differential influence of the various ApoE alleles and their corresponding ApoE isoforms on brain disorders and injury repair in the immature brain has therefore also been a focus of interest for research into the aetiology of CP. However, studies searching for associations between ApoE polymorphisms and the risk of CP have given conflicting results.3,6–10
Using another approach, Blackman et al. recently tested the hypothesis that specific ApoE alleles may be associated with different severities of CP, independent of the insult.9 These authors found borderline non-significant evidence that children with CP who were carriers of the APOEε4 allele were less severely impaired in their gross motor function than children without this allele, suggesting a protective effect of APOEε4. This finding would be consistent with the hypothesis put forward by Corbo et al., who proposed that APOEε4 could be characterized as a `thrifty' allele; that is an allele with an evolutionary beneficial effect in a young individual, but with detrimental consequences in an organism past its reproductive period.11 This hypothesis was also in line with a review of the cognitive and physical development of children with severe diarrhoea in early childhood by Oria et al.12 The lack of clear results in the study by Blackman et al. could be a result of the low statistical power or to a genetically heterogeneous population, or both.9
In this study, therefore, we wanted to investigate the association between APOEε4 and the severity of CP in a larger and more ethnically homogeneous population. In line with the study by Blackman et al. and the concept of APOEε4 being a `thrifty' allele as proposed by Corbo et al., our main hypothesis was that children with CP who were carriers of the APOEε4 allele would be less severely impaired in their gross motor function than children without this allele.9,11 In addition, we studied the association with other indicators of severity such as poorer fine motor function, epilepsy, and eating difficulties requiring gastrostomy tube feeding (GTF). Finally, we studied the association between ApoE polymorphisms and these indicators of severity within each of the most common CP subtypes.
METHOD
Study design
In this cross-sectional study, all children recorded with detailed information in the Cerebral Palsy Register of Norway (CPRN) as of February 1st 2011 and born between 1996 and 2003 were eligible for participation. Details of this informed consent and population-based register have been reported elsewhere.1 DNA was analysed on specimens collected by the parents on buccal swabs, and the genotyping results were linked to clinical data in the CPRN.
Participants
Among the 703 invited children and families recorded in the CPRN at the time of the study, 281 (40%) returned swabs. Of these, 26 were of poor quality and could not be used, resulting in a final study population of 255 children with reliable ApoE genotype (36.3%).
Variables
CP was defined and further classified into spastic (unilateral and bilateral), dyskinetic, and ataxic subtypes according to the guidelines proposed by the network for the Surveillance of Cerebral Palsy in Europe.13
The main outcome variable, severity of CP, was defined by the Gross Motor Function Classification System (GMFCS).14 The system classifies motor function into five levels, with level I indicating the least severe and level V the most severe activity limitation. As GMFCS levels were retrospectively estimated in the CP register for children born between 1996 and 1998, we were not able to distinguish between level I and II.15 Thus, we studied this variable as a four-level category variable, as well as a dichotomous variable by contrasting levels I and II with levels III to V.
A secondary variable was fine motor ability, according to the Bimanual Fine Motor Function (BFMF) scale.16 As with the GMFCS, the BFMF scale classifies fine motor function into five levels, with level I indicating the least severe and level V the most severe activity limitation. Other indicators of the severity of sequelae after the brain insult were active epilepsy (defined as current use of antiepileptic drugs) and the requirement for GTF. Severe impairments in vision and hearing were not included as secondary outcomes owing to low numbers, and cognitive abilities were also not studied because of the insufficient quality of the data.
Other variables were the child's age, sex, gestational age in completed weeks at birth and birthweight. The last two of these variables were prospectively recorded and provided by the Medical Birth Register of Norway.
DNA analyses
Catch-All™ Buccal Swabs (Epicentre® Biotechnologies, Madison, WI, USA, were used to collect buccal cells for DNA extraction and analysis. Buccal swabs were sent by regular mail to the children's families. A detailed instruction sheet, information about the study and a consent form were enclosed with the swabs. In addition to the written instructions, an instructional video was made and linked to the CPRN home page.
