Abstract
Objectives
Pancreatic stellate cells are source of dense fibrotic stroma, a constant pathological feature of chronic pancreatitis (CP) and pancreatic adenocarcinoma (PDAC). We observed correlation between levels of cyclooxygenase-2 (COX-2) and its product prostaglandin E2 (PGE2) and the extent of pancreatic fibrosis. Aim of this study was to delineate the effects of PGE2 on immortalized human pancreatic stellate cells (HPSC) and to identify the receptor involved.
Methods
IHC, RT-PCR and Q-RT-PCR were used to assess COX-2, extracellular matrix (ECM) and matrix metalloproteinases (MMP) gene expression. Eicosanoid profile was determined by LC/MS/MS. HPSC proliferation was assessed by MTS assay; migration by Boyden chamber assay and invasion using an invasion chamber. Transient silencing was obtained by siRNA.
Results
HPSC express COX-2 and synthesize PGE2. PGE2 stimulated HPSC proliferation, migration and invasion; stimulated expression of both ECM and MMP genes. HPSC expressed all four EP receptors. Only blocking the EP4 receptor resulted in abrogation of PGE2 mediated HPSC activation. Specificity of EP4 for the effects of PGE2 on stellate cells was confirmed using specific antagonists.
Conclusion
Our data indicate that PGE2 regulates PSC profibrotic activities via EP4 receptor thus suggesting EP4 receptor as useful therapeutic target for pancreatic cancer to reduce desmoplasia.
Keywords: PGE2, Pancreatic Cancer, Pancreatic Stellate cells, EP4 receptor
Introduction
Pancreatic fibrosis occurs as the result of a complex process of stellate cell activation, expression of matrix molecules followed by their synthesis, secretion, deposition, maturation and remodeling leading ultimately to a dense fibrous connective tissue (1,2). Such a dense fibrotic stroma is the best known hallmark of chronic pancreatitis and pancreatic cancer. This process which occurs after injury, a reparative or reactive process may be protective. However, when the dense stroma becomes extensive it can interfere with normal pancreatic function (3). Recently it has been suggested that this fibrotic stroma may also act as a physical barrier to drug delivery in pancreatic cancer therapy (4). Therefore, this fibrotic response is generally considered pathological and efforts to reduce the extent of this phenomenon are constantly being sought. However, the specific mechanisms involved in the initiation, remodeling, and resolution of pancreatic fibrosis are poorly understood (3). Fibrotic initiation is generally associated with inflammation and several inflammatory molecules are able to stimulate extracellular matrix production from the pancreatic stellate cells and several have been considered potential therapeutic targets (5). However, which regulators are most critical and might be the best therapeutic targets remains unclear.
Pancreatic inflammation is associated with a high level of COX-2 activity (6,7) which increases the production of prostaglandins including PGE2 within the inflamed pancreas. PGE2 has both pro-inflammatory and cell-protective activities (8). COX-2 and PGE2 are correlated with worse prognosis in many cancers including lung, gastric and pancreatic (9,10). Prostaglandin endoperoxide synthase, commonly referred to as cyclooxygenase (COX), catalyzes the reduction of arachidonic acid (AA), to form prostaglandin E2 by microsomal PGE2 synthases (11). Currently, there are three known COX isoforms COX-1, COX-2 and COX-3 (a splice variant of COX-1). COX-1 is a ubiquitously and constitutively expressed isoform that is postulated to have “housekeeping” functions with basal production of prostaglandins under homeostatic conditions. In contrast, COX-2 is encoded by an early-response gene and can be rapidly induced by growth factors, cytokines, inflammatory mediators and tumor promoters (12). By immunohistochemistry (IHC), COX-2 was found to be highly expressed in chronic pancreatitis (6), pancreatic adenocarcinoma (7, 13-15) and pancreatic intraepithelial neoplasia (PanIN) (16). The expression of COX-2 in chronic pancreatitis is primarily localized in the cytoplasm of pancreatic acinar cells, islet cells, and ductal cells (6), while in pancreatic adenocarcinoma it is localized primarily to cancer cells (7). Transgenic over-expression of COX-2 in the pancreas led to the development of pancreatic fibrosis and even cellular transformation (17). However, despite the strong correlation between COX-2 and pancreatic fibrosis, the mechanisms involved in COX-2 mediated effects on the stroma are unclear.
Stellate cells are resident cells of the pancreas, located at the basolateral aspect of acinar cells under control conditions (2). In the normal pancreas, stellate cells are quiescent, identifiable by the presence of vitamin-A containing lipid droplets in the cytoplasm and positive immunostaining for cytoskeletal proteins such as desmin and glial acidic fibrillary protein (18). In health, PSC play a role in extracellular matrix turnover via their ability to both synthesize as well as degrade matrix molecules. During inflammatory injury, PSCs become activated and assume a myofibroblast-like phenotype characterized by the loss of vitamin A droplets, the production of α-SMA and extracellular matrix proteins such as collagen I and III, fibronectin and laminin (3). Factors known to be up-regulated during pancreatic injury such as TGF-β, plateletderived growth factor (PDGF) as well as proinflammatory cytokines, stimulate PSC proliferation and production of extracellular matrix proteins (19). Notably, activated PSC also produce increased amounts of matrix metalloproteinase-2 (MMP2) (20), known to degrade collagen IV, an essential component of the basement membrane, thereby facilitating the deposition of fibrillar collagenla as observed in pancreatic fibrosis. Despite this information, the major mechanisms regulating stellate cells and their development of pancreatic desmoplasia are not fully understood.
In the current study, we sought to elucidate the role of PGE2 on pancreatic fibrosis in order to determine whether PGE2 or its receptor might be a useful target for novel therapies designed to interfere with inflammation and reduce fibrosis. Hence, we have investigated the effects of PGE2 on pancreatic stellate cells. Most of these studies utilized immortalized primary human pancreatic stellate cells (HPSC) which were previously characterized (21). However, key experiments were repeated with primary stellate cells which confirmed the observations. Our studies support the suggestion that HPSCs cells express COX-2 and secrete PGE2(22). More importantly, we found that PGE2 is a powerful regulator of stellate cells that increases their proliferation, migration and the production of extra cellular matrix (ECM) molecules. We further identified EP4 as the key prostaglandin receptor using both siRNA and specific antagonists. Our data indicate for the first time that EP4 is of key importance in PSC regulation. Because EP4 is a therapeutically tractable target this may facilitate the development of new approaches to improve therapies for chronic pancreatitis and pancreatic cancer.
Materials and Methods
Materials
Culture media and fetal bovine serum (FBS) were obtained from Life Technologies, Inc. (Rockville, MD). PGE2, EP1 antagonist (cat # SC18220) and EP2 antagonist (cat # AH 6809) were purchased from Cayman Chemicals (Ann Arbor, MI). The EP4 antagonist (ONOAE3208) was obtained from ONO pharmaceuticals (Osaka, Japan).
Cell Culture
Immortalized human pancreatic stellate cells (HPSC) were isolated using the outgrowth method from pancreatic adenocarcinoma samples from patients undergoing surgical resection and were immortalized (21). HPSCs (HPSC developed from two different patients - lines 1 and 2) were maintained at 37°C in a humidified atmosphere of 5% CO2 and were grown in 10% DMEM containing 1% antibiotic.
Immunohistochemical Staining for COX-2
Unstained 4micron tissue sections from human patients were deparaffinized with xylene and rehydrated with ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol and non specific binding sites were blocked with protein blocking solution (5% normal horse and 1% normal goat serum). Primary antibody against COX-2 (1:800 dilution; cat # HPA001335, Sigma Chemical) was added and the samples were incubated overnight at 4°C. The secondary antibody was added and incubated for 1 hour at room temperature. Finally, slides were developed with 3,3 diaminobenzidine substrate (DAB) and counterstained with hematoxylin. Then the slides were dehydrated with ethanol, fixed with xylene and mounted. Immunohistochemistry was analyzed using an inverted light microscope (Olympus, Center valley, PA). Images were digitally captured using a charge coupled device camera (Hamamatsu, Bridgewater, NJ) and smartcapture software (Digital Scientific, Cambridge, UK).
LS/MS/MS
Liquid chromatography tandem mass spectrometry (LC/MS/MS) was performed to determine the profile of eicosanoids present in HPSC cellular extracts or HSPC conditioned medium. Media bathing the HPSC cells were collected after 15-20 hours of serum starving and used for the study.
Transient transfection of small interfering RNA
HPSC was plated on 100-mm dishes and transiently transfected with siControl, siEP2 and siEP4 (siRNA IDs # SI03650325, SI02757587, SI00019208, Qiagen, Valencia, CA) at a final concentration of 10 nmol/L with Hiperfect transfection reagent (Qiagen, Valencia, CA), serum starved overnight and treated with and without PGE2 (100nM) and RNA was prepared after 24h for RT-PCR and Q-RT-PCR. Relative expression was calculated after normalizing against β-Actin.
Invasion and Migration assays
For studies of cell invasiveness, BIOCOAT Matrigel invasion chambers (BD Biosciences, Chicago, IL) were used. Briefly, 2 × 104 HPSC cells with and without siRNA transfection were re-suspended in 100ul of serum-free medium were added to the upper chamber and different concentrations of PGE2 (1–1000 nM) in 0.5% serum containing DMEM were added into the lower chamber. The cells were allowed to invade the Matrigel for 22 h at 37°C in a 5% CO2 atmosphere. DMEM containing 0.5% serum was used as control. The non-invading cells on the upper surface of the membrane were removed with a cotton swab and the invading cells on the lower surface of the membrane were fixed and stained with a Diff-Quick stain kit (BD Biosciences), washed twice with water and air-dried. Invading cells in three adjacent microscope fields for each membrane were imaged at 20× magnification. To assess cellular migratory potential, the protocol described above was used, except that migration chambers devoid of matrigel was used (BD Biosciences, Chicago, IL). Experiments were performed at least in triplicate, and the results were shown as mean ± SD of three independent experiments.
RT-PCR and Quantitative RT-PCR
Total RNA was isolated from HPSC with and without siRNA transfection. DNAse was used to remove contaminating genomic DNA and RNA was purified. Quality of the RNA was confirmed by running on a denaturing gel, and we have observed clear 28S and 18S rRNA bands. RT-PCR and Q-RT-PCR were conducted and the amplified products were separated on 1.5% agarose gels and visualized by ethidium bromide. Primers designed for β-actin was used as a loading control for the PCR reactions. A non-reverse transcribed control was used to assure that no genomic DNA was amplified. Primers were designed for RT-PCR (Sigma, St.Louis, MO) and Q-RT-PCR (TaqMan Probes, Applied Biosystems, Foster City, CA) Table 1.
Table 1. Primer Sequences.
| RT-PCR Primers: | |
|---|---|
| EP1: (5′ atc gct tcg gcc tcc acc ttc ttt 3′) | (3′ gcc agc gcc acc aac acc a 5′) |
| EP2: (5′ctc gct gcc gct gct gga cta tgg3′) | (3′gca ggc gag cac cga gac aat gag 5′) |
| EP3: (5′ ggc gct ggc gat gaa caa cga g 3′) | (3′ ggc gct gga gat gaa caa cga g 5′) |
| EP4: (5′ ccg ccc cca ggt agc cag gag 3′) | (3′tgc ggg agg aca gcg ttc agg t 5′) |
| MMP-1: (5′ att cta ctg ata tcg ggg ctt tga3′) | (3′ tgt cct tgg ggt atc cgt gtg tag5′) |
| MMP-2: (5′ ctt ctt gtc gcg gtc gta gtc ctc3′) | (3′ tgg cga tgg ata ccc cct tga5′) |
| MMP-7: (5′aaa ctc ccc gcg tca tag aaa taa t3′) | (3′ tga gtt gca gca tac agg aag tt 5′) |
| MMP-9: (5′ gcg ctg ggc tta gat cat tcc tca 3′) | (3′ gca gcg cgg gcc att gtc 5′) |
| MMP-11: (5′ctg gcg ggc gct ggg aga aga c3′) | (3′cag ggc tgg cca tat agg tgt tga5′) |
| TIMP-1: (5′ cgt cat cag ggg cca agt tcg tg 3′) | (3′ gag gca ggc agg caa ggt gac 5′) |
| TIMP-2: (5′ ctg gcg ggc gct ggg aga agac3′) | (3′cag ggc tgg cca tat agg tgt tga3′) |
| Fibronectin: (5′ccg cca cgt gcc agg att acc3′) | (5′agg ggc tcg ctc ttc tga tta ttc 3′) |
| Collagen1A1: (5′tgt cca ccg agg ctc cca gaa c3′) | (5′ ccc agg ctc cgg tgt gac tcg tg 3′) |
| Vimentin: (5′ ggt ccg tgt cct cgt cct cct ac 3′) | (3′ cgc ggg ctt tgt cgt tgg tta 5′) |
| Elastin: (5′gga ccc ctg act cac gac ctc 3′) | (5′ act tgg ccg ctc ccc tct tgt ttc 3′) |
| HSPG2: (5′ ccg cca ggg cag gtc a 3′) | (3′ ggt ggg cag cgg tag gaa gga gta 5′) |
| COX-2: (5′ ggt ctg gtg cct ggt ctg atg atg 3′) | (5′ gtc ctt tca agg aga atg gtg c 3′) |
| β-actin: (5′ atg ata tcg ccg cgc tcg tcg tc 3′) | (5′ cgc tcg gcc gtg gtg gtg aa 3′) |
| Q-RT-PCR Primers: | |
| EP4: | Hs00168761 |
| MMP2: | Hs00234422 |
| MMP9: | Hs00234579 |
| COL1A1: | Hs00164004 |
| β-actin: | 4352935E |
Statistical analysis
All experiments were conducted in triplicates. Data presented are means of the three or more independent experiments +/− standard error mean (SEM). Statistical analysis was done using GraphPad Prism (GraphPad Software). Comparisons were made using two-tailed Student's t test and significant difference was defined as P < 0.05.
Results
COX2 levels are elevated within several cellular compartments of injured pancreas
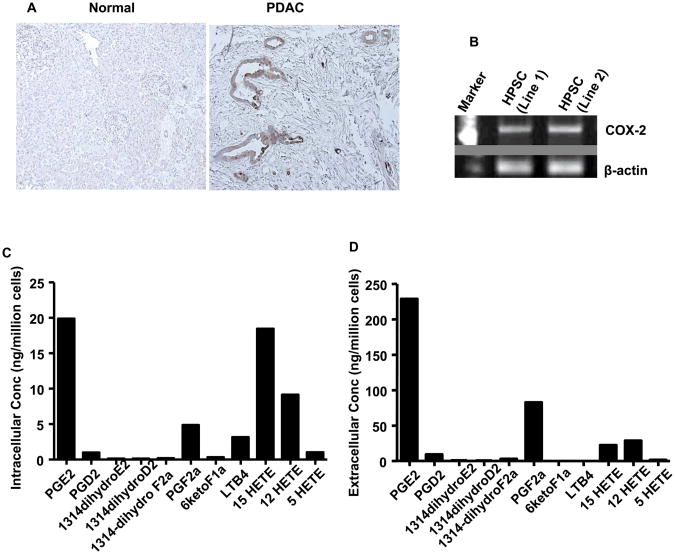
Immunohistochemical analysis revealed high levels of COX-2 in tissue sections of human pancreatic tumors compared to normal pancreas tissues (Fig. 1A), as previously described (14,23). Additional micrographs of tissue sections are shown in Supplementary Figure 1A&B. Although COX-2 levels in PDAC cells were high, significant levels were also observed in the stroma. To more closely address the expression of COX-2 in pancreatic stroma, in isolated HPSC, we examined COX-2 expression by RT-PCR. We found that HPSC (lines 1 and 2) expressed COX-2 mRNA (Fig. 1B). Full-length gels are shown in Supplementary Figure 1C. To determine whether HPSC COX-2 was functional, we measured the level of PGE2 produced by HPSC in culture (Fig. 1C). We observed an intracellular concentration of 20 ng/million cells of PGE2 in stellate cell lysates, which was the highest among the eicosanoids examined. We also examined levels of secreted eicosanoids and again PGE2 levels were found to be the highest (230 ng/million cells) (Fig. 1D). Similar results were obtained with both the lines of HPSC developed (line 2 data not shown). These data confirm that PGE2 within pancreatic tumor is derived from both tumor cells and PSCs, as has previously been reported (24). Although, PGE2 is present in the pancreatic tumor microenvironment, its influence on stellate cell behavior has not been well investigated.
Fig.1. COX-2 expression and eicosanoid levels in PDAC and the stroma.
(A) Immunohistochemistry showing the levels of COX-2 in normal and PDAC tissue sections from human samples. (B) RT-PCR showing the expression of COX-2 in HPSC. Liquid chromatography tandem mass spectrometry (LC/MS/MS) was performed to determine the profile of eicosanoids present in HPSC cellular extracts (C) or HSPC conditioned medium (D).
PGE2 stimulates stellate cell proliferation, migration, invasion and stromal gene expression
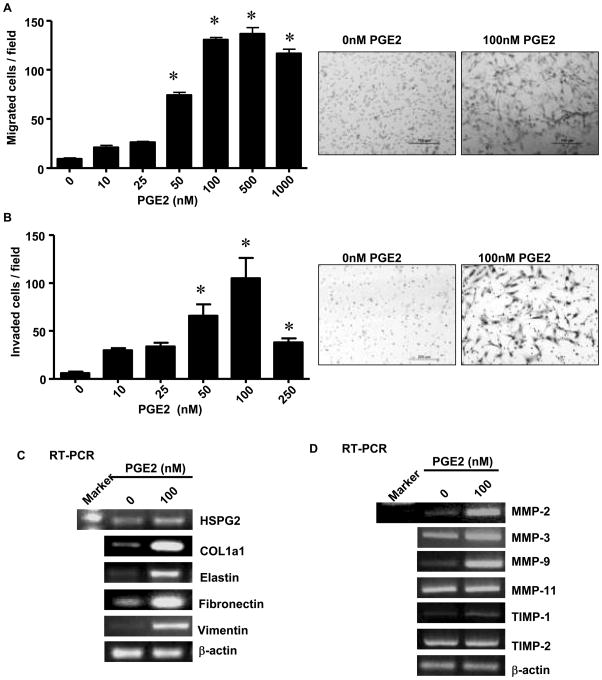
To determine the effects of PGE2 on stellate cells, we treated HPSC (line 1) with exogenous PGE2 in vitro. HPSCs re-suspended in 0.5% serum containing media (2×104) were transferred to migration and invasion chambers and treated with and without PGE2 (0-1000nM). Exogenous PGE2 induced a dose-dependent increase in HPSC cell migration (Fig 2A) and invasion (Fig 2B). Significant effects were consistently noted at 50 nM and maximal effects occurred around 100nM of PGE2. Higher concentrations of PGE2 tended to generate reduced effects suggesting high dose inhibition. Similar results were obtained with both the lines of HPSC developed (line 2 data not shown).
Fig.2. PGE2 stimulates migration, invasion and gene expression.
Cell migration and invasion were assessed by counting the number of stained cells that penetrated the migration membrane after 24h following PGE2 (100nM) stimulation. (A) membrane alone (right, stained cells; left migration numbers p<0.05 versus control) (B) matrigel coated membrane (right, stained cells; left migration numbers p<0.05 versus control) (C) RT-PCR showing the expression of HSPG2, COL1A1, elastin, fibronectin, vimentin and (D) the expression of MMP-2, 3, 9, 11 and TIMP-1, 2 before and after stimulation with PGE2 (100nM). β-actin served as control.
In order to see if stimulation of HPSC with PGE2 influenced stromal gene expression, RNA was extracted from HPSC treated with or without PGE2 (100nM) and mRNA levels of several key molecules were analyzed. PGE2 stimulated expression of several structural genes involved in extra cellular matrix formation including collagen1a1, heparan sulfate proteoglycan 2 (HSPG2), fibronectin and elastin as well as vimentin, an intermediate filament cytoskeletal protein found in mesenchymal cells (Fig 2C). Full-length gels are shown in Supplementary Figure 2A-E. Exogenous PGE2 also stimulated genes involved in matrix turnover and cellular invasion. These molecules included the increased expression of matrix metalloproteinases (MMPs) MMP-2, MMP-3, MMP-9, and tissue inhibitors of metalloproteinases-1 (TIMP-1) mRNA, whereas the mRNA levels of MMP-11 and TIMP-2 remained unchanged (Fig 2D). ull-length gels are shown in Supplementary Figure 3A-D.
The EP4 receptor is required for the effects of PGE2 on stellate cells
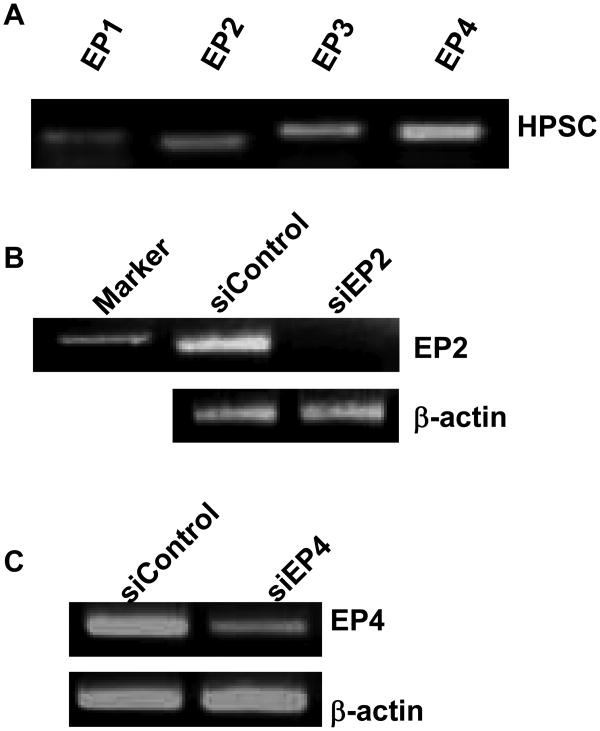
To determine the receptor(s) which might be responsible for the effects of PGE2 on stellate cells, we first identified which receptors were expressed by these cells. RNA was prepared from HPSC cells and RT-PCR was conducted with primers specific for each of the four types of EP receptors. We observed that mRNA for all four receptors, EP1-4 was expressed in the HPSC cells (Fig 3A). Based on what is known about the effects of PGE2 on pancreatic cancer cells, we decided to examine the importance of the most likely candidates for these effects, EP2 and EP4 receptors, using siRNA mediated silencing. The siRNA silencing was highly effective at reducing the appropriate mRNAs as indicated by RT-PCR (Fig 3B, C). Full-length gels are shown in Supplementary Figure 4A&B. No effects of these specific siRNAs were noted on the non-targeted EP receptors (data not shown).
Fig.3. EP receptor differential expression by HPSC and silencing by siRNA.
(A) EP1, 2, 3, and 4 receptor mRNA are differentially expressed by HPSC as determined by RT-PCR (B) silencing of mRNA expression was achieved by transient transfection with human siControl and siEP2 (B) or siEP4 (C) β-actin served as control.
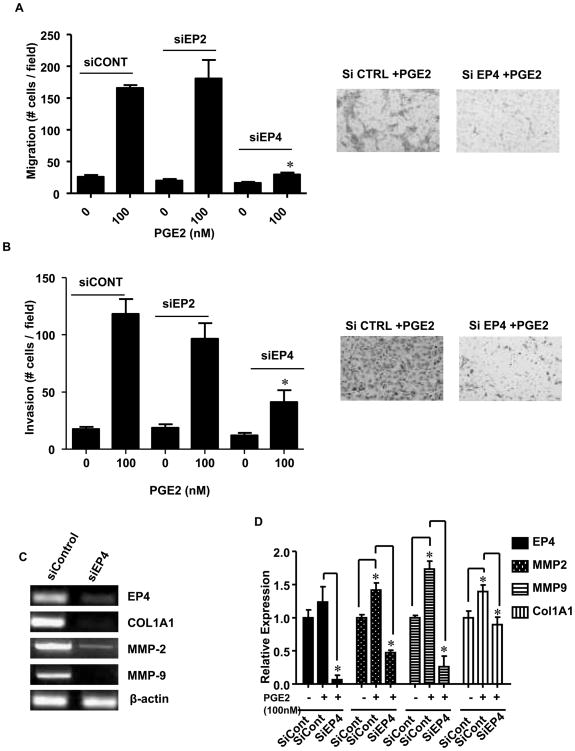
Next, we investigated the effects of silencing EP2 and EP4 receptors on PGE2 mediated biological effect on stellate cells. We observed that transfection with a control siRNA had no effect to reduce PGE2 stimulated HPSC migration or invasion (Fig 4A, B). Likewise, silencing of EP2 did not show a reduction in these parameters. However, silencing of EP4 dramatically reduced the effects of exogenous PGE2 on both migration and invasion. We also examined the effects of silencing EP4 receptors on the ability of exogenous PGE2 to induce expression of Collagen 1A1, MMP-2 and MMP-9 (Fig 4C). Transfection with EP4 targeting siRNA significantly reduced EP4 expression as well as PGE2 stimulated expression of Collagen 1A1 and the matrix metalloproteinases, MMP 2 & 9. Full-length gels are shown in Supplementary Figure 5A-C. The same results were confirmed by performing quantitative RT-PCR also (Fig 4D). Exogenous addition of PGE2 significantly stimulated the expression of MMP-2 (0.4 fold), MMP-9 (0.75 fold) and Collagen 1A1 (0.4 fold), while silencing EP4 receptor resulted in significant decrease in the expression of MMP-2 (1 fold), MMP-9 (1 fold) and Collagen 1A1 (0.5 fold). EP4 silencing was also confirmed to be significantly reduced (1 fold).
Fig.4. Effects of EP2 and EP4 silencing on PGE2 mediated HPSC functions.
HPSC were transiently transfected with siControl, siEP2 or siEP4. After 48 hours, cell migration and invasion were assessed by counting the number of stained cells that penetrated the migration membrane after 24h following PGE2 (100nM) stimulation. (A) membrane alone (right, stained cells; left migration numbers p<0.05 versus control) (B) matrigel coated membrane (right, stained cells; left migration numbers p<0.05 versus control) (C) RT-PCR shows the effects of PGE2 mediated stimulation of COL1A1, MMP-2 and 9 after the silencing of EP4 receptor. β-actin served as control. (D) Q-RT-PCR shows the effects of PGE2 mediated stimulation of COL1A1, MMP-2 and 9 and decrease after the silencing of EP4 receptor. β-actin served as control.
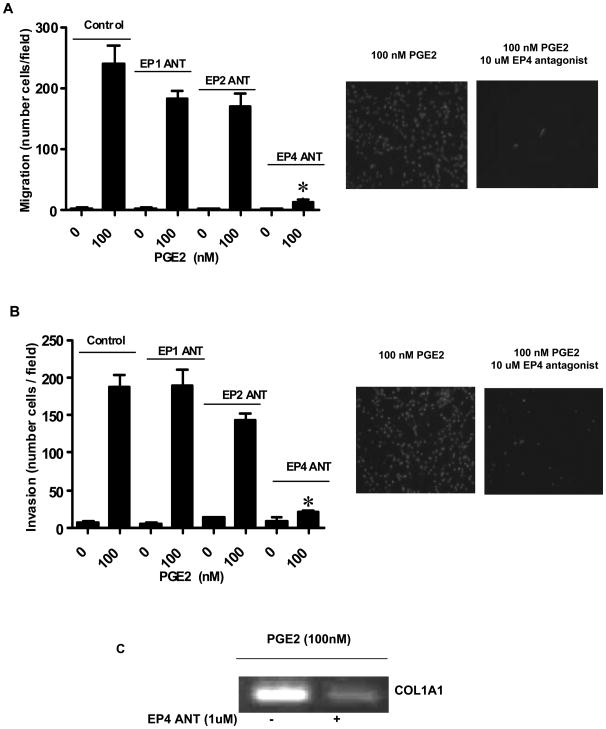
The role of EP4 as the critical receptor for stellate cell regulation by PGE2, was confirmed pharmacologically using receptor specific small molecule inhibitors. In these Boyden chamber migration assays, 10μM concentrations of selective EP antagonists (25) were added to the lower chambers 1 hour prior to PGE2 treatment in serum free media. Treatment of HPSCs with antagonists for either EP1 or EP2 receptors did not affect cell migration as quantified by DAPI staining. In contrast, HPSC pre-treated with the specific antagonist against the EP4 receptor (ONO-AE3-208) significantly reduced cell migration (P<0.05) (Fig 5A). Likewise, when HPSCs were treated independently with specific antagonists against either EP1 or EP2 receptors, neither affected matrigel invasion. However, again the EP4 antagonist significantly reduced HPSC invasion through matrigel (P<0.05) (Fig 5B).
Fig.5. Effects of specific EP receptor antagonists on PGE2 mediated HPSC functions.
HPSC were plated and allowed to settle overnight. Serum containing media was re-plated with serum free media for 24 hours before addition of EP1, EP2 or EP4 antagonists at a dose of 10μM. 1 hour after the antagonist addition, cell migration and invasion were assessed by counting the number of DAPI stained cells that penetrated the migration membrane after 24 h following PGE2 (100nM) stimulation. (A) membrane alone (right, stained cells; left migration numbers p<0.05 versus control) (B) matrigel coated membrane (right, stained cells; left migration numbers p<0.05 versus control). Addition of EP4 antagonist showed a significant reduction of PGE2 mediated HPSC migration and invasion. (C) RT-PCR showing the reduction in collagen 1A1 expression on treatment with EP4 antagonist (10μM) as compared to increase on treatment with PGE2 (100nM) alone.
Discussion
PSCs have recently become the focus of much attention due to their importance in PDAC. The fibrotic stroma that they elaborate serves as the specific niche in which the cancer cells develop. Furthermore, this stroma supports the survival of the cancer cells (21,26) and may provide a physical barrier to some therapies (4). However, stellate cells also play a critical role in chronic pancreatitis (1,27). In both diseases, PSCs produce an abundant desmoplastic response that ends up replacing normal parenchyma and contributing to pancreatic insufficiency. This stroma can also impinge on nerves to cause pain and can block the common bile duct leading to jaundice. Many factors regulate PSC activity including cytokines and several growth factors such as TGFβ, PDGF, and CTGF (28). However, the regulation of PSCs by prostaglandins has not been extensively evaluated say for one study using normal rat stellate cells in combination with human tumor cells (24). The use of PANC-1 conditioned medium (CM) stimulated rat stellate cells to produce COX-2 that was prevented by U0126, an extracellular signal-regulated kinase (ERK) 1/2 inhibitor. Furthermore, NS398, a selective COX-2 inhibitor, reduced the growth of PSCs by PANC-1 CM. Several studies have indicated that COX-2 expression is high in both chronic pancreatitis and PDAC therefore suggesting a potential role in fibrosis (6,29). COX-2 levels are also known to positively correlate with the aggressiveness of PDAC (30) and that blocking COX-2 significantly reduces pancreatic tumor size and metastatic potential (31) in animal models. No studies to date, however, have examined the downstream role of PGE2 or the EP receptors in HPSCs. Our data are the first to suggest that targeting EP4 receptors may be a useful way to reduce stellate cell activity associated with pancreatic inflammation and fibrosis.
Previous studies have shown that inhibiting the COX-2 pathway suppresses the development of many cancers including pancreatic cancer (32). Also, COX-2 gene expression and its primary metabolite prostaglandin E2 (PGE2) were highly expressed in 90% of pancreatic tumors (7). The majority of studies have focused on the tumor cells. Our studies by contrast, highlight the importance of COX-2 and PGE2 in the profound desmoplastic reaction associated with PDAC. As a pharmacologic intervention, celecoxib is a non-steroidal anti-inflammatory drug (NSAID) and a highly selective COX-2 inhibitor. Traditional NSAIDs can inhibit both COX-1 and COX-2, to varying degrees. Selective inhibition of COX-2 by celecoxib was found to effectively suppress the growth of pancreatic cancer cells in vitro (17) and to inhibit PDAC tumor growth and angiogenesis in orthotopic implantation tumor models (33). More recently it was shown that Celecoxib could slow or prevent the progression of early PanIn lesions in mouse models to metastatic disease (32). These data strongly support inhibition of this pathway as a therapeutic approach for PDAC.
Unfortunately, Celecoxib has been found to be a poor therapeutic. We now know that COX-2 inhibition impacts negatively on cardiac and renal, which causes to serious side effects of prolonged inhibition of this pathway (34). In pancreatic cancer clinical trials, celecoxib in combination with standard chemotherapeutic drugs was found to be modestly effective in some studies but in other studies the effectiveness was limited by toxicity (35-39). From all of the available data it appears that inhibition of COX-2/PGE2 pathway is effective, but celecoxib does not appear to be the best therapeutic to inhibit this pathway. In the current study, we found that the effects of PGE2 on PSC were specifically mediated by the EP4 receptor. These data contrast with those reported for the PDAC cells where the EP2 receptor is thought to be most important (40). Stromal formation in lung cancer has also been reported to involve EP4 receptors (41). Targeting EP4 receptors has recently been found to decrease foci formation, metastasis and tumor incidence in colon cancer (42). To date several toxicity studies have been conducted and no toxicity has been reported for EP4 antagonist. Barring any toxicity issues associated with selective downstream targeting of EP4, this may be an important new approach to control pancreatic fibrosis.
Although the importance of stroma in PDAC is well known, the molecular mechanisms that regulate stromal activity and the downstream signaling remain poorly understood. Our study provides the first evidence that PGE2 induces major behavioral changes in PSCs. These changes include contribution to the proliferation, migration and activity of PSCs in the formation of the collagen I matrix. These effects are mediated by the EP4 receptor. Furthermore, that fibrotic activity of the stellate cells is tightly regulated by the EP4 receptor as a potential target for the prevention of pancreatic fibrosis or as adjuvant treatment administered along with treatments for PDAC.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by funds from DK052067 (to CDL), by the Lockton Endowment (to CDL), by the UTMDACC IRG-Basic/Translational Research fund (to VR), NIH MERIT award R37 DK47297 (to RND) and Cancer Center Support Core grant CA16672 (to MDACC).
Footnotes
The authors have no any potential conflict of interest including any financial, personal, or other relationships with other people or organizations that could inappropriately influence this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chantale Charo, Department of Cancer Biology, UT MD Anderson Cancer Center, Houston, TX.
Vijaykumar Holla, Department of Cancer Biology, UT MD Anderson Cancer Center, Houston, TX.
Thiruvengadam Arumugam, Department of Cancer Biology, UT MD Anderson Cancer Center, Houston, TX.
Rosa Hwang, Department of Surgical Oncology, UT MD Anderson Cancer Center, Houston, TX.
Peiying Yang, Department of Cancer Biology, UT MD Anderson Cancer Center, Houston, TX.
Raymond N. Dubois, Department of Cancer Biology and Gastrointestinal Oncology, UT MD Anderson Cancer Center, Houston, TX.
David G. Menter, Department of Cancer Biology, UT MD Anderson Cancer Center, Houston, TX.
Craig D. Logsdon, Department of Cancer Biology and Medical Oncology, UT MD Anderson Cancer Center, Houston, TX.
Vijaya Ramachandran, Department of Cancer Biology, UT MD Anderson Cancer Center, Houston, TX.
References
- 1.Masamune A, Watanabe T, Kikuta K, et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7:S48–54. doi: 10.1016/j.cgh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 3.Apte MV, Wilson JS. Mechanisms of pancreatic fibrosis. Dig Dis. 2004;22:273–279. doi: 10.1159/000082799. [DOI] [PubMed] [Google Scholar]
- 4.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by pro inflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–541. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlosser W, Schlosser S, Ramadani M, et al. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas. 2002;25:26–30. doi: 10.1097/00006676-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Tucker ON, Dannenberg AJ, Yang EK. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 8.Robert A, Nezamis JE, Lancaster C, et al. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77:433–443. [PubMed] [Google Scholar]
- 9.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krysan K, Reckamp KL, Sharma S, et al. The potential and rationale for COX-2 inhibitors in lung cancer. Anticancer Agents Med Chem. 2006;6:209–220. doi: 10.2174/187152006776930882. [DOI] [PubMed] [Google Scholar]
- 11.Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- 12.Wu WK, Sung JJ, Lee CW, et al. Dysregulation of cellular signaling in gastric cancer. Cancer Lett. 2010;295:7–16. doi: 10.1016/j.canlet.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Kokawa A, Kondo H, Gotoda T, et al. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333–338. doi: 10.1002/1097-0142(20010115)91:2<333::aid-cncr1006>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Koshiba T, Hosotani R, Miyamoto Y, et al. Immunohistochemical analysis of cyclooxygenase-2 expression in pancreatic tumors. Int J Pancreatol. 1999;26:69–76. doi: 10.1007/BF02781733. [DOI] [PubMed] [Google Scholar]
- 15.Merati K, Saidaty M, Andea A, et al. Expression of inflammatory modulator COX-2 in pancreatic ductal adenocarcinoma and its relationship to pathologic and clinical parameters. Am J Clin Oncol. 2001;24:447–452. doi: 10.1097/00000421-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Maitra A, Ashfaq R, Gunn CR, et al. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol. 2002;118:194–201. doi: 10.1309/TPG4-CK1C-9V8V-8AWC. [DOI] [PubMed] [Google Scholar]
- 17.Colby JK, Klein RD, McArthur MJ, et al. Progressive metaplastic and dysplastic changes in mouse pancreas induced by cyclooxygenase-2 overexpression. Neoplasia. 2008;10:782–796. doi: 10.1593/neo.08330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrow B, Rowley D, Dang T, et al. Characterization of tumor-derived pancreatic stellate cells. J Surg Res. 2009;157:96–102. doi: 10.1016/j.jss.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 19.Menke A, Adler G. TGFbeta-induced fibrogenesis of the pancreas. Int J Gastrointest Cancer. 2002;31:41–46. doi: 10.1385/IJGC:31:1-3:41. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Erkan M, Abiatari I, et al. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol Ther. 2007;6:218–227. doi: 10.4161/cbt.6.2.3623. [DOI] [PubMed] [Google Scholar]
- 21.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki H, Ohnishi H, Hama K, et al. Cyclooxygenase-2 is required for activated pancreatic stellate cells to respond to proinflammatory cytokines. Am J Physiol Cell Physiol. 2007;292:C259–268. doi: 10.1152/ajpcell.00030.2006. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Li YM, Yu C, et al. Expression of p53, p16 and COX-2 in pancreatic cancer with tissue microarray. Hepatobiliary Pancreat Dis Int. 2006;5:138–142. [PubMed] [Google Scholar]
- 24.Yoshida S, Ujiki M, Ding XZ, et al. Pancreatic stellate cells (PSCs) express cyclooxygenase-2 (COX-2) and pancreatic cancer stimulates COX-2 in PSCs. Mol Cancer. 2005;4:27. doi: 10.1186/1476-4598-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X, Kundu N, Rifat S, et al. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006;66:2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 26.Fujita H, Ohuchida K, Mizumoto K, et al. Tumor-stromal interactions with direct cell contacts enhance proliferation of human pancreatic carcinoma cells. Cancer Sci. 2009;100:2309–2317. doi: 10.1111/j.1349-7006.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apte MV, Pirola R, Wilson J. New insights into alcoholic pancreatitis and pancreatic cancer. J Gastroenterol Hepatol. 2009;24:S51–56. doi: 10.1111/j.1440-1746.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- 28.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 29.Okami J, Yamamoto H, Fujiwara Y, et al. Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res. 1999;5:2018–2024. [PubMed] [Google Scholar]
- 30.Albazaz R, Verbeke CS, Rahman SH, et al. Cyclooxygenase-2 expression associated with severity of PanIN lesions: a possible link between chronic pancreatitis and pancreatic cancer. Pancreatology. 2005;5:361–369. doi: 10.1159/000086536. [DOI] [PubMed] [Google Scholar]
- 31.Arjona-Sanchez A, Ruiz-Rabelo J, Perea MD, et al. Effects of capecitabine and celecoxib in experimental pancreatic cancer. Pancreatology. 2010;10:641–647. doi: 10.1159/000288708. [DOI] [PubMed] [Google Scholar]
- 32.Funahashi H, Satake M, Dawson D, et al. Delayed progression of pancreatic intraepithelial neoplasia in a conditional Kras (G12D) mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res. 2007;67:7068–7071. doi: 10.1158/0008-5472.CAN-07-0970. [DOI] [PubMed] [Google Scholar]
- 33.Raut CP, Nawrocki S, Lashinger LM, et al. Celecoxib inhibits angiogenesis by inducing endothelial cell apoptosis in human pancreatic tumor xenografts. Cancer Biol Ther. 2004;3:1217–1224. doi: 10.4161/cbt.3.12.1221. [DOI] [PubMed] [Google Scholar]
- 34.Dajani EZ, Islam K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. 2008;59:117–133. [PubMed] [Google Scholar]
- 35.El-Rayes BF, Zalupski MM, Shields AF, et al. A phase II study of celecoxib, gemcitabine, and cisplatin in advanced pancreatic cancer. Invest New Drugs. 2005;23:583–590. doi: 10.1007/s10637-005-1028-z. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari V, Valcamonico F, Amoroso V, et al. Gemcitabine plus celecoxib (GECO) in advanced pancreatic cancer: a phase II trial. Cancer Chemother Pharmacol. 2006;57:185–190. doi: 10.1007/s00280-005-0028-1. [DOI] [PubMed] [Google Scholar]
- 37.Lipton A, Campbell-Baird C, Witters L, et al. Phase II trial of gemcitabine, irinotecan, and celecoxib in patients with advanced pancreatic cancer. J Clin Gastroenterol. 2010;44:286–288. doi: 10.1097/MCG.0b013e3181cda097. [DOI] [PubMed] [Google Scholar]
- 38.Pino MS, Milella M, Gelibter A, et al. Capecitabine and celecoxib as second-line treatment of advanced pancreatic and biliary tract cancers. Oncology. 2009;76:254–261. doi: 10.1159/000205388. [DOI] [PubMed] [Google Scholar]
- 39.Xiong HQ, Plunkett W, Wolff R, et al. A pharmacological study of celecoxib and gemcitabine in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2005;55:559–564. doi: 10.1007/s00280-004-0916-9. [DOI] [PubMed] [Google Scholar]
- 40.Pino MS, Nawrocki ST, Cognetti F, et al. Prostaglandin E2 drives cyclooxygenase-2 expression via cyclic AMP response element activation in human pancreatic cancer cells. Cancer Biol Ther. 2005;4:1263–1269. doi: 10.4161/cbt.4.11.2138. [DOI] [PubMed] [Google Scholar]
- 41.Katoh H, Hosono K, Ito Y, et al. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am J Pathol. 2010;176:1469–1483. doi: 10.2353/ajpath.2010.090607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty GA, Byrne SM, Molloy ES, et al. Proneoplastic effects of PGE2 mediated by EP4 receptor in colorectal cancer. BMC Cancer. 2009;9:207. doi: 10.1186/1471-2407-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.