Abstract
Research suggests that structural properties of drug users’ social networks can have substantial effects on HIV risk. The purpose of this study was to investigate if the structural properties of Appalachian drug users’ risk networks could lend insight into the potential for HIV transmission in this population. Data from 503 drug users recruited through respondent-driven sampling were used to construct a sociometric risk network. Network ties represented relationships in which partners had engaged in unprotected sex and/or shared injection equipment. Compared to 1,000 randomly generated networks, the observed network was found to have a larger main component and exhibit more cohesiveness and centralization than would be expected at random. Thus, the risk network structure in this sample has many structural characteristics shown to be facilitative of HIV transmission. This underscores the importance of primary prevention in this population and prompts further investigation into the epidemiology of HIV in the region.
Keywords: HIV, Social network analysis, Rural, Injection drug users, Sociometric
Introduction
Over the past two decades, there has been a surge in the number of studies applying social network analysis (SNA) to research in public health [1], particularly to the study of behaviors that increase individuals’ risk for HIV and other blood borne and sexually transmitted infections (STIs) (e.g., [2–4]). This methodological trend both reflects and promotes a paradigmatic shift from understanding HIV risk as a product of individual risk behavior to one that encompasses the recognition that “human beings copulate in context” [5, p. S148]. Risk networks can promulgate or mitigate HIV transmission in ways that often cannot be anticipated from individual-level behavior alone. For example, a seminal study to apply SNA to the assessment of HIV and STI transmission conducted in Colorado Springs [6, 7] found that, based on individual-level data, the risk of HIV transmission appeared to be low (i.e. the prevalence of HIV was low and risk behavior was relatively uncommon). However, examination of network data revealed that risk was higher than anticipated, as everyone in the main component was within just a few steps of a HIV infected person [7]. Follow-up analyses of the data suggested that the lack of HIV transmission over time in the network may be attributable to a network structure that was decreasingly conducive to widespread transmission (i.e. decreased size and connectivity) [8] and to the peripheral location of individuals who were HIV positive [6].
Since the publication of these seminal studies, further research has provided insight into network measures that are most appropriate for understanding HIV transmission and engagement in HIV risk behaviors [9–11]. A systematic review by De et al. [2] identified that larger network size, higher density, and greater centrality were all associated with equipment sharing (e.g., sharing of needles, cookers, cottons, and/or rinse water) among injection drug users. In a longitudinal study of network structure, risk behavior, and HIV transmission, Rothenberg et al. [8] found that needle sharing was inversely associated with an increase in the number and decrease in the size of network components. Several studies have also examined the role of microstructures, or interconnected subgroups within networks, in affecting HIV and other STI transmission (e.g., [7, 8, 12–14]). Cohesive microstructures in risk networks can act as “incubators” for epidemics [15, p. 62], and in some cases can be particularly conducive to HIV and STI transmission [11, 16].
SNA can also expose a network’s vulnerability to HIV transmission and its amenability to network-based intervention. Systematic reviews indicate that network-based interventions can be successful in promoting HIV risk-reduction, such as condom use and decreased drug equipment sharing [3, 17]. However, because communities have their own social structures, networks, norms, and leaders, intervention approaches should be tailored to align with the needs and risks of particular communities [18]. The lack of understanding of the structure of risk networks in rural areas, therefore, could present a major impediment to intervention development. Thus, the purpose of the current study was to describe the risk network structure among high-risk, rural drug users residing in Appalachia in order to determine the potential for HIV transmission as well as the amenability of the network to intervention.
Methods
Research Site and Data Collection
From November 2008 to August 2010, data were collected for the social networks among Appalachian people (SNAP) study from drug users in a rural county (population 28,712 [19]) located in Appalachian, Kentucky. Inclusion criteria for the study included being at least 18 years of age, residing in an Appalachian Kentucky county, and having used at least one of the following to get high in the past 30 days: prescription opioids, heroin, crack/cocaine or methamphetamine. The overall goal of the SNAP study was to examine risk factors for HIV, hepatitis C (HCV), and herpes simplex-2 virus (HSV-2) using a social network approach. HIV testing was performed using the OraQuick® ADVANCE™ Rapid HIV-1/2 Antibody Test (OraSure, Bethlehem, PA), which is a manually performed, visually read, 20 min immunoassay for the detection of HIV-1 and HIV-2 antibodies in human oral fluid. The test has been shown to have high sensitivity and specificity [20]. All participants were also tested for antibodies to HCV and HSV-2; baseline prevalence was identified to be 44.1 and 11.7 %, respectively. Pre-and post-test counseling was provided, following CDC protocols [21].
Participants were recruited using respondent driven sampling (RDS), which is an especially useful technique for recruiting hard-to-reach populations such as drug users [22, 23], particularly in rural settings [24, 25]. A total of 107 seeds for RDS were identified through outreach workers and community informants and through flyers posted in town, including outside the study field office. Upon completion of an interview-administered questionnaire, seeds were given three coupons to sample their peers. Individuals who redeemed the coupons and completed the interview were then given coupons to recruit additional members. This process continued through 14 waves of recruitment until the desired sample size was achieved.
For each redeemed coupon, the distributor of the coupon was given $10. Participants were also compensated $50 for their time completing the interview-administered questionnaire. A total of 506 participants completed the interviews, but three reported discrepant substance use information on their eligibility screening assessment and interview and were excluded them from analysis. Thus, the final sample size for analysis was 503. The protocol was approved by the University of Kentucky Institutional Review Board and a Certificate of Confidentiality was obtained from the National Institutes of Health.
Social Network Data Collection
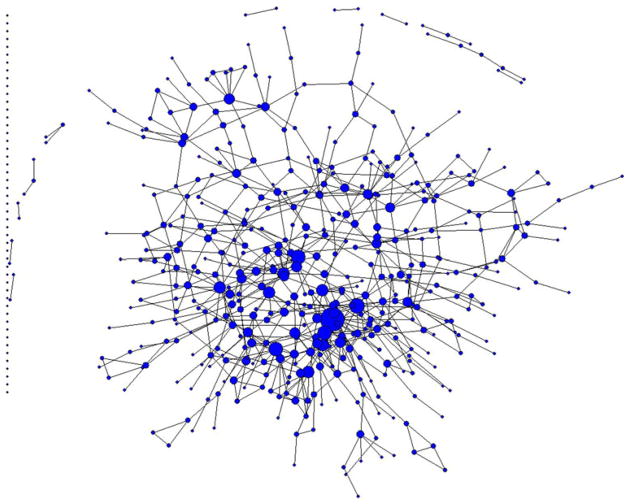
A name-generating questionnaire was used to collect data on participants’ drug and sex (risk) networks. The complete network is shown in Fig. 1. Participants gave the first name and last initial of anyone with whom they had sex and/or used drugs (excluding only alcohol and marijuana) during the past 6 months. For each network member named, additional demographic information was gathered (e.g., gender, race, approximate age, and employment status). Participants’ reported sexual activity and drug use with network members was assumed to be reciprocal (i.e. one participant’s report of drug co-usage or sex with a network member was sufficient to constitute a network linkage).
Fig. 1.
Complete network of sexual ties (including protected and unprotected sex) and drug-sharing ties (including drug equipment sharing and non-sharing) among 503 non-medical users of prescription drugs in rural Appalachian. Note Nodes are sized by degree centrality
A series of steps were followed to confirm ties between the network members. First, names and demographic information were compared to that of other participants enrolled in the study and to other participants’ named network members. If this did not result in a confirmed linkage, names and demographic information were compared to the detailed demographic information provided by individuals screened for eligibility (n = 939). If the eligibility screen could not confirm a tie, the community-based study staff were queried for their knowledge of existing network linkages. If none of the aforementioned steps resulted in a confirmed a linkage, the named network member was excluded from the network. Only individuals who were eligible to do so and consented to be in the study (n = 503) were included in the final network analysis. These techniques were similar to those used in previous studies involving disease transmission within social networks [6, 7, 11, 26].
HIV Risk Network
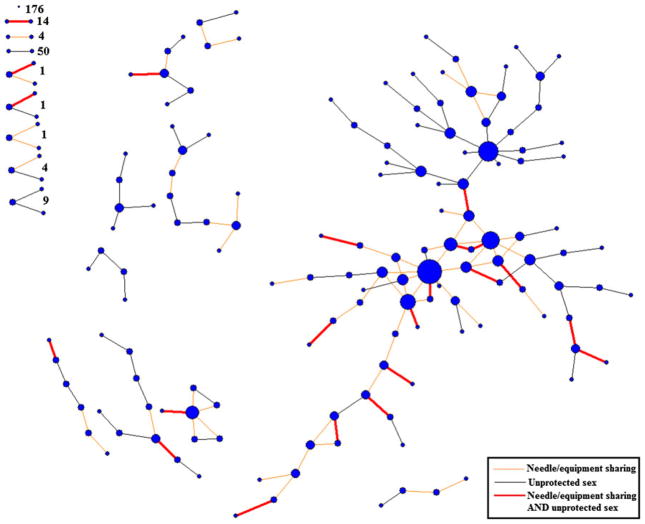
Once a list of sex and drug network members was elicited, eligible participants were queried on recent (past 6 months) risk behaviors they engaged in with each named risk network member. These data included information regarding whether network members had engaged in injection drug use and the frequency with which they shared illicit drugs, as well as syringes, cottons and cookers. For each sex network member, participants reported the frequency with which they had engaged in unprotected sex in the past 6 months. From these data, a “HIV risk network” was constructed in which each tie represented a relationship in which partners had engaged in unprotected sex and/or shared drug equipment (e.g., syringes and/or cookers) at least once in the past 6 months (Fig. 2). All subsequent analyses were based on this HIV risk network. Social network data were compiled and calculated using UCInet 6.361 [27] and network visualizations were produced using NetDraw 2.117 (Harvard, MA) [28].
Fig. 2.
HIV risk network of 503 nonmedical users of prescription drugs in rural Appalachia. Note Nodes are sized by degree centrality
Random Network Generation Compared to Observed HIV Risk Network
To our knowledge, there are currently no published studies involving a sample of rural drug users that could serve as an appropriate comparison for this study. Therefore, the most objective method for evaluating the observed risk network for anomalous properties and structures was to compare it to random simulated networks. Random networks (1,000) were generated using the Erdos-Renyi [29] method in UCInet [27]. This method generates networks based on network density and the number of nodes in the observed network (0.001965 and 503, respectively). By holding density and the number of nodes constant, the number of ties and mean degree centrality were also mathematically constant (496 and 0.98608, respectively). The structure of the observed HIV risk network was then compared to the simulated random networks to determine if the observed network structure was substantially different from that which would be expected under random-mixing (i.e. if all ties within the network were distributed randomly). This approach was modeled after methods used in previous social network research on disease transmission [15]. Specifically, network comparisons were made using indexes demonstrated in previous research to be relevant to transmission of HIV or other sexually transmitted infections (described below). Visual examples of the indexes are shown in Fig. 3.
Fig. 3.
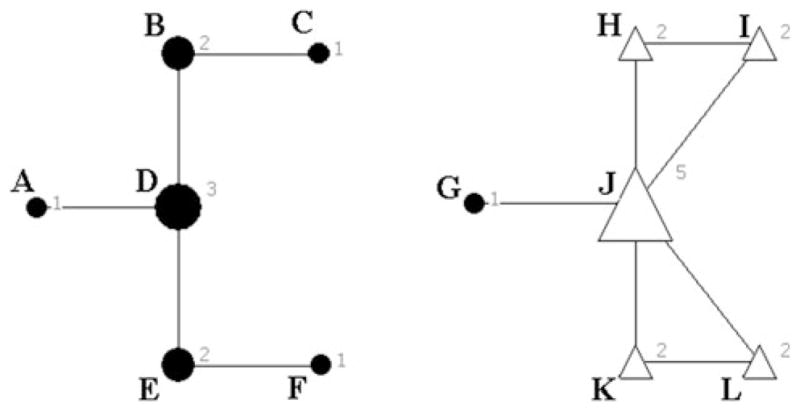
Visual examples of network indexes. Note This figure displays examples of two components, both of which have a component size of 6. A–F form component 1 and G–L form component 2. The numbers and corresponding node size show the degree centrality of each individual. For example, A only has a degree of 1 given they have only one connection (to D), while J has a degree of 5, as it is tied to G, H, I, K, and L. In component 2, two transitive triads, marked by triangles, exist between J, H, and I, and also J, K, and L. H, I, J, K, and L also form a 2core, highlighted by hollow symbols, as each individual is tied to at least two others within the subgroup. In component 1 the diameter or longest geodesic path would be 4, representing the number of steps it would take A to reach C or F
Most networks are comprised of several sub-networks, or components. A component is a fundamental network structure within which all pairs of individuals are connected directly or indirectly through one or multiple path(s) [30]. Numerous studies have examined the importance of component structure for HIV and STI transmission [8, 15, 16] and amenability of networks to intervention [31]. It has been suggested in previous research that changes in the number and size of components in a network could facilitate and/or inhibit HIV transmission over time [8]. In the present study, two measures were used to evaluate network components: main component size and average component size. Main Component Size represented the number of nodes located in the largest connected component in the network. Average Component Size was the average size of all components within a network [8, 32].
Average Geodesic Distance (AGD) was used to assess participants’ proximity to other actors in their risk network. Geodesic distance is the length of the shortest path between a given pair of members in the network [33]. AGD was computed by summing all geodesic lengths and dividing by the number of geodesics. Distances between individuals who were “unreachable” (i.e. they lack any direct or indirect connections to one another) were excluded from analysis of geodesic distance. The Diameter of a network is the longest geodesic path between a pair of individuals in a network [33]. The diameter would expose if network members were connected in a long, chain-like structure, or if network members were interconnected at short geodesic distances.
Network Centralization [34] was used to calculate the degree to which the network was centralized around one or a few actors [35]. Degree centrality [34] was used for the computation of network centralization in this study given its common use in similar studies (e.g., [8, 10, 12, 16]) and its demonstrated relevance to HIV vulnerability [9]. Degree centrality represents the number of individuals adjacent to a given individual [34]. Subsequently, degree centralization (CD) was calculated as , where {CD(ni)}are the actors’ degree indices and CD(n*) is the largest observed degree index [33, p. 180]. Therefore, the centralization value reflects the extent to which the network resembles a maximally centralized network in which all network members are connected through one maximally central actor (i.e. visualized in the shape of a star) [33, 34]. Degree centralization indices range from 0 to 1 with 0 indicating that all degree centralities are equal in the network and 1 indicating that one actor has chosen all other actors and the other actors interact only with this one, central actor [33, 34]. Thus, high centralization characterizes a more hierarchical network, while low centralization characterizes a network in which ties are evenly distributed among network members [35]. In summary, the centrality of a drug user, as well as the overall shape of the network (in terms of degree centralization), may play a role in individual- and network-level risk behavior [2, 36].
K-cores [37] were used to examine subsets within the networks that were more connected than others. K-cores are connected groups in which all members are also connected to k other members of the group. Social networks with many individuals within large k-cores suggest a structure where many individuals are in ‘tight-knit’ groups [38]. Previous research has demonstrated that 2-cores are particularly conducive to HIV and STI transmission [16, 39]; thus, for the present analysis, a specific computation was made to determine the percentage of participants located in 2-cores.
Transitivity is the extent to which triadic closure occurs in the network. Triadic closure can be visualized as a triangle, in which persons A, B and C all know each other, whereas non-closure can be visualized as a 3-node chain, in which person A knows person B and person B knows person C. The transitivity measure reflects the percentage of all triads in the network that exhibit closure [33]. Transitive triads are the smallest cyclical structure possible in a social network [33]. Cyclical structures have been examined in similar studies, and are relevant to HIV transmission given that they are indicative of a cohesive network in which individuals can be exposed to infection via multiple paths [15, 40, 41].
Results
Table 1 displays demographic and behavioral characteristics of the sample. More detailed information about participants’ substance abuse is described elsewhere [42, 43]. Approximately 43 % of the sample was female and the vast majority (94 %) was white. The median age was 31 years (range: 18–65), and the median number of years in which participants had lived in rural Appalachia was 29 years. Just over half (57 %) had graduated from high school, and 58 % were employed. The median monthly income was under $700 and two thirds of participants were uninsured. The majority were not currently married (74 %) and none of the participants tested positive for HIV.
Table 1.
Demographic characteristics of nonmedical users of prescription drugs in rural Appalachia (n = 503)
| n (%) | |
|---|---|
| Demographic characteristics | |
| Female | 217 (43.1) |
| White | 474 (94.2) |
| Age (years)–median (IQR) | 31 (26–38) |
| High school graduate | 284 (56.5) |
| Years lived in rural Appalachia-median (IQR) | 29 (23–35) |
| Marital status | |
| Married/remarried | 132 (26.2) |
| Never married | 170 (33.8) |
| Widowed/separated/divorced | 201 (40.0) |
| Employeda | 289 (57.5) |
| Median monthly income (IQR)b | $677 (300–1,200) |
| Uninsured | 333 (66.2) |
| Unstable living arrangementsc | 25 (5.0) |
| Lifetime number of incarcerations-median (IQR) | 3 (1–6) |
| Behavioral characteristics | |
| Recent (past 30 days) | |
| Injection drug use | 242 (48.1) |
| Syringe sharing (receptive and/or distributive) | 84 (16.7) |
| Median number of unprotected sex acts (IQR) | 10 (2–27) |
| Median number of sex partners (IQR) | 1 (0.0–1.1) |
| Lifetime | |
| Injection drug use | 394 (78.3) |
| Median number of sex partners (IQR) | 15 (8–31) |
IQR interquartile range
Described their “usual employment pattern” in the past 3 years as full- or part-time employed
Includes income from all legal and illegal sources
Described their “usual living arrangement” in the past 3 years as having “no stable arrangements”
As shown in Fig. 2, various risk relationships are present in the network, including bidirectional ties representing syringe/equipment sharing and unprotected sex (“multiplex ties”, n = 35), unprotected sex only (n = 145), and syringe/equipment sharing only (n = 68). There were 176 isolates, 68 dyads, and 16 triads present. Eighty-nine participants (17.7 %) were connected within one large, main component of the network. Individuals who have many concurrent relationships are often engaging in more than one type of risk relationship across different partnerships.
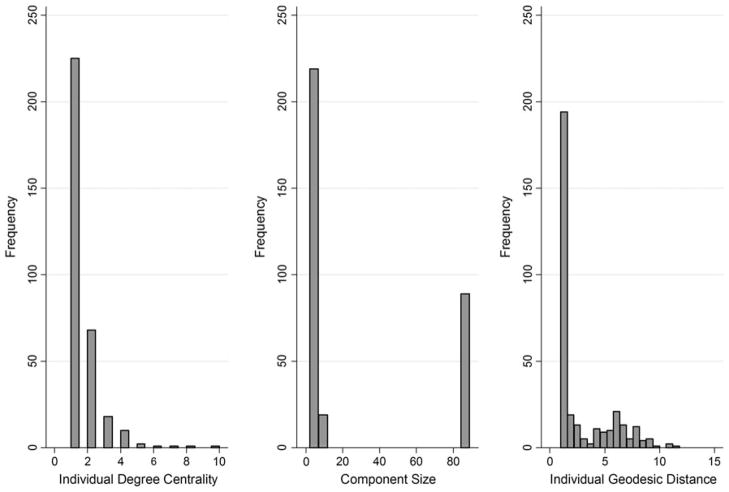
Boxplots (with the observed network indicated) for each network measure are displayed in Fig. 4. The size of the observed main component was in the 90th percentile of values from the random networks (Table 2). The maximum number of ‘steps’ between any two members (i.e. diameter) of the main component was 17. On average, any network member was approximately six steps from any other given network member (AGD = 6.37), which was near the average computed from the random networks. As shown in Fig. 5, the distribution of individual-level AGDs of members of the observed network had a strong positive skew.
Fig. 4.
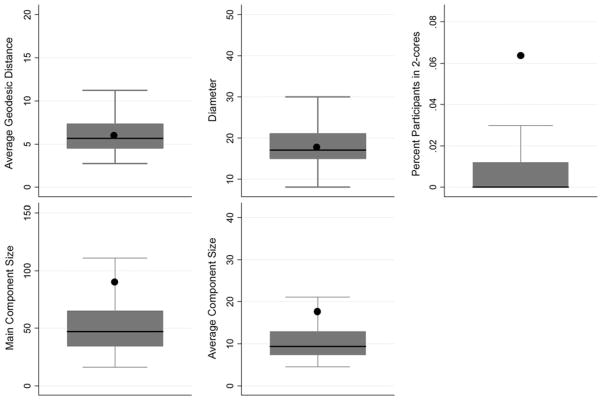
Boxplots of indexes computed from 1,000 random generated networks (n = 503) with observed network (n = 503) indicated by the symbol black circle. Note Boxplots for centralization and transitivity were unable to be constructed due to lack of variance in values generated from the random networks
Table 2.
Comparison of observed HIV risk network structure of rural drug users (n = 503) to structure of simulated random networks (1,000 permutations)
| Characteristic | Observed network Mean (SD) | Random networks Mean (SD) | Rangea | Percentileb |
|---|---|---|---|---|
| AGD | 6.37 (3.22) | 6.12 (2.05) | 2.76–16.83 | 50th |
| Diameter | 17 (−) | 18.55 (5.30) | 8.00–48.00 | 25th |
| Centralization | 1.80 % (−) | 0.82 % (0.14) | 0.60–1.40 | 95th |
| Percentage of network members located in 2-cores | 6.36 % (−) | 0.83 % (0.01) | 0.00–8.35 | 95th |
| Transitivity | 4.56 % (−) | 0.06 % (0.17) | 0.00–0.94 | 95th |
| Average Component Size | 17.64 (33.13) | 10.79 (5.05) | 4.50–41.06 | 75th |
| Main Component Size | 89 (−) | 51.63 (22.23) | 16.00–141.00 | 90th |
SD standard deviation, AGD average geodesic distance
Range of values across random networks only
Percentile in which value from observed network fell in relation to values from all networks (percentiles: 5th, 10th, 25th, 50th, 75th, 90th, 95th)
Fig. 5.
Distribution of degree centrality, component size, and geodesic distance within the observed HIV risk network (n = 327). Note Individual degree centrality, component size, and individual geodesic distance was computed only on those individuals who were not isolates (number of isolates = 176) in the HIV risk network
The average size of components in the observed network (mean: 17.64, SD: 33.17) was not substantially larger than that observed in the random networks (mean: 10.79, SD: 5.05). The distribution of component sizes in the observed network demonstrates that outside of the main component (n = 89), the majority were located in very small components (Fig. 5). Transitivity in the network was also examined. Nearly 5 % of triads were transitive (i.e. exhibited closure) in the observed network; this percentage was substantially higher than that of any of the random networks (mean: 0.06 %; range: 0–0.94 %).
The degree of centralization in the observed network is 1.80 %. The centralization of the observed network was higher than that observed across the random networks (mean: 0.82 %, SD: 0.14, maximum: 1.40 %). The observed network was a statistical outlier in terms of the percentage of participants located in 2-cores (6.36) compared to the average observed across random networks (0.83 %; SD: 0.01). On individual-level degree centrality, the distribution of values in the observed network showed a strong positive skew, with the majority of participants having a degree centrality of 1 (Fig. 5). The average degree centrality in the observed network was 1.52 (SD: 1.06).
Discussion
Implications for HIV Risk
The size of the observed network’s main component was notably larger than that of the random simulated networks. The main component in the observed network encompassed 89 participants. Thus, if a member of the main component in this network of rural drug users was infected with HIV, the infection would have the potential to spread to nearly 18 % of the entire sample. Whereas if the network was more fragmented, the spread of infection would be bound to smaller risk groups and may have less widespread impact [5].
On the other hand, membership in the large, cohesive main component could also be protective against HIV acquisition. The network structure observed in this study resembles the sexual network identified by Helleringer and collegues [14, 40, 44] in a study on Likoma Island, Malawi. The Likoma Island community was highly insular and, with the exception of a few “bridge populations” who traveled to the mainland, had very limited mobility [14, 44]. Helleringer S, Kohler H-P [40] identified a sexual network with a large, cohesive main component in which the HIV prevalence was lower than that of network members on the periphery. This finding could be due to a number of factors, including the demographic characteristics of members of the main component, endemic stage of the HIV epidemic, and/or social forces that distanced HIV infected individuals from the core network [40, 45–47]. However, the insularity of the community and of the main network component could have also served as a protective factor from introduction of HIV from the ‘bridge populations’ [40]. The latter mechanism may be relevant to understanding the epidemiology of HIV in rural Appalachia, which has historically been considered as geographically and socially isolated [48]. Several studies of high-risk individuals in Central Appalachia have found no cases of HIV, even amongst those involved in the criminal justice system [49–51] and those injecting prescription and other illicit drugs [50, 52]. According to statewide surveillance data, the eight-county area development district in which the recruitment catchment area is located has reported only 13 cases of HIV since 2005 [53]. Much more qualitative and longitudinal research is needed to explore the role of mobility in shaping network structure and HIV prevalence in Appalachia, as many scholars have argued that the region has not been as isolated as was once assumed [54].
The observed network was also characterized by a high number of cohesive microstructures. Nearly 5 % of triads in the observed network were transitive, which was in the 95th percentile compared to the random networks. This indicates that the observed network exhibited much more cohesion than would be expected at random. Cohesive networks have been shown to be more conducive to HIV and STI transmission than are non-cohesive networks [7, 8, 12, 13]. The network also had a large percentage of participants located in 2-cores relative to that found in the simulated random networks. Two-cores have been specifically identified as a microstructure conducive to HIV [39] and STI transmission [16]. Friedman et al. found that among HIV seronegative injection drug users, those located in 2-cores were more likely to report having shared rinse water and engaged in unprotected sex. The study also found that in the main component, location in a 2-core was significantly associated with HIV infection. However, the authors warned against concluding that 2-cores were a risk factor for infection in a network that has not been penetrated by HIV; instead, they suggested that 2-cores should be considered as a network location that may serve as a loci of transmission once HIV has been established within a network [39]. Thus, the prevalence of 2-core membership and high degree of transitivity in the observed network, indicate that should HIV be introduced into this population, the prevalence of microstructures could not only facilitate transmission, but also provide niches for its persistence.
The observed network was substantially more centralized than that which would be expected at random. Network centrality is associated with increased engagement in risk behaviors (e.g., [2, 16, 39, 55, 56]), and transmission of HIV [9] and other STIs [12, 16]. While centrality is an important measure for individual risk, centralization is important to understanding risk in the overall network. Centralized networks can facilitate diffusion [35]; in a HIV risk network, for example, infection of central members can result in quicker diffusion to others than would occur in a decentralized network. The behavior of central members within a centralized network can also impact HIV transmission. For example, the review by De et al. [2] suggested that one mechanism by which centrality influences HIV transmission is through the role that central individuals have in acting as brokers in drug equipment exchange.
Implications for HIV Intervention
Though several characteristics of the observed network would appear to place it at increased risk for HIV transmission, it should also be noted that certain network structures may also increase its amenability to intervention. Network-driven HIV interventions among drug users have been shown in previous research to be successful in reducing risk behavior [3, 57] and in some situations, very cost-effective [58]. Peer intervention strategies capitalize on the indigenous social resources available in drug-using networks, and through involving individuals embedded in these networks, create a more dynamic intervention strategy that can be responsive to local changes in the drug-using environment [59].
The large main component and high centralization identified in the present study may present an ideal situation for conducting peer-to-peer interventions. Previous research has shown that central members of a large network component may be more effective peer educators given that there is potential for broad dissemination of prevention messages [31]. In centralized HIV risk networks, individuals with high centrality may be ideal peer-interventionists, in that they can reach a higher share of network members than if the network was decentralized. Some research has shown that drug users involved in peer outreach may themselves gradually adopt more harm reduction strategies [60]; this is important given that adoption of harm reduction behavior by a central individual in a risk network could elicit behavioral changes not only through active diffusion, but also through social modeling. Also, the removal of central members (through enrollment in drug treatment programs, for example) from centralized networks creates component fragmentation and longer pathways for infection to travel, thus slowing or eliminating transmission of infection across the network [61]. In addition to important insights gained from visual inspection of the networks, recent innovations such as KeyPlayer algorithms [62, 63] may offer methods for identifying sets of individuals who, if removed from the network, would create the most disruption to possible disease transmission pathways.
Selecting peer-interventionists on the basis of membership in a central location of large components also has its limitations, however, as it precludes dissemination to individuals who are isolates in the network. Also, centrality and network location do not reflect individuals’ skills as educators or their willingness to engage in peer-intervention [31]. The effectiveness of selecting peer-interventionists on the basis of their centrality can also be impacted by the degree to which central individuals are clustered with each other within the network [35, 62]. In this case, the dissemination of risk reduction information will be concentrated in areas occupied by individuals with high centrality and will not diffuse through the network as intended. Again, KeyPlayer [62, 63] algorithms may offer a mechanism for identifying sets of individuals who could achieve the broadest diffusion of intervention information throughout the entire network. Nevertheless, much more research is needed to understand how network structures can facilitate and/or hinder implementation of HIV prevention interventions [64].
Though this study fills an important gap in the extant literature on risk network structure and HIV risk behavior, there are limitations. The cross-sectional nature of the data limits the ability to make causal inferences about the ways in which the observed network structure affects disease transmission. Social networks are dynamic; therefore, accounting for relationship timing in disease diffusion presents challenges that need to be addressed [65]. It is also possible that some key network members were not captured in the current study due to potential recall and response bias in the self-reported network data. Another concern may be that the RDS sampling strategy would influence the structural properties of the network and limit the inferences that could be drawn from the results; however, only 1 % (n = 5) of the total 496 bidirectional ties observed in the HIV risk network overlapped with ties in the RDS referral chains.
In this article, the social network structure of Appalachian drug users at risk for HIV is compared to random networks of similar dimensions (i.e. size and density). Random networks often have innate properties, such as low transitivity, that differ from human networks (see Newman et al. [66] for a full discussion). Recent methods, such as Exponential Random Graph Modeling (also known as P* models) [67, 68], attempt to address these differences by parameterizing networks using Monte Carlo Markov chain maximum likelihood estimations [67, 68]. However, these methods are still in their infancy for networks of the size used in the current study [69]; the vast majority of existing studies have used datasets of smaller sizes, often of less than 50 nodes. Thus, the current study highlights opportunities to develop alternative ways to create baseline models with specific properties against which to compare empirical networks.
Conclusions
As previous research has demonstrated, the determining factor in the network’s ultimate vulnerability to widespread HIV infection is the network location of the initial cases [5, 7]. Thus, the importance of risk network structure to HIV transmission cannot be overstated. As Rothenberg et al. [5, p. S146] explain, “…The requisite behaviors for HIV transmission, in the absence of an appropriate temporal, geographic, and social setting (i.e. the right network), may not result in disease propagation”. In this sample of rural drug users, the requisite behaviors for transmission are present, as are the network structures. The lack of HIV in this population, therefore, is surprising and deserving of further research. This study does highlight the vulnerability of this population to HIV establishment, however, underscoring the need for immediate implementation of prevention programs, possibly through network-level intervention strategies.
Acknowledgments
This study was funded by the National Institute on Drug Abuse (R01DA024598).
Contributor Information
A. M. Young, Department of Behavioral Science, Center on Drug and Alcohol Research, University of Kentucky College of Medicine, 333 Waller Avenue, Lexington, KY 40504, USA. Department of Behavioral Sciences and Health Education, Emory University Rollins School of Public Health, Atlanta, GA, USA
A. B. Jonas, LINKS Center for Social Network Analysis, Gatton College of Business and Economics, University of Kentucky, Lexington, KY, USA
U. L. Mullins, Department of Behavioral Science, Center on Drug and Alcohol Research, University of Kentucky College of Medicine, 333 Waller Avenue, Lexington, KY 40504, USA
D. S. Halgin, LINKS Center for Social Network Analysis, Gatton College of Business and Economics, University of Kentucky, Lexington, KY, USA
J. R. Havens, Email: jennifer.havens@uky.edu, Department of Behavioral Science, Center on Drug and Alcohol Research, University of Kentucky College of Medicine, 333 Waller Avenue, Lexington, KY 40504, USA
References
- 1.Luke D, Harris J. Network analysis in public health: history, methods, and applications. Annu Rev Public Health. 2007;28:69–93. doi: 10.1146/annurev.publhealth.28.021406.144132. [DOI] [PubMed] [Google Scholar]
- 2.De P, Cox J, Boivin J-F, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102(11):1730–9. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Brown K, Shen S-Y, Tucker J. Social network-based interventions to promote condom use: a systematic review. AIDS Behav. 2011;15:1298–308. doi: 10.1007/s10461-011-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liljeros F, Edling CR, Nunes Amaral LA. Sexual networks: implications for the transmission of sexually transmitted infections. Microbes Infect/Institut Pasteur. 2003;5(2):189–96. doi: 10.1016/s1286-4579(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg RB, Potterat JJ, Woodhouse DE. Personal risk taking and the spread of disease: beyond core groups. J Infect Dis. 1996;174(Suppl 2):S144–9. doi: 10.1093/infdis/174.supplement_2.s144. [DOI] [PubMed] [Google Scholar]