Cells with DNA for analysis were collected from February to April 2011. Returned swabs were stored in a refrigerator at 4°C to 6°C until analysis in April and May 2011. DNA extraction and ApoE genotyping were performed at the DNA Science Core, University of Virginia School of Medicine, Charlottesville, VA, USA following procedures as previously described.9 Briefly, single nucleotide polymorphisms genotyping by the TaqMan Allelic Discrimination method was employed to determine the allele compositions of ApoE Cd112 (rs429358) and Cd158 (rs7412). All the genotyping was performed in triplicate on an Applied Biosystems 7900HT Sequence Detection Systems by Life Technologies Corp, Carlsbad, CA USA. Data failing a consistency check at a confidence level of 95% were subjected to repeat analysis. Samples failing even after repeat were removed from further analyses.
Sample size estimation
A priori power calculations indicated that a study population of 200 children would allow us to demonstrate a twofold difference in prevalence of APOEε4 between the groups, with a two-sided significance level of 5% and 80% power.
Statistical analysis
STATA software (version 11; College Station, TX, USA) was used to analyse the data. Differences in the proportions of study participants were compared using χ2 statistics, or Fisher's exact test when appropriate. Where GMFCS and BFMF levels were analysed as multilevel outcomes, ordinal logistic regression was used to calculate odds ratios (OR) with 95% confidence intervals (CIs) that children with APOEε4 and or APOEε2/ε3 had differing levels of risk. In each case, levels of outcome were compared with the reference lowest group. Further post-hoc pairwise comparisons were made for each level of the outcome. Where the independent variable had more than two levels, a trend test was performed by including the variable as a continuous predictor. Where GMFCS and BFMF level along with secondary variables were categorized as dichotomous variables, a parallel analysis was performed using binary logistic regression; p values of less than 0.05 were considered statistically significant.
The present study was approved by the Regional Ethics Committee in Mid-Norway.
RESULTS
Characteristics of the participating and the non-participating children are shown in Table I. The table shows that the distribution of GMFCS levels varied slightly between the groups (p=0.013). The main difference was a lower proportion of children in GMFCS level V among participants than among non-participants. The participants had a mean age of 12 years (SD 2y 3mo) at the time of the genotyping.
Table I.
Characteristics of the children recorded on the Cerebral Palsy Register of Norway, diagnosed with cerebral palsy, and born between 1996 and 2003. Children who returned swabs are compared with non-participants
| Participants in study (n=281) | Non-participants (n=422) | |
|---|---|---|
|
|
||
| n (%) | n (%) | |
| Sex | ||
| Males | 156 (55.5) | 244 (57.8) |
| Females | 125 (44.5) | 178 (42.2) |
| CP subtype according to SCPE classification | ||
| Unilateral | 115 (42.4) | 154 (38.1) |
| Bilateral | 129 (47.6) | 190 (47.0) |
| Dyskinetic | 16 (5.9) | 35 (8.7) |
| Ataxic | 11 (4.1) | 25 (6.2) |
| Non-classified | 10 (3.5) | 18 (4.2) |
| GMFCS level | ||
| I–II | 182 (64.8) | 247 (58.5) |
| III | 36 (12.8) | 44 (10.4) |
| IV | 40 (14.2) | 58 (13.7) |
| V | 23 (8.2) | 61 (14.5) |
| Missing | 0 | 12 (2.8) |
| BFMF level | ||
| I | 88 (31.3) | 103 (24.4) |
| II | 91 (32.4) | 122 (28.9) |
| III | 36 (12.8) | 32 (7.5) |
| IV | 29 (10.3) | 44 (10.4) |
| V | 29 (10.3) | 69 (16.3) |
| Missing | 8 (2.8) | 52 (12.3) |
| Use of antiepileptic drugs | ||
| Yes | 67 (23.8) | 117 (27.7) |
| No | 121 (43.0) | 186 (44.0) |
| Missing | 94 (33.4) | 120 (28.4) |
| Gastrostomy tube feeding | ||
| Yes | 26 (9.2) | 68 (16.1) |
| No | 242 (86.1) | 328 (77.7) |
| Missing | 13 (4.6) | 26 (6.1) |
CP, cerebral palsy; SCPE, Surveillance of Cerebral Palsy in Europe; GMFCS, Gross Motor Function Classification System; BFMF, Bimanual Fine Motor Function.
Table II shows the distribution of ApoE alleles in the study population. The proportion of children being hetero- or homozygous regarding the APOEε4 allele did not differ from the proportion reported in a study of 798 healthy Norwegian blood donors (p=0.37).17 The allele frequencies were tested and found to be in Hardy–Weinberg equilibrium.
Table II.
Distribution of ApoEalleles among 255 children with cerebral palsy in Norway
| Genotype | n (%) study population |
|---|---|
| E2/E3 | 25 (9.8) |
| E2/E4 | 5 (2) |
| E3/E3 | 149 (58.4) |
| E3/E4 | 61 (23.9) |
| E4/E4 | 15 (5.9) |
| E2/E2 | 0 |
There was no association between GMFCS levels and the presence of the APOEε4 allele regardless of whether the outcome (GMFCS) was studied as a dichotomous variable (Table III) or as a categorical variable (data not shown). Also, in analyses stratified by CP subtypes there was no association between any of the ApoE alleles and the severity of gross motor impairment (data not shown).
Table III.
Proportion of children with gross motor function corresponding to GMFCS levels I–II and III–V, epilepsy, and gastrostomy tube feeding according to the presence or absence of the APOEε4 allele with corresponding odds ratios and 95% confidence intervals
| no ε4 | ε4 | Odds ratio (CI)a | p | |
|---|---|---|---|---|
| GMFCS level | ||||
| I–II | 115 | 51 | 1.15 (0.66–1.99) | 0.63 |
| III–V | 59 | 30 | ||
| Epilepsy | ||||
| No | 81 | 27 | 2.19 (1.13–4.24) | 0.02 |
| Yes | 37 | 27 | ||
| Gastrostomy | ||||
| No | 154 | 68 | 2.72 (1.12–6.59) | 0.027 |
| Yes | 10 | 12 |
Children without ε4 allele used as reference with odds ratio of 1. CI, confidence interval.
Moreover, there was no association between fine motor function (BFMF levels) and the APOEε4 allele in the total CP population (OR 1.39; 95% CI 0.86–2.25; p=0.176). In contrast, the presence of the ε4 allele was associated with active epilepsy (OR 2.19; 95% CI 1.13ε4.24; p=0.02) and a requirement for GTF (OR 2.72; 95% CI 1.12–6.59; p=0.027).
However, in stratified analyses we found that the presence of the ε4 allele was strongly associated with more severe fine motor impairment (i.e. a higher BFMF level) among children with spastic unilateral CP (Table IV). No similar trend was seen for children with spastic bilateral CP, dyskinetic, or ataxic CP (data not shown).
Table IV.
The proportion of children with fine motor function corresponding to Bimanual Fine Motor Function (BFMF) levels I–III, according to the presence of APOEε4 allele among children with unilateral CP.
| BFMF level | no ε4 | ε4 | OR (CI)a | p | OR (CI); p-value for trend test |
|---|---|---|---|---|---|
| I | 36 | 10 | 1 | ||
| II | 34 | 12 | 1.27 (0.49–3.32) | 0.630 | 2.60 (1.31–6.88); 0.007 |
| III | 5 | 10 | 7.20 (1.99–25.95) | 0.003 |
The corresponding odds ratio is given with 95% confidence intervals that the presence of the e4 allele was associated with more severe fine motor impairment, using children without the allele as reference (n=107)
Children with BFMF level I used as reference with an odds ratio of 1. OR, odds ratio; CI, confidence interval.
DISCUSSION
In this study, we could not confirm our hypothesis that carrying the APOEε4 allele would have a protective effect on the severity of CP, whether assessed by impairments in gross or fine motor function or by associated impairments. Instead, we found significant associations between the presence of the APOEε4 allele and the presence of epilepsy or the need for GTF. Among children with unilateral CP we also found an association between carrying the APOEε4 allele and more severe impairments of fine motor function.
We consider chance to be an unlikely explanation for the lack of evidence of a protective effect of the APOEe4 allele in brain injuries leading to CP in this study, since all effects were in the opposite direction. Although statistically significant, some of the subgroup analyses were statistically underpowered and these results should, therefore, be interpreted with caution. Nonetheless, the finding of more impaired fine motor function among children with unilateral CP was statistically highly significant.
Approximately 40% of the families returned swabs; the proportion of children with the most severe gross motor impairments was lower among participants than among non-participants, suggesting some selection bias. However, it is most unlikely that the ApoE genotype affected the response rate, and we therefore consider it unlikely that selection bias could explain our results. This is further supported by the lack of a trend in any direction (no association either with more or less severe GMFCS levels). Finally, the distribution of ApoE alleles was very similar to the general Norwegian population, suggesting that participants were representative of the background population.
The retrospective classification of children born between 1996 and 1998 into GMFCS levels may have led to misclassification of patients. However, this would not be expected to affect the results when we dichotomized our data into those in GMFCS levels I and II and III to V, as the main difference between these two groups is the ability to walk without assistive devices. Confounding owing to differences in ethnicity and thereby a different distribution of ApoE genotypes is unlikely in this Norwegian population, which consisted mainly of white Europeans.
Our main findings are in conflict with the previous study by Blackman et al. which showed weak evidence of a protective effect of the APOEε4 allele.9 This difference could be because of differences in ethnicity and sample size. Our results are also not consistent with those of Oria et al., who studied cognitive and physical development in poor Brazilian favela children with heavy burdens of diarrheal diseases.12 In that study, carrying the APOEε4 allele was shown to be a developmental advantage. However, it was thought that the protective effect of ApoE4 could possibly be related to a better intestinal absorption of cholesterol and not just a specific protective effect of ApoE4 in the central nervous system.
Our results did not provide evidence in favour of a protective effect of the APOEε4 allele. Instead, we found some evidence of an adverse effect of the APOEε4 allele on the severity of CP, as indicated by more epilepsy, a greater need for GTF, and more severe fine motor impairment among children with unilateral CP. In the following section, we will discuss the plausibility of these results, which, to our knowledge, have not been described earlier.
The results are consistent with the majority of research reporting an association between the presence of the APOEε4 allele and adverse outcome following traumatic brain injury in adults.18 Similar results after traumatic brain injury in children and young adults have also been reported.19,20
The general molecular mechanisms underlying the poorer outcome associated with the presence of the lipoprotein ApoEε4 may be a reduced ability to modulate the inflammatory response resulting in increased neuronal damage, less dendritic branching and axonal sprouting with limited connectivity compared with the other ApoE lipoproteins.21,22 In addition, new synapse formation, essential for reestablishing communication between neurons is a process that seems less efficient in the brains of APOEε4 carriers.23
The association between the presence of the APOEε4 allele and epilepsy in our study may be consistent with a few smaller studies on the genetic background of epilepsy in children without CP.24 However, most of these studies have been done in children with chronic temporal lobe epilepsy, and most reports on the association between APOEε4 and epilepsy have been inconclusive.25,26 At the same time, there are several studies in adults with post-traumatic seizures pointing to a seemingly unfavourable effect of the APOEε4 allele.27
Although the exact mechanisms underlying epilepsy are poorly understood, an association between the extent of the brain damage and epilepsy may be biologically plausible. In a recent study, Aboud et al.28 described different neuronal resilience from ApoE genotypes ε3/ε3 and ε4/ε4 in surgically resected material from patients with intractable epilepsy. Their findings indicate that neurons from individuals with the APOEε3/3 genotype are better able than their APOEε4/ε4 counterparts to mount appropriate and more liberal repair responses to the hyperexcitability induced neuronal damage of epilepsy. This suggests that APOEε3, but not APOEε4, alleles confer resilience to host neurons no matter the type of injury.
The need for GTF in children with CP is linked to extensive neuronal damage in subcortical areas of the brain and is subsequently used for children with the most impairment. The association between APOEε4 and a greater need for GTF can accordingly be regarded as an expression of more widespread brain injury in the carriers of this allele. It can be further argued that this finding supports the concept of less efficient neuronal repair mechanisms provided by APOEε4.
The apparent discrepancy between the adverse effect of APOEε4 leading to epilepsy and increased frequency of gastrostomy feeding, and no detectable effect on motor impairment may be explained by differences in the localization and extent of the injury. Epilepsy and feeding difficulties necessitating gastrostomy can also conceivably be caused by neuronal damage at an extent too small to be detected by changes in GMFCS level, but still extensive enough to influence these complications of CP.
The association between carrying the APOE4 allele and more severe fine motor impairment in the participants with unilateral CP has also not been described earlier. However, in a review of apolipoprotein E in Alzheimer disease and other neurological disorders, Verghese et al.25 describe a suggestive association between poor long-term outcome in patients with intracerebral haemorrhage for carriers of APOEε4, but not following ischaemic stroke. We are not able to differentiate between these two mechanisms in our study. The biological mechanism underlying this association in our study is difficult to explain. An explanation must include the concept that the molecular processes leading to unilateral CP are in some way different from the ones underlying bilateral CP. One speculation could be that this genotype-specific effect is due to differences in the timing and extent of the insult. Although most cases of spastic unilateral CP are due to antenatal insults (stroke), most cases of bilateral CP are related to preterm birth or severe hypoxic-ischaemic injury at term birth. Thus, the more limited injury occurring in utero may be more accessible to endogenous repair than insults occurring at birth, regardless of whether the child is born preterm or at term. Under the more favourable conditions in utero, the presence or absence of the effect of APOEε4 may play an additional role.
CONCLUSION
The present study did not show effects of the various ApoE genotypes large enough to influence the levels of the GMFCS and did not support our main hypothesis of a protective effect of the APOEε4 allele on the severity of CP. Instead, subgroup analyses suggested a detrimental effect of the APOEε4 allele on fine motor function, epilepsy, and the ability to feed independently, consistent with findings of a worse outcome in patients recovering from traumatic brain injury.
Figure 1.
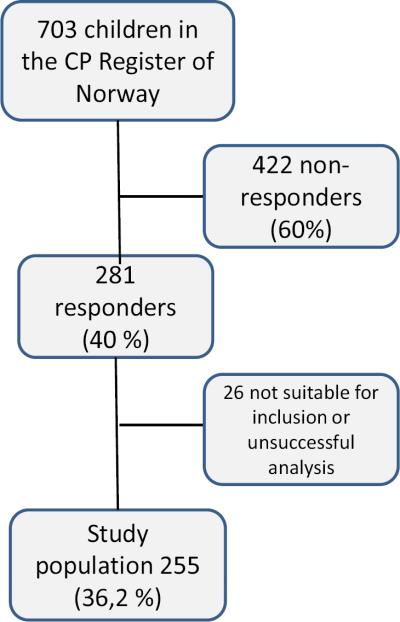
Flow chart of the study population of 255 children, starting with the 703 children in the Cerebral Palsy Register of Norway, born between 1996 and 2003.
AKNOWLEDGEMENTS
This study was supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (R03HD65826), University of Virginia Children's Hospital Grant-in-Aid, the University of Virginia Pratt Research Fund, and the Health Authority of Central Norway.
We would also like to thank Ms Jodi Darring at the University of Virginia, and Ms Sandra Julsen Hollung at the CPRN for their assistance, and all the children and their families who contributed to the study.
ABBREVIATIONS
- ApoE
Apolipoprotein E
- BFMF
Bimanual Fine Motor Function
- CPRN
Cerebral Palsy Register of Norway
- GTF
Gastrostomy tube feeding
REFERENCES
- 1.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–6. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 2.Stoknes M, Andersen GL, Elkamil AI, et al. The effects of multiple pre- and perinatal risk factors on the occurrence of cerebral palsy. A Norwegian register based study. Eur Journal Paediatr Neurol. 2012;16:56–63. doi: 10.1016/j.ejpn.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 3.O'Callaghan ME, MacLennan AH, Haan EA, Dekker G. The genomic basis of cerebral palsy: a HuGE systematic literature review. Hum Genet. 2009;126:149–72. doi: 10.1007/s00439-009-0638-5. [DOI] [PubMed] [Google Scholar]
- 4.Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res. 2011;50:62–74. doi: 10.1016/j.plipres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan BD. Genetic influences on outcome following traumatic brain injury. Neurochem Res. 2007;32:905–15. doi: 10.1007/s11064-006-9251-3. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda MM, Weck ME, Sarwark JF, Hamidullah A, Wainwright MS. Association of apolipoprotein E genotype and cerebral palsy in children. Pediatrics. 2007;119:306–13. doi: 10.1542/peds.2006-1083. [DOI] [PubMed] [Google Scholar]
- 7.Braga LW, Borigato EV, Speck-Martins CE, et al. Apolipoprotein E genotype and cerebral palsy. Dev Med Child Neurol. 2010;52:666–71. doi: 10.1111/j.1469-8749.2009.03465.x. [DOI] [PubMed] [Google Scholar]
- 8.McMichael GL, Gibson CS, Goldwater PN, et al. Association between Apolipoprotein E genotype and cerebral palsy is not confirmed in a Caucasian population. Hum Genet. 2008;124:411–6. doi: 10.1007/s00439-008-0564-y. [DOI] [PubMed] [Google Scholar]
- 9.Blackman JA, Gurka MJ, Bao Y, Dragulev BP, Chen WM, Romness MJ. Apolipoprotein E and functional motor severity in cerebral palsy. J Pediatr Rehabil Med. 2009;2:67–74. doi: 10.3233/PRM-2009-0063. [DOI] [PubMed] [Google Scholar]
- 10.Meirelles Kalil Pessoa de B, Rodrigues CJ, de Barros TE, Bevilacqua RG. Presence of apolipoprotein E epsilon4 allele in cerebral palsy. J Pediatr Orthop. 2000;20:786–9. doi: 10.1097/01241398-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE *4 a `thrifty' allele? Ann Hum Genet. 1999;63:301–10. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 12.Oria RB, Patrick PD, Blackman JA, Lima AA, Guerrant RL. Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med Hypotheses. 2007;68:1099–107. doi: 10.1016/j.mehy.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surveillance of Cerebral Palsy in Europe Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE) Dev Med Child Neurol. 2000;42:816–24. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 14.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 15.Andersen GL, Irgens LM, Haagaas I, Skranes JS, Meberg AE, Vik T. Cerebral palsy in Norway: prevalence, subtypes and severity. Eur J Paediatr Neurol. 2008;12:4–13. doi: 10.1016/j.ejpn.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Beckung E, Hagberg G. Neuroimpairments, activity limitations, and participation restrictions in children with cerebral palsy. Dev Med Child Neurol. 2002;44:309–16. doi: 10.1017/s0012162201002134. [DOI] [PubMed] [Google Scholar]
- 17.Kumar T, Liestol K, Maehlen J, Hiorth A, Jettestuen E, Lind H, et al. Allele frequencies of apolipoprotein E gene polymorphisms in the protein coding region and promoter region (−491A/T) in a healthy Norwegian population. Hum Biol. 2002;74:137–42. doi: 10.1353/hub.2002.0006. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008;25:279–90. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]
- 19.Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005;128:2556–61. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- 20.Brichtova E, Kozak L. Apolipoprotein E genotype and traumatic brain injury in children – association with neurological outcome. Childs Nerv Syst. 2008;24:349–56. doi: 10.1007/s00381-007-0459-6. [DOI] [PubMed] [Google Scholar]
- 21.Dumanis SB, Tesoriero JA, Babus LW, et al. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–22. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo L, LaDu MJ, Van Eldik LJ. A dual role for apolipoprotein e in neuroinflammation: anti- and pro-inflammatory activity. J Mol Neurosci. 2004;23:205–12. doi: 10.1385/JMN:23:3:205. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kauffman MA, Consalvo D, Moron DG, Lereis VP, Kochen S. APOE epsilon4 genotype and the age at onset of temporal lobe epilepsy: a case-control study and meta-analysis. Epilepsy Res. 2010;90:234–9. doi: 10.1016/j.eplepsyres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–52. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briellmann RS, Torn-Broers Y, Busuttil BE, et al. APOE epsilon4 genotype is associated with an earlier onset of chronic temporal lobe epilepsy. Neurology. 2000;55:435–7. doi: 10.1212/wnl.55.3.435. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Arrastia R, Gong Y, Fair S, et al. Increased risk of late posttraumatic seizures associated with inheritance of APOE epsilon4 allele. Arch Neurol. 2003;60:818–22. doi: 10.1001/archneur.60.6.818. [DOI] [PubMed] [Google Scholar]
- 28.Aboud O, Mrak RE, Boop F, Griffin ST. Apolipoprotein epsilon3 alleles are associated with indicators of neuronal resilience. BMC Med. 2012;10:35. doi: 10.1186/1741-7015-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]


