Abstract
We compared the Autonomic Symptom Profile results in 16 women with chronic pelvic pain (CPP) and 15 age-matched healthy subjects. Moderately severe generalized autonomic symptomology occurs in women with CPP, but not in controls. Further study including autonomic testing is needed to confirm results and explore the mechanism of dysfunction.
Introduction
Despite it’s high prevalence of nearly 6%, chronic pelvic pain (CPP) still has no formally accepted definition [1]. Pain lasting more than 6 months in the pelvic region and affecting quality of life is a reasonable descriptor. Since multiple co-morbid conditions such as migraine, fibromyalgia and irritable bowel syndrome accompany some types of pelvic pain, we hypothesized that changes in autonomic function may occur and that these would be manifested by autonomic symptomology in this patient group. We used a validated questionnaire in a pilot evaluation of autonomic function in a small group of women suffering from CPP.
Materials and Methods
This IRB-approved prospective case-controlled study included adult women with CPP suffering from pelvic pain ≥ 6 months duration and age-matched healthy women seen during annual health examinations. All patients completed a question set asking for specific characteristics of pelvic pain (table 1), and specific co-morbid conditions, listed in the results section, as well as the original long form Autonomic Symptom Profile© [3]. Scores were compared with those reported in the original publication and analyzed, using SPSS 11.0 using the Student’s t-test, Pearson Chi-Square, and one-way analysis of variance as appropriate.
Table 1.
Chronic pelvic pain symptomatology and co-morbid conditions. The number in the denominator represents the number of total responses to the item.
| Chronic Pelvic Pain (n = 16) | Control (n = 15) | P-value1 | |
|---|---|---|---|
| Pelvic pain between menses | 16/16 | 4/15 | < 0.001 |
| Dyspareunia | 14/14 | 2/14 | < 0.001 |
| Pain with walking | 11/16 | 0/15 | < 0.001 |
| Pain with lifting | 10/15 | 0/15 | < 0.001 |
| Pain with full bladder | 10/15 | 1/15 | < 0.001 |
| Previous laparoscopy | 16/16 | 4/10 | = 0.001 |
| Pain with bowel movement | 10/15 | 2/15 | < 0.01 |
| Other medical diagnosis | 11/16 | 3/15 | < 0.01 |
| Dysmenorrhea | 10/12 | 6/14 | < 0.05 |
P-values were determined by Pearson Chi-square test.
Results
Mean age and gravida were not different (CPP 36.9, 2.5 NS; Healthy 36.4, 1.6 NS). As expected, the CPP group more frequently experienced pain in every circumstance and had a higher rate of past laparoscopy (Table 1) but not other past surgery (not shown). Not surprisingly, CPP patients more often reported coexisting medical conditions, including 7 subjects with irritable bowel syndrome, 6 with depression, 5 with anxiety disorders, 4 with migraines, and 1 each with fibromyalgia, interstitial cystitis, asthma, post-traumatic stress disorder, and reflex sympathetic dystrophy. In contrast, within the healthy group 1 subject reported migraine headaches, 1 fibromyalgia, 2 hypertension, and 1 breast cancer.
The Autonomic Symptom Profile score was 8.5 ± 5.4 for healthy subjects (similar to Mayo group healthy controls 9.8 ± 9), and 27.4 ± 9 for the CPP group. Subcategory scores (table 2) were higher for the CPP group in all categories except for autonomic diarrhea, sleep, and syncope. Finally, absence of difference in validation scale scores (under/over scoring) between CPP and healthy groups suggested no difference in any non-organic component.
Table 2.
Mean raw scores for subcategories of the Autonomic Symptom Profile and Validation Scales.
| Chronic pelvic pain (n = 16) | Control (n = 15) | P-value1 | |
|---|---|---|---|
| Vasomotor | 5.5 ± 4.0 | 0.5 ± 2.1 | < 0.001 |
| Secretomotor disorder | 3.6 ± 2.5 | 0.7 ± 0.3 | < 0.001 |
| Gastroparesis | 1.8 ± 1.5 | 0.1 ± 0.3 | < 0.001 |
| Orthostatic intolerance | 6.0 ± 3.2 | 2.2 ± 2.6 | < 0.001 |
| Constipation | 2.7 ± 2.2 | 0.7 ± 1.0 | < 0.01 |
| Bladder dysfunction | 2.5 ± 2.5 | 0.6 ± 0.7 | < 0.01 |
| Pupillomotor | 3.2 ± 2.1 | 1.5 ± 1.9 | < 0.05 |
| Autonomic diarrhea | 1.0 ± 1.2 | 0.5 ± 0.6 | NS |
| Sleep disorder | 1.0 ± 2.0 | 1.7 ± 3.0 | NS |
| Syncope | 0.1 ± 0.5 | 0.0 ± 0.0 | NS |
| Total | 27.4 ± 9.0 | 8.5 ± 5.4 | <0.000001 |
| Validation scales | |||
| Under scoring | 0.6 ± 1.2 | 1.1 ± 1.3 | NS |
| Over scoring/psychosomatic | 0.3 ± 0.7 | 0.0 ± 0.0 | NS |
P-values were determined using one-way analysis of variance.
