Abstract
Background
The efficacy of Roux-en-Y gastric bypass surgery to produce weight loss has been well documented, but few studies have measured the key components of energy balance, food intake, and energy expenditure longitudinally.
Methods
Male Sprague-Dawley rats on a high-fat diet underwent either RYGB, sham operation, or pair feeding, and were compared to chow-fed lean controls. Body weight and composition, food intake and preference, energy expenditure, fecal output, and gastric emptying were monitored before and up to 4 months after intervention.
Results
Despite recovery of initially decreased food intake to levels slightly higher than before surgery and comparable to sham-operated rats after about 1 month, RYGB rats maintained a lower level of body weight and fat mass for 4 months that was not different from chow-fed age-matched controls. Energy expenditure corrected for lean body mass at 1 and 4 months after RYGB was not different from pre-surgical levels and from all other groups. Fecal energy loss was significantly increased at 6 and 16 weeks after RYGB compared to sham operation, and there was a progressive decrease in fat preference after RYGB.
Conclusions
In this rat model of RYGB, sustained weight loss is achieved by a combination of initial hypophagia and sustained increases in fecal energy loss, without change in energy expenditure per lean mass. A shift away from high-fat towards low-fat/high-carbohydrate food preference occurring in parallel suggests long term adaptive mechanisms related to fat absorption.
Keywords: high-fat diet, body composition, adiposity, fat preference, fecal energy loss, gastric emptying
Introduction
Roux-en-Y gastric bypass surgery (RYGB) is highly effective in the treatment of obesity and remission of type-2 diabetes [1–5]. Unlike calorie restriction by dieting, the weight-reduced state after RYGB does not appear to trigger the powerful metabolic adaptations resulting in reduced energy expenditure and increased hunger [6, 7], thus resulting in sustained weight loss up to 16 years [8, 9]. A number of clinical studies concluded that RYGB patients lost the preoccupation with thoughts about food and the desire to eat that beleaguered them before surgery [10, 11]. Thus, understanding the mechanisms suppressing these counter-regulatory adaptive processes that allow sustained weight loss over many years should lead to powerful novel treatment strategies in the fight against obesity and metabolic diseases.
A number of candidate mechanisms have been proposed for the beneficial effects of RYGB, including elevated circulating levels of anorexigenic gut hormones GLP-1 and PYY as well as reduced levels of the orexigenic hormone ghrelin [12–15]. However, given the complexity of signaling from the gut to the brain and other organs, there is likely to be more than one mechanism involved, and there may be a temporal evolution of signaling changes. In addition to decreased food intake, increased energy expenditure also starts to emerge as a contributing factor to the sustained weight loss after RYGB. Human studies have produced conflicting data with either reduced [16–21], unchanged [22–25], or increased [26] total or resting energy expenditure at various times after RYGB.
Recent studies in rodent models lean toward an increased energy expenditure after surgery [27–29] or at least less of a decrease that could be expected from weight loss-induced metabolic adaptation [30, 31] [32]. Differences in findings are likely due to the different times of measurement relative to surgery and/or the use of different correction factors.
The aim of the present study was, therefore, to monitor changes in body composition, food and water intake, energy expenditure, fecal production and energy loss, and gastric emptying longitudinally, before and at various times after RYGB surgery. We carried out RYGB or sham surgery on rats made obese on two-choice high- and low-fat diet to closely mimic the human situation. Control groups on the same two-choice diet included sham-operated rats and sham-operated rats pair-fed to the caloric intake of RYGB rats. An additional control group consisted of age-matched chow-fed rats without any surgical intervention.
Materials and Methods
Animals and housing
Male Sprague-Dawley rats initially weighing ~200 g (Harlan Industries, Indianapolis, IN) were housed individually in wire-mesh cages at a constant temperature of 21–23° C with a 12h light-dark cycle (lights on 07:00, off at 19:00). Food and water were provided ad libitum unless otherwise indicated. Animals were made obese by putting them on a two-choice diet for 23–26 weeks consisting of normal laboratory chow (Kcal%: Carb, 58; Fat, 13.5; Prot, 28.5, # 5001, Purina LabDiet, Richmond IN) and high-sucrose, high-fat diet (sweet HF diet; Kcal%: Carb, 35; Fat, 45; Prot, 20, D12451, Research Diets, New Brunswick, NJ), with each of the diets containing sufficient minerals and vitamins. Liquid Ensure diet (Kcal%: Carb, 64; Fat, 21.6; Prot, 14.4, Abbott Laboratories, Columbus, OH) was also provided before the surgery for habituation to the diet. They were then randomly assigned to either sham surgery, RYGB surgery, or sham surgery pair-fed to RYGB animals. After surgery, only liquid Ensure was provided as a source of food for the first 10 days, and then regular chow and sweet HF diet were made available thereafter. A lean control group without surgery was placed on a regular chow diet throughout the experiment.
All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institutes of Health.
Roux-en-Y gastric bypass surgery
Details of the RYGB surgical procedure have been reported earlier [15, 31]. Briefly, the procedure connected a gastric pouch that was approximately 20% of the total gastric volume to a Roux limb about 15 cm-long, a biliopancreatic limb about 40 cm-long, and a common limb that was 25 cm-long.
Sham-surgery was similar to the RYGB procedure with the exceptions that there were incisions in the jejunum about 25 cm from the ileocecal valve and another in the gastric fundus both of which were closed with sutures, the transected jejunum was re-anastomosed, and the stomach had the cutting stapler laid over it without firing. This process produced a similar amount of surgical trauma in the sham-operated rats while preserving the normal flow of nutrients.
To overcome potential deficits in iron absorption and development of anemia, rats were administered a macromolecular dextran-iron complex (Iron Dextran injectable, catalog # 93963, 5 mg, sc; Town and Country, Ashland, OH) once a week for the first two weeks after RYGB surgery. Additional doses were administered to individual anemic animals if indicated.
Measurement of body composition and body weight
Body composition was initially measured 23 weeks before surgery before introducing the high-fat diet, again at 3 weeks before surgery after 20 weeks of the high-fat diet, and then at 1, 3, 6, and 15 weeks post-surgery. A Minispec LF 90 NMR Analyzer (Bruker Corp., The Woodlands, TX), which uses whole-body magnetic resonance relaxometry in non-anesthetized rodents, was used to measure the body composition with excellent linearity and reproducibility [35].
Body weight was measured daily for the first two weeks followed by weekly recording. Body weight was monitored daily for the first two weeks, and then was recorded weekly. Body composition was measured before introduction of the high-fat diet (23 weeks before surgery), after 20 weeks of high-fat diet (3 weeks before surgery), and at 1, 3, 6, and 15 weeks after surgery, by using a Minispec LF 90 NMR Analyzer (Bruker Corporation, The Woodlands, TX). This method uses whole body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility.
Measurement of food intake and high-fat preference
Food and water intake was measured for 3 weeks before surgery and throughout the postoperative period. During days 2–10, Ensure intake was measured daily, and for later time points, intake of each component of the two-choice diet, regular chow and high-fat diet was measured by taking spillage into account. All intake is reported in kcal/day, and fat preference was calculated as calories from high-fat diet/(calories from high-fat + regular chow) × 100.
Measurement of energy expenditure
Energy expenditure was measured by indirect calorimetry (Comprehensive Lab Animal Monitoring System [CLAMS]; Columbus Instruments, Columbus, OH), 4 weeks before surgery as well as 5 and 17 weeks after surgery. The rats were habituated to the chambers for 1–2 days before collecting data. Then VO2 consumption and respiratory exchange ratio (RER) were measured for a three-day period with ad libitum access to food. Resting energy expenditure (REE) was defined as the bottom 10% of the energy expenditure during the 3-day period. Four months after surgery, the same measurements were taken when the animals were subjected to fasting for 24h and re-feeding afterwards.
Measurement of fecal output
Feces were collected over a period of 3 days at −20, 40, and 110 days relative to surgery. Both wet weight and dry weight, after dried in a convection oven at 50 °C, were recorded. Gross fecal energy content (kcal/g) was determined by bomb calorimetry in the Analytical Laboratory of Kansas State University (Manhattan, KS).
Measurement of gastric emptying
Animals were adapted to ingest a prepared pancake 3–5 days before testing. After an overnight fast, rats were placed into 7 L Plexiglass chambers. Air flow rate into the chamber was at 0.65 L/min. After stabilization of the baseline, rats received 1 g of pancake containing 5 μl [13C]-octanoic acid. All animals finished the pancake within 5 min. [13C]- to [12C]-carbon dioxide ratio was analyzed from the exhaled air in the chamber using the nondispersive Infra Red Isotope Analyzer (IRIS; Wagner Analysen Technik, Bremen, Germany) [36]. After initial 5 min sampling for 1h, air samples were taken at 15 min intervals for a total duration of 6h. The time needed to reach 50% recovery of the administered [13C] substrate was used to determine gastric half-emptying time (T1/2). Latency to the peak in fractional dose per hour (Tmax) was also determined.
Statistical analyses
Body weight, body composition, food intake, fat preference, water intake, fecal energy output, energy expenditure, and respiratory exchange rate across time were analyzed by two-way repeated measures ANOVA, with treatment as a between-subject factor and days as a repeated within-subject factor, followed by Bonferroni’s post-hoc multiple comparison test. Food intake for food choice/acceptance was analyzed by a three-way repeated measures ANOVA, with treatment and diet as a between-subject factor and days as a repeated within-subject factor, followed by Bonferroni’s multiple comparison test. Gastric emptying was analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test. Data are presented as mean ± SEM. Statistical significance was set at p < 0.05.
Results
Body weight and body composition
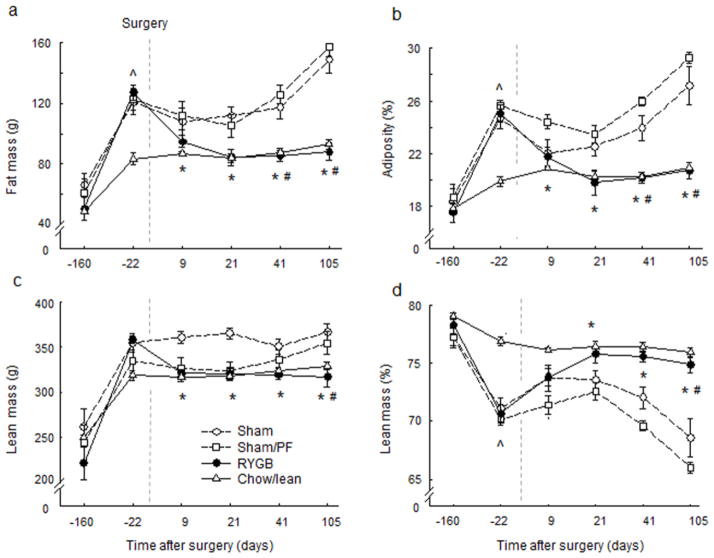
Our high-fat diet-exposed rats weighed ~550g, while the age-matched, chow-fed control rats weighed ~450g at the time of surgery (Fig. 1a). The high-fat diet-induced weight gain was due to both fat mass gain from 61g to 123g (+102%) and lean mass gain from 240g to 345g (+44%), resulting in an increase in adiposity from 18.0% to 25.0% (+39%) and reduction in leanness from 77% to 70% (−9%) (Fig. 2). The chow-fed control rats gained less fat mass (+66%) and lean mass (+28%), and their adiposity increased only from 17.7 to 19.8 (+ 12%) in the same period of time.
Fig. 1. Body weight.
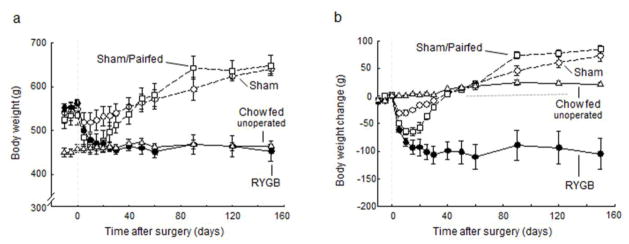
and body weight change of rats with Roux-en-Y gastric bypass surgery (RYGB, filled circles, n = 6), sham surgery (Sham, open circles, n = 7), sham surgery, pair-fed to RYGB (PF, open squares, n = 6), all fed a high-fat diet, and lean control rats fed a regular chow diet (Chow, open triangles, n = 6).
Fig. 2. Body composition.
(Means ± SEM of total and relative fat and lean mass) of rats with Roux-en-Y gastric bypass surgery (RYGB, filled circles, n = 6), sham surgery (Sham, open circles, n = 7), sham surgery, pair-fed to RYGB (PF, open squares, n = 6), all fed a high-fat diet, and lean control rats fed a regular chow diet (Chow, open triangles, n = 6). * p < 0.05, RYGB vs. Sham; # p < 0.05, RYGB vs. PF; ^ p < 0.05, chow vs. all other groups; based on ANOVA, followed by Bonferroni-corrected multiple comparisons.
After RYGB surgery, body weight decreased steeply for the first 10 days and then after 20 days reached a plateau of around 110 g (−20%) below pre-surgical levels (Fig. 1b). Both fat mass and lean mass significantly decreased after RYGB surgery, reaching levels exhibited by the chow-fed controls by 3 weeks after surgery (Fig. 2), after sham surgery, body weight decreased only transiently by about 25 g and then continued to steadily increase for the remainder of the observation period to about 75g (+13%) above presurgical levels (Fig. 1). This increase was mainly due to an increase in fat mass, as reflected by the further increase in adiposity to 27.5 ± 1 % at 4 months post-surgery (Fig. 2). Sham-operated rats that were pair-fed to the intake of RYGB rats lost weight more rapidly than ad libitum-fed sham-operated rats and similarly to RYGB rats, but then gained weight back rapidly as pair-feeding was no longer restricting their intake. By 6 weeks after surgery, sham-operated, pair-fed rats had caught up with sham-operated rats, and at the end of the observation period their adiposity and percent lean mass were not significantly different from each other.
Food and water intake
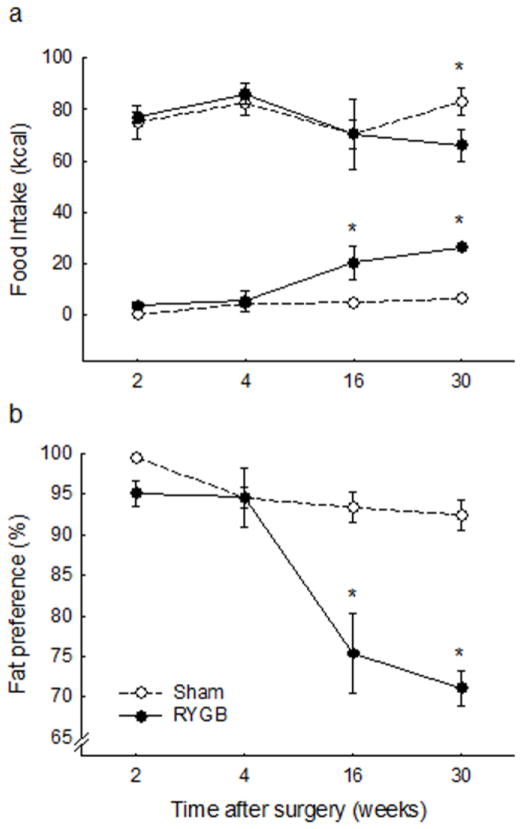
Energy intake from Ensure was severely suppressed for the first few days after RYGB compared with sham-operated rats, but recovered within about 2 – 3 weeks after surgery, when they were given a choice between high-fat pellets and regular fat chow (Fig. 3a). Pair-feeding was halted 6 weeks after surgery, when RYGB rats no longer exhibited hypophagia.
Fig. 3. Food and water intake of.
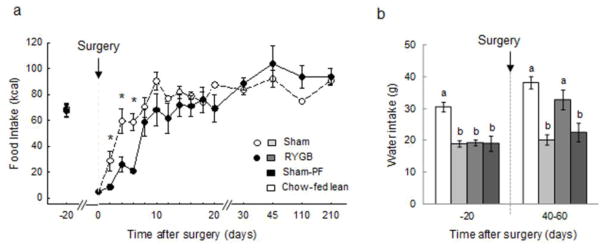
rats with Roux-en-Y gastric bypass surgery (RYGB, filled circles or medium gray bars, n = 6), sham surgery (Sham, open circles or light gray bars, n = 7), sham surgery, pair-fed to RYGB (Sham-PF, dark gray bars, n = 6), all fed a high-fat diet, and lean control rats fed a regular chow diet (Chow-fed lean, white bars, n = 6). * in a, p < 0.05. Bars in b that do not share the same letters are significantly different from each other, based on ANOVA, followed by Bonferroni-corrected multiple comparisons.
Water intake before surgery was similar for RYGB, Sham, and PF rats, significantly lower than for chow-fed controls (Fig. 3b). At 6 – 9 weeks after surgery, RYGB rats consumed significantly more water than sham and PF rats, an amount not significantly different from chow-fed controls.
Fat preference
If given a choice between high-fat diet and regular (low-fat) chow, rats designated to undergo RYGB surgery had a similar very high preference (>95%) for the high-fat diet (data not shown). After surgery, sham-operated rats continued with this very high preference for 45% fat diet, and their chow intake remained very low (Fig. 4). In contrast, the RYGB rats gradually increased chow intake and decreased high-fat intake, resulting in a significantly lower fat preference by about 3 months after surgery.
Fig. 4.
Fat preference of rats with Roux-en-Y gastric bypass surgery (RYGB, filled circles, n = 6), or sham surgery (Sham, open circles, n = 7), on a two-choice high-fat/regular chow maintenance diet. (a) intake of high fat diet (top) and chow (bottom) expressed in calories. (b) Preference ratio for high-fat diet intake. * p < 0.05, based on ANOVA, followed by Bonferroni-corrected multiple comparisons.
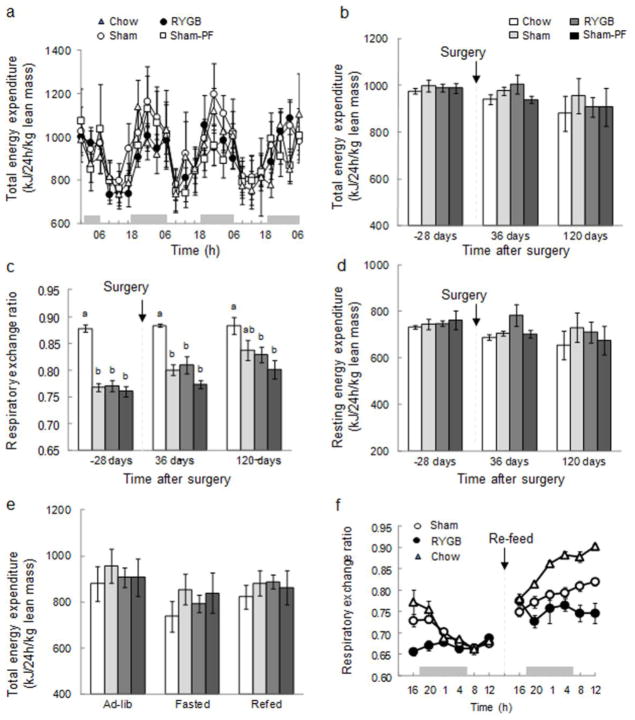
Energy expenditure and substrate utilization
Energy expenditure and substrate utilization was assessed by indirect calorimetry under ad libitum food intake conditions, 4 weeks before, as well as 5 and 17 weeks, after surgery. Continuous 72-h records of energy expenditure corrected for lean body mass showed the typical diurnal pattern in all groups, but no significant differences between groups at 5 weeks after surgery (Fig. 5a). There were also no significant differences in average daily total and resting energy expenditure corrected for lean body mass between RYGB and any other groups before surgery and at 5 and 17 weeks after surgery (Fig. 5b, d). To differentiate diet-induced thermogenesis from REE, we compared ad libitum, fasting, and re-feeding conditions at 17 weeks after surgery. As expected, 20-h fasting slightly decreased total energy expenditure across all groups, but there were no significant differences between the groups (Fig. 5e). Re-feeding the fasted rats brought energy expenditure back to higher levels, but again, there were no significant differences between RYGB and any other groups.
Fig. 5. Energy expenditure and RER.
of rats with Roux-en-Y gastric bypass surgery (RYGB, filled circles or medium gray bars, n = 6), sham surgery (Sham, open circles or light gray bars, n = 7), sham surgery, pair-fed to RYGB (Sham-PF, open squares or dark gray bars, n = 6), all fed a high-fat diet, and lean control rats fed a regular chow diet (Chow, open triangles or white bars, n = 6). (a) Energy expenditure during 3 successive day-night cycles, averaged over 4 h periods. (b) Total energy expenditure for the 4 groups before and after surgery. (c) Respiratory exchange ratio (RER) before and after surgery. Bars that do not share the same letters are significantly different from each other, based on ANOVA, followed by Bonferroni-corrected multiple comparisons. (d) Resting energy expenditure before and after surgery. (e) Effect of fasting and re-feeding on total energy expenditure 17 weeks after surgery. (f) Effect of fasting and re-feeding on RER, 17 weeks after surgery.
If energy expenditure was corrected for total body mass (as in most previously published RYGB studies), ANOVA showed significant treatment (F[3,42] = 11.4, p < 0.001) and time effects (F[2,42] = 8.2, p = 0.001), and follow up comparisons showed that average total daily energy expenditure was significantly higher in RYGB rats compared with sham (+17%) and pair-fed rats (+20%) 5 weeks after surgery (not shown). At 17 weeks after surgery, there was still a trend for increased energy expenditure in RYGB rats (+ 13%), compared to sham rats, and a significant increase +(19%), compared to pair-fed rats. Finally, if expressed per kg0.75 body weight, only the difference between RYGB (618 ± 18.1) and pair-fed rats (534 ± 18.2 kJ/24h/kg0.75, p < 0.05) at 5 weeks after surgery remained significant.
The respiratory exchange ratio which increases with increasing carbohydrate utilization showed the expected higher values in chow-fed lean control rats compared with the three other groups mostly consuming high-fat diet throughout the observation period (Fig. 5c). ANOVA showed significant treatment (F [3,42] = 21.9, p < 0.001) and time effects (F[2,42] = 23.3, p < 0.001), and a significant interaction (F[6,42] = 2.7, p = 0.025). Follow up comparisons showed that, although there was a trend for increasing respiratory exchange ratios after RYGB and sham surgery, there were no significant differences between RYGB, sham, and pair-fed rats either 5 or 17 weeks after surgery. As expected, the respiratory exchange ratio of all groups gradually converged to a lower level of between 0.66 and 0.68 during the fast, indicating a shift toward fat utilization, and it increased again after re-feeding (Fig. 5f). However, there were no significant differences observed between RYGB and sham rats.
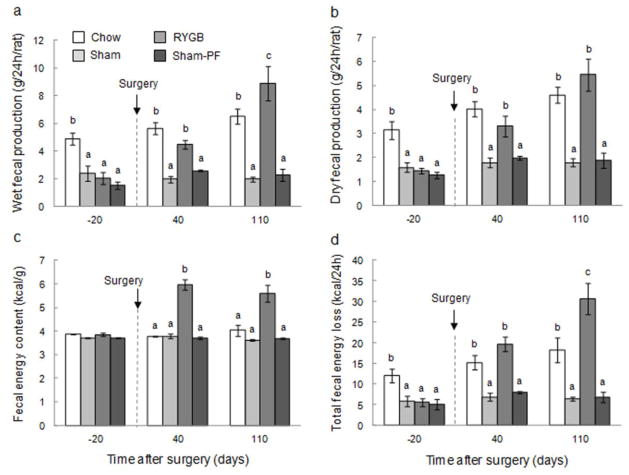
Fecal production and energy content
Before surgery, wet fecal output was similar in high-fat consuming RYGB, sham, and PF rats, but significantly higher in chow-consuming controls (Fig. 6a). At 6 and 16 weeks after surgery, both wet and dry fecal output significantly increased from pre-surgical levels in RYGB rats, but not in any other group. At 16 weeks after surgery, dry fecal output of RYGB rats was, however, similar to chow-fed lean rats (Fig. 6b). Fecal gross energy content was significantly greater by about 2 kcal/g (+50%) in RYGB rats both 6 and 16 weeks after surgery compared with all other groups (Fig. 6c). The combined effect of increased production and energy content resulted in significantly increased total fecal energy output both 6 and 16 weeks after RYGB compared with sham surgery and pair-feeding (Fig. 6d). While at 6 weeks after surgery, the total energy output of RYGB and chow-fed control rats was similar, around 15–20 kcal/day, it further increased to around 30 kcal/day in RYGB rats at 16 weeks after surgery, significantly higher than chow-fed rats.
Fig. 6. Fecal production and energy output.
of rats with Roux-en-Y gastric bypass surgery (RYGB, medium gray bars, n = 6), sham surgery (Sham, light gray bars, n = 7), sham surgery, pair-fed to RYGB (Sham-PF, dark gray bars, n = 6), all fed a high-fat diet, and lean control rats fed a regular chow diet (Chow, white bars, n = 6). Bars that do not share the same letters are significantly different from each other, based on ANOVA, followed by Bonferroni-corrected multiple comparisons.
Gastric emptying
ANOVA showed a significant main effect of treatment condition for lag time (F[3,43] = 6.36, p<0.001) (Fig. 7a), and for half-emptying time (F[3,43] = 3.65, p = 0.02) (Fig. 7b). Follow-up tests with Bonferroni correction revealed a significantly delayed lag time for obese rats compared with all three other conditions (p<0.05), and a significantly slower half-emptying time for obese rats compared with chow-fed lean control rats (p < 0.05). Follow-up tests with Fisher’s LSD test additionally revealed significantly shorter half-emptying time of RYGB compared with obese rats (p < 0.05).
Fig. 7. Gastric emptying.
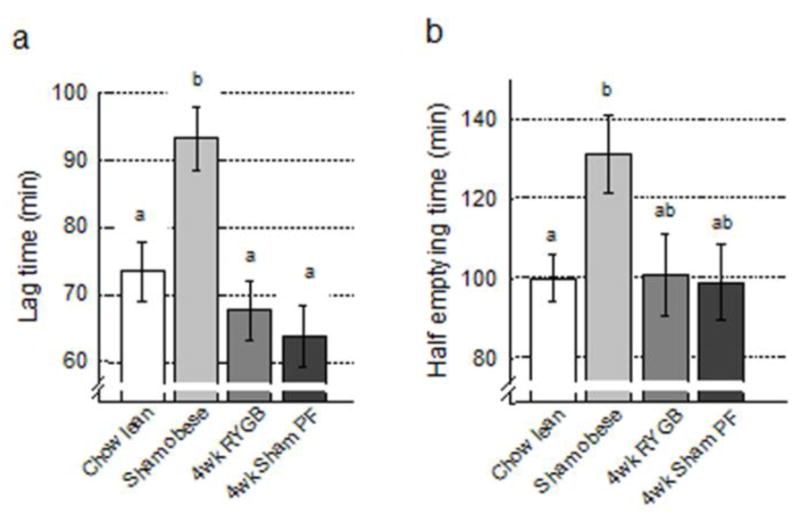
of rats with Roux-en-Y gastric bypass surgery (RYGB, medium gray bars, n = 6), sham surgery (Sham/obese, light gray bars, n = 13), sham surgery, pair-fed to RYGB (PF, dark gray bars, n = 6), all fed a high-fat diet, and lean control rats fed a regular chow diet (Chow, white bars, n = 19). Time to reach maximal emptying rate (lag time, a) and time to empty half of the food ingested (half-emptying time, b) are shown. Bars that do not share the same letters are significantly different from each other, based on ANOVA, followed by Bonferroni-corrected multiple comparisons.
Discussion
The results of this longitudinal study in a Sprague-Dawley rat model of RYGB show that several mechanisms contribute to the surgery-induced sustained reduction in body weight and fat mass. The initial hypophagia leads to rapid weight loss which is later defended by reduced fat absorption but not enhanced energy expenditure if it is expressed per lean body mass. These findings confirm some but not other earlier reports with various rat models of RYGB [14, 27–29, 37]. The present study is also unique in that it systematically assessed body composition and energy balance in the same animals before and at several time points after surgery. Also, in addition to sham-operated controls, the study included sham-operated, pair-fed rats and age-matched lean rats maintained exclusively on chow. Limitations of the study include the relatively low number of RYGB rats included, the somewhat incomplete measurements of total food intake, and the lack of any intervention approach towards identifying the potential underlying mechanisms.
Clearly, the surgery was successful in completely reversing and preventing further obesity as indicated by the flat body weight and composition from about 3 weeks after surgery to the end of the 4 month observation period. We have also shown in a previous study that obesity-induced elevated leptin levels are completely reversed by RYGB in this model [15]. Complete reversal of obesity is typically not observed in humans, where BMI often remains in the overweight or even obese range around 30 kg/m2 [38]
As in most other studies [14, 27, 28, 31, 37], food intake was initially greatly reduced, and although some of this reduction was associated with the nonspecific trauma of surgery as indicated by a sizable dip in sham-operated animals, the other part of this initial hypophagia was likely due to the restrictive component of RYGB. A meal pattern analysis during this period revealed a drastically reduced meal size and an unsuccessful attempt to compensate by increased meal frequency [31]. This is the equivalent of early satiety, thus resorting to snacking or grazing behavior seen in RYGB patients. In only one rat study there was no apparent reduction in food intake, and this might be explained by the fact that the animals were not obese and exclusively fed regular chow [29].
Not only did the hypophagia disappear after about 3–4 weeks, but RYGB rats started to ingest more calories than sham controls. Therefore, pair-feeding was stopped at this time, and pair-fed rats caught up with the body weight and body composition of sham-operated rats quite rapidly. The presence of slight hyperphagia in the face of stable lower body weight can either be explained by a reduction of nutrient absorption or an increase of energy expenditure, or both.
In contrast to several other reports in rodent models of RYGB [27–29, 39], we do not find energy expenditure to be increased in our rat model. However, this discrepancy is likely the result of different normalization procedures, with most of these studies using normalization per total body mass or a derivative of total body mass such as kg 0.75. If corrected for total body mass, we also find significantly higher energy expenditure after RYGB compared with sham-operated and pair-fed animals, but if corrected for lean body mass only, no significant differences are observed. This latter conclusion is in agreement with at least two other studies in rodents; no change in energy expenditure per rat was reported in obese Zucker rats, 2 weeks after RYGB surgery [32], and energy expenditure per rat was initially decreased after combination duodenal bypass-sleeve gastrectomy surgery in Wistar rats, before normalizing after 6 weeks [30].
Cross-sectional studies in humans reported either increased [23], no change in [17], or even decreased [40] energy expenditure, but in none of these studies was energy expenditure normalized to lean mass.
The caveats of reporting energy expenditure in rodents with large differences in fat mass have been discussed [41, 42], and although there is no perfect way to do it, it is clear that correction for lean body mass is superior to correction for total body mass or body mass to any power. This is because metabolic activity of fat tissue, although not negligible, is much less than metabolic activity of lean tissue. Thus, it is likely that the increased energy expenditure of RYGB compared with sham-operated rats in the Bueter et al.[28] and Stylopoulos et al. [27] studies was due to normalization to either total body weight or total body weight 0.75. In a recent study in mice, Nestoridi et al. also concluded that energy expenditure is significantly higher 2–8 weeks after RYGB compared to sham surgery, even though it was not different on an uncorrected per mouse basis [39]. However, a careful energy expenditure component analysis taking into account not only energy intake and expenditure, but also fecal energy loss, body energy gain, and energy cost for storage, suggests that “apparent energy expenditure” was significantly higher by about 10% after RYGB than after control surgery.
Taken together, it is clear that if correcting for lean body mass, no rodent or human study has unambiguously demonstrated significantly increased energy expenditure after RYGB compared with sham-operation. However, the many methodological differences including species, surgery, postsurgical time, and diet, as well as the fact that fat tissue is not completely metabolically inert, make it impossible to rule out a role of for energy expenditure in RYGB-induced weight loss. Future studies using more detailed energy expenditure component analysis in rodents [39], and comprehensive human studies using doubly-labeled water will be necessary to obtain definitive answers.
Fecal output and energy loss were clearly increased in our rat RYGB model. In RYGB rats, but not the other groups, dry fecal production significantly increased from a low level ~1.5 g before surgery, to ~3 g at 6 weeks and ~5 g at 16 weeks after surgery, and it was similar in weight to the considerable dry fecal production of chow-fed control rats. Although we have not measured macronutrient composition of fecal output, we speculate that RYGB rats lost mainly fat in the feces, while chow-fed rats lost mainly non-metabolizable fiber. This assumption is supported by the significantly increased fecal energy density in RYGB rats compared to all other groups, and findings in other rat models of RYGB using non-obese, chow-fed rats, in which no differences in fecal output and energy density were found between gastric bypass and sham-operated rats [14, 28, 29]. Consistent with our observations of increased fecal energy loss in high-fat fed rats after RYGB, Guijarro et al. [37] reported that in their rat model fat absorption was significantly reduced by ~ 30% after RYGB. Furthermore, a recent study in mice reported a more than three-fold higher fecal energy loss after RYGB compared with sham-operation [39]. In contrast, Stylopoulos et al. [27] reported a relatively small fecal energy loss of ~ 4% in yet another rat model of RYGB surgery. The smaller fecal energy loss in this latter study appears to be accounted for by the considerably longer common limb (~70% of total intestinal length) compared to our model and the model of Guijarro (~30%). Although fecal energy loss is not considered a major factor in humans, it can contribute up to 170 kcal/d to the total energy deficit at 14 months after long-limb RYGB [43], which is comparable to the relatively long biliopancreatic limb in our rat model.
Gastrointestinal transit time could play an important role in the efficiency of nutrient absorption. Delayed gastric emptying has been reported in obese subjects [44], and this could be reversed by RYGB. Accelerated delivery of undigested nutrients, particularly fat, to the Roux and common limbs could contribute to malabsorption. In agreement with the observation in obese subjects [44], gastric emptying of a mixed meal tended to be delayed in sham-operated obese rats, and this effect was reversed 4 weeks after RYGB. However, it is not clear why the equally obese pair-fed rats also showed this accelerated emptying trend, and the interpretation of the results is limited by the fact that measuring emptying of high-fat diet would have been more revealing. Furthermore, exactly the opposite was observed in a human study with accelerated gastric emptying in obese subjects compared to normal weight control subjects and reversal to slower gastric emptying 9 months after jejunoileal bypass [45]. Therefore, additional studies will be necessary to determine changes in gastric emptying after RYGB and its potential role in malabsorption and the control of food intake.
Conclusions
In this longitudinal study, we confirm the sustained effectiveness of RYGB in high-fat diet-induced obese rats to reduce body weight and adiposity. Reduced food intake appears to be responsible only for the initial rapid weight loss, but additive effects of fecal energy loss and perhaps slightly elevated energy expenditure keep the body weight at these lower levels for an extended period of time. The mechanisms responsible for each of these three components remain to be determined.
Acknowledgments
Grant Support: Supported by NIH grants #DK47348 and #DK071082
We thank Abby Duhé for expert technical support. The work was partially funded by the National Institutes of Health Grants DK47348 and DK071082.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Conflict of Interest Disclosure
None of the authors declares a conflict of interest
References
- 1.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52:1098–1103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- 4.Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31 (Suppl 2):S290–296. doi: 10.2337/dc08-s271. [DOI] [PubMed] [Google Scholar]
- 5.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–481. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 6.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 7.Redman LM, Heilbronn LK, Martin CK, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–423. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 10.Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr. 2010;92:277–283. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- 11.Olbers T, Bjorkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buscemi S, Caimi G, Verga S. Resting metabolic rate and postabsorptive substrate oxidation in morbidly obese subjects before and after massive weight loss. Int J Obes Relat Metab Disord. 1996;20:41–46. [PubMed] [Google Scholar]
- 17.Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 18.Tamboli RA, Hossain HA, Marks PA, et al. Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2010;18:1718–1724. doi: 10.1038/oby.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gastaldi G, Russell A, Golay A, et al. Upregulation of peroxisome proliferator-activated receptor gamma coactivator gene (PGC1A) during weight loss is related to insulin sensitivity but not to energy expenditure. Diabetologia. 2007;50:2348–2355. doi: 10.1007/s00125-007-0782-1. [DOI] [PubMed] [Google Scholar]
- 20.Das SK, Roberts SB, McCrory MA, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 21.Bobbioni-Harsch E, Morel P, Huber O, et al. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–4700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 22.de Castro Cesar M, de Lima Montebelo MI, Rasera I, Jr, de Oliveira AV, Jr, Gomes Gonelli PR, Aparecida Cardoso G. Effects of Roux-en-Y gastric bypass on resting energy expenditure in women. Obes Surg. 2008;18:1376–1380. doi: 10.1007/s11695-008-9460-8. [DOI] [PubMed] [Google Scholar]
- 23.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 24.Carrasco F, Rojas P, Ruz M, et al. Energy expenditure and body composition in severe and morbid obese women after gastric bypass. Rev Med Chil. 2008;136:570–577. [PubMed] [Google Scholar]
- 25.Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg. 2006;16:1602–1608. doi: 10.1381/096089206779319347. [DOI] [PubMed] [Google Scholar]
- 26.Flancbaum L, Choban PS, Bradley LR, Burge JC. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery. 1997;122:943–949. doi: 10.1016/s0039-6060(97)90336-6. [DOI] [PubMed] [Google Scholar]
- 27.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009;17:1839–1847. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bueter M, Lowenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Furnes MW, Tommeras K, Arum CJ, Zhao CM, Chen D. Gastric bypass surgery causes body weight loss without reducing food intake in rats. Obes Surg. 2008;18:415–422. doi: 10.1007/s11695-007-9392-8. [DOI] [PubMed] [Google Scholar]
- 30.Nadreau E, Baraboi ED, Samson P, et al. Effects of the biliopancreatic diversion on energy balance in the rat. Int J Obes (Lond) 2006;30:419–429. doi: 10.1038/sj.ijo.0803166. [DOI] [PubMed] [Google Scholar]
- 31.Zheng H, Shin AC, Lenard NR, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277–285. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- 34.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 35.Kunnecke B, Verry P, Benardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res. 2004;12:1604–1615. doi: 10.1038/oby.2004.200. [DOI] [PubMed] [Google Scholar]
- 36.Sanaka M, Yamamoto T, Kuyama Y. Retention, fixation, and loss of the [13C] label: a review for the understanding of gastric emptying breath tests. Dig Dis Sci. 2008;53:1747–1756. doi: 10.1007/s10620-007-0103-z. [DOI] [PubMed] [Google Scholar]
- 37.Guijarro A, Suzuki S, Chen C, et al. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1474–1489. doi: 10.1152/ajpregu.00171.2007. [DOI] [PubMed] [Google Scholar]
- 38.Bjorklund P, Laurenius A, Een E, Olbers T, Lonroth H, Fandriks L. Is the roux limb a determinant for meal size after gastric bypass surgery? Obes Surg. 2010;20:1408–1414. doi: 10.1007/s11695-010-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestoridi E, Kvas S, Kucharczyk J, Stylopoulos N. Resting energy expenditure and energetic cost of feeding are augmented after Roux-en-Y gastric bypass in obese mice. Endocrinology. 2012;153:2234–2244. doi: 10.1210/en.2011-2041. [DOI] [PubMed] [Google Scholar]
- 40.del Genio F, Alfonsi L, Marra M, et al. Metabolic and nutritional status changes after 10% weight loss in severely obese patients treated with laparoscopic surgery vs integrated medical treatment. Obes Surg. 2007;17:1592–1598. doi: 10.1007/s11695-007-9286-9. [DOI] [PubMed] [Google Scholar]
- 41.Himms-Hagen J. On raising energy expenditure in ob/ob mice. Science. 1997;276:1132–1133. doi: 10.1126/science.276.5315.1132. [DOI] [PubMed] [Google Scholar]
- 42.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odstrcil EA, Martinez JG, Santa Ana CA, et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92:704–713. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 44.Jackson SJ, Leahy FE, McGowan AA, Bluck LJ, Coward WA, Jebb SA. Delayed gastric emptying in the obese: an assessment using the non-invasive (13)C-octanoic acid breath test. Diabetes Obes Metab. 2004;6:264–270. doi: 10.1111/j.1462-8902.2004.0344.x. [DOI] [PubMed] [Google Scholar]
- 45.Naslund E, Melin I, Gryback P, et al. Reduced food intake after jejunoileal bypass: a possible association with prolonged gastric emptying and altered gut hormone patterns. Am J Clin Nutr. 1997;66:26–32. doi: 10.1093/ajcn/66.1.26. [DOI] [PubMed] [Google Scholar]