- 6.Rothenberg RB, Woodhouse DE, Potterat JJ, Muth SQ, Darrow WW, Klovadahl AS. Social networks in disease transmission: The Colorado Springs Study. NIDA Res Monogr. 1995;151:3–19. [PubMed] [Google Scholar]
- 7.Klovdahl AS, Potterat JJ, Woodhouse DE, Muth JB, Muth SQ, Darrow WW. Social networks and infectious disease: The Colorado Springs Study. Soc Sci Med. 1994;38(1):79–88. doi: 10.1016/0277-9536(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 8.Rothenberg RB, Potterat JJ, Woodhouse DE, Muth SQ, Darrow WW, Klovdahl AS. Social network dynamics and HIV transmission. AIDS. 1998;12(12):1529–36. doi: 10.1097/00002030-199812000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Bell DC, Atkinson JS, Carlson JW. Centrality measures for disease transmission networks. Soc Netw. 1999;21(1):1–21. [Google Scholar]
- 10.Rothenberg RB, Potterat JJ, Woodhouse DE, Darrow WW. Choosing a centrality measure: epidemiologic correlates in the Colorado Springs study of social networks. Soc Netw. 1995;17(3–4):273–97. [Google Scholar]
- 11.Friedman SR, Neaigus A, Jose B, et al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997;87(8):1289–96. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothenberg R, Sterk C, Toomey K, et al. Using social network and ethnographic tools to evaluate syphilis transmission. Sex Transm Dis. 1998;25(3):154–60. doi: 10.1097/00007435-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Potterat JJ, Rothenberg RB, Muth SQ. Network structural dynamics and infectious disease propagation. Int J STD AIDS. 1999;10(3):182–5. doi: 10.1258/0956462991913853. [DOI] [PubMed] [Google Scholar]
- 14.Helleringer S, Kohler H-P, Chimbiri A, Chatonda P, Mkandawire J. The Likoma Network Study: context, data collection, and initial results. Demogr Res. 2009;21:427–68. doi: 10.4054/DemRes.2009.21.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bearman PS, Moody J, Stovel K. Chains of Affection: the structure of adolescent romantic and sexual networks. Am J Sociol. 2004;110(1):44–91. [Google Scholar]
- 16.De P, Singh AE, Wong T, Yacoub W, Jolly A. Sexual network analysis of a gonorrhoea outbreak. Sex Transm Infect. 2004;80(4):280–5. doi: 10.1136/sti.2003.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neaigus A. The network approach and interventions to prevent HIV among injection drug users. Public Health Rep. 1998;113:140–50. [PMC free article] [PubMed] [Google Scholar]
- 18.Amirkhanian YA, Kelly JA, McAuliffe TL. Identifying, recruiting, and assessing social networks at high risk for HIV/AIDS: methodology, practice, and a case study in St Petersburg, Russia. AIDS Care. 2005;17(1):58–75. doi: 10.1080/09540120412331305133. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. [Accessed June 17, 2011.];Kentucky Quick Facts: Perry County 2010. 2011 http://quickfacts.census.gov/qfd/states/21/21193.html.
- 20.Delaney K, Branson B, Uniyal A, et al. Performance of an oral fluid rapid HIV-1/2 test: experience from four CDC studies. AIDS. 2006;20(12):1655–60. doi: 10.1097/01.aids.0000238412.75324.82. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. HIV Counseling with Rapid Tests. 2007. [Google Scholar]
- 22.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–99. [Google Scholar]
- 23.Heckathorn DD. Respondent-driven sampling II: deriving valid population estimated from chain-referral samples of hidden populations. Soc Probl. 2002;49(1):11–34. [Google Scholar]
- 24.Wang J, Falk RS, Li L, Rahman A, Carlson RG. Respondent-driven sampling in the recruitment of illicit stimulant drug users in a rural setting: findings and technical issues. Addict Behav. 2007;32(5):924–37. doi: 10.1016/j.addbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Draus P, Siegal H, Carlson R, Falck R, Wang J. Cracking the cornfields: recruiting Illicit Stimulant Drug Users in Rural Ohio. Sociol Q. 2005;46(1):165–89. [Google Scholar]
- 26.Woodhouse DE, Rothenberg RR, Potterat JJ, et al. Mapping a social network of heterosexuals at high risk for human immunodeficiency virus infection. AIDS. 1994;8:1331–6. doi: 10.1097/00002030-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Borgatti S, Everett M, Freeman L. Ucinet for Windows: software for social network analysis. Version 6.303. Harvard: Analytic Technologies; 2002. [Google Scholar]
- 28.Netdraw network visualization [computer program]. Version 2.095. Harvard: Analytic Technologies; 2002. [Google Scholar]
- 29.Erdos P, Renyi A. On the evolution of random graphs. Publ Math Inst Hung Acad Sci. 1960;5:17–61. [Google Scholar]
- 30.Hannerman RA, Riddle M. Introduction to social network methods. Riverside, CA: 2005. [Accessed August 22, 2011.]. http://www.faculty.ucr.edu/*hanneman/nettext/ [Google Scholar]
- 31.Weeks MR, Clair S, Borgatti SP, Radda K, Schensul JJ. Social Networks of Drug Users in High Risk Sites: finding the Connections. AIDS Behav. 2002;6(2):193–206. [Google Scholar]
- 32.Latkin C, Mandell W. People and places: behavioral settings and personal network characteristics as correlates of needle sharing. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(3):273. doi: 10.1097/00042560-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 33.Wasserman S, Faust K. Social network analysis: methods and applications. New York: Cambridge University Press; 1994. [Google Scholar]
- 34.Freeman LC. Centrality in social networks: conceptual clarification. Soc Netw. 1979;1:215–39. [Google Scholar]
- 35.Valente TW. Social networks and health. Oxford: Oxford University Press; 2010. [Google Scholar]
- 36.Miller M, Neaigus A. Networks, resources and risk among women who use drugs. Soc Sci Med. 2001;52(6):967–78. doi: 10.1016/s0277-9536(00)00199-4. [DOI] [PubMed] [Google Scholar]
- 37.Seidman S. Network structure and minimum degree. Soc Netw. 1983;5:269–87. [Google Scholar]
- 38.Doreian P, Woodard KL. Defining and locating cores and boundaries of social networks. Soc Netw. 1994;16(4):267–93. [Google Scholar]
- 39.Friedman SR, Neagius A, Jose B, et al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997;87(8):1289–96. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helleringer S, Kohler H-P. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island. Malawi AIDS. 2007;21:2323–32. doi: 10.1097/QAD.0b013e328285df98. [DOI] [PubMed] [Google Scholar]
- 41.Potterat J, Muth S, Rothenberg R, et al. Sexual network structure as an indicator of epidemic phase. Sex Transm Infect. 2002;78:i152–8. doi: 10.1136/sti.78.suppl_1.i152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: a cross-sectional comparison and retrospective survival analysis. Addiction. 2012;107:587–96. doi: 10.1111/j.1360-0443.2011.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonas AB, Young AM, Oser CB, Leukefeld CG, Havens JR. OxyContin® as currency: OxyContin® use and increased social capital among rural Appalachian drug users. Soc Sci Med. 2012;74:1602–9. doi: 10.1016/j.socscimed.2011.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helleringer S, Kohler H-P, Chimbiri A. Characteristics of external/bridge relationships by partner type and location where sexual relationship took place. AIDS. 2007;21(18):2560–1. doi: 10.1097/QAD.0b013e3282f112bd. [DOI] [PubMed] [Google Scholar]
- 45.Rothenberg R. HIV transmission networks. Curr Opin HIV AIDS. 2009;4(4):260–5. doi: 10.1097/COH.0b013e32832c7cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brewer D, Potterat J, Muth S, Gisselquist D, Brody S. Disconnects in presumed heterosexual HIV transmission in Malawi. AIDS. 2008;22:1377–87. doi: 10.1097/QAD.0b013e3282fc7306. [DOI] [PubMed] [Google Scholar]
- 47.Helleringer S, Kohler H-P. Cross-sectional research design and relatively low HIV incidence, rather than blood exposures, explain the peripheral location of HIV cases within the sexual networks observed on Likoma. AIDS. 2008;22(11):1378. [Google Scholar]
- 48.Photiadas J, Schwarzweller H. Changes in rural Appalachia: implications for action programs. Philadelphia: University of Pennsylvania Press; 1970. [Google Scholar]
- 49.Oser C, Leukefeld C, Tindall M, et al. Male and female rural probationers: HIV risk behaviors and knowledge. AIDS Care. 2006;18(4):339–44. doi: 10.1080/02652040500200491. [DOI] [PubMed] [Google Scholar]
- 50.Havens J, Oser C, Leukefeld C. Injection risk behaviors among rural drug users: implications for HIV prevention. AIDS Care. 2011;23(5):638–45. doi: 10.1080/09540121.2010.516346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oser C, Smiley-McDonald H, Havens J, Leukefeld C, Webster J, Cosentino A. Lack of HIV seropositivity among a group of rural probationers: explanatory factors. J Rural Health. 2006;22(3):273–5. doi: 10.1111/j.1748-0361.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 52.Havens J, Oser C, Crosby R, Leukefeld C. Social network factors predict engagement in HIV risk behaviors among rural Appalachian drug users in the United States. 18th international AIDS conference; Vienna. July 2010. [Google Scholar]
- 53.Kentucky Cabinet for Health and Family Services. An integrated epidemiologic profile for HIV/AIDS prevention and care planning for Kentucky, 2010. Frankfort: Department for Public Health, HIV/AIDS Branch; 2012. [Google Scholar]
- 54.Lewis R, Billings D. [Accessed February 11, 2012];Appalachian Culture and Economic Development. http://www.rri.wvu.edu/pdffiles/lewisarc.pdf. Regional Research Institute, West Virginia University Web Site.
- 55.Ghani AC, Swinton J, Garnett GP. The role of sexual partnership networks in the epidemiology of gonorrhea. Sex Transm Dis. 1997;24(1):45–56. doi: 10.1097/00007435-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Lovell AM. Risking risk: the influence of types of capital and social networks on the injection practices of drug users. Soc Sci Med. 2002;55(5):803. doi: 10.1016/s0277-9536(01)00204-0. [DOI] [PubMed] [Google Scholar]
- 57.Coyle S, Needle RH, Normand J. Outreach-based HIV prevention for injecting drug users: a review of published outcome data. Public Health Rep. 1998;113(Suppl 1):219–30. [PMC free article] [PubMed] [Google Scholar]
- 58.Broadhead RS, Heckathorn DD, Weakliem DL, et al. Harnessing peer networks as an instrument for AIDS prevention: results from a peer-driven intervention. Public Health Rep. 1998;113:42–57. [PMC free article] [PubMed] [Google Scholar]
- 59.Weeks MR, Convey M, Dickson-Gomez J, et al. Changing drug users’ risk environments: peer health advocates as multi-level community change agents. Am J Community Psychol. 2009;43:330–44. doi: 10.1007/s10464-009-9234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weeks MR, Li J, Dickson-Gomez J, et al. Outcomes of a peer HIV prevention program with injection drug and crack users: the risk avoidance partnership. Subst Use Misuse. 2009;44:253–81. doi: 10.1080/10826080802347677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valente T. Network models of the diffusion of innovations. Creskill, NJ: Hampton Press, Inc; 1995. [Google Scholar]
- 62.Borgatti S. Identifying sets of key players in a network. Comput Math Organ Theory. 2006;12(1):21–34. [Google Scholar]
- 63.Borgatti S. [Accessed January 5, 2012.];KeyPlayer Version 1.44. http://www.analytictech.com/keyplayer/keyplayer.htm.
- 64.Gest SD, Osgood W, Feinberg M, Bierman K, Moody J. Strengthening Prevention program theories and evaluations: contributions from Social Network Analysis. Prev Sci. 2011 doi: 10.1007/s11121-011-0229-2. Published online 5 July 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moody J. The importance of relationship timing for diffusion: indirect connectivity and std infection risk. Soc Forces. 2002;81:25–56. [Google Scholar]
- 66.Newman MEJ, Strogatz S, Watts D. Random graphs with arbitrary degree distributions and their applications. Phys Rev E. 2001;64:1–17. doi: 10.1103/PhysRevE.64.026118. [DOI] [PubMed] [Google Scholar]
- 67.Snijders T. Markov, chain Monte Carlo estimation of exponential random graph models. J Soc Structure. 2002;3(2) [Google Scholar]
- 68.Robins G, Snijders T, Wang P, Handcock M, Pattison P. Recent developments in exponential random graph (p*) models for social networks. Soc Netw. 2007;29:192–215. [Google Scholar]
- 69.Pattison P, Robins G, Snijders T, Wang P. Conditional estimation of exponential random graph models from snowball sampling designs. Technical Report. 2011 Dec 31; [Google Scholar]