Discussion
This exploratory study based only on questionnaire responses of patients with chronic pelvic pain compared to healthy subjects suggests significant levels of autonomic dysfunction. These findings are in keeping with the increasing body of evidence linking autonomic dysfunction with chronic pain of every type. The surprising finding was the magnitude of the change. Compared to the original study of neurologic disorders, CPP scores (27.4 ± 9) were similar to peripheral neuropathy scores (25.9 ± 17.9), and even exceeded autonomic disorders subjects in 3 categories, constipation, vasomotor and pupillomotor dysfunction, the last 2 being far removed from the pelvic region.
The limitations of our study should be clearly stated. The sample size of 31 is small, though admittedly this is more likely to mask a true difference than to create the illusion of a difference that is not present. Second, the data are based on structured questionnaire self-report with no external examiner or autonomic testing corroboration, clearly the next step in confirming these findings. Importantly, the CPP group is likely to be on more medications than the healthy group, many of which may influence autonomic function and its subjective symptom manifestations. Finally, the instrument we used was validated in a different population of patients with neurologic disorders, and its application in this population may of may not be reliable.
Autonomic symptoms could occur in CPP because they predispose to CPP, as a consequence of chronic pain, or reflecting a third underlying neurologic abnormality. Neither the literature nor clinical experiences emphasize CPP in patients with autonomic disorders to support the first option. In contrast, other forms of chronic pain do manifest generalized autonomic dysfunction. However, if pain were directly causative, one might expect focal autonomic dysfunction that matches the area of pain. This was not the case in our population, suggesting some underlying central neurologic dysfunction. An anatomic basis for such an association is emerging in the reciprocal connections between the periaquaductal gray region which controls descending modulating pain and the locus coeruleus and raphe nuclei which influence autonomic outflow [5].
In conclusion, moderately severe autonomic symptoms occur with CPP, but not in pain-free women. Further work using more elaborate methods such as formal autonomic testing may confirm and extend these findings.
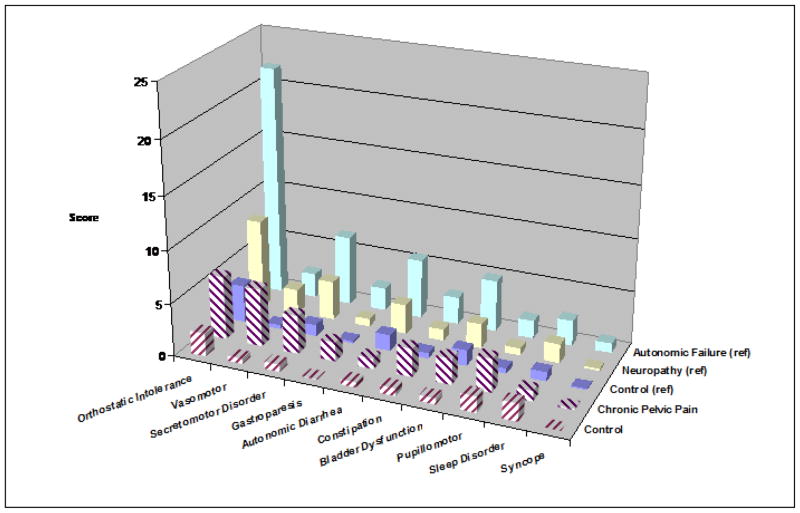
Figure 1.
Autonomic Symptom Score by Category in the 5 Patient Groups
Autonomic symptom scores for each domain across the 5 patient groups. The 3 groups from the original paper are solid bars, while the 2 groups from this study are hashed. Note the very high levels of vasomotor and pupillomotor dysfunction in the CPP patients, even higher for the group with neurogenic autonomic failure.
References
- 1.Williams RE, et al. Prevalence and characteristics of irritable bowel syndrome among women with chronic pelvic pain. Obstet Gynecol. 2004;104(3):452–8. doi: 10.1097/01.AOG.0000135275.63494.3d. [DOI] [PubMed] [Google Scholar]
- 2.Shipton EA. Pain Acute and Chronic. 2. London: Arnold; 1999. [Google Scholar]
- 3.Longstreth GF, Preskill DB, Youkeles L. Irritable bowel syndrome in women having diagnostic laparoscopy or hysterectomy. Relation to gynecologic features and outcome. Dig Dis Sci. 1990;35(10):1285–90. doi: 10.1007/BF01536421. [DOI] [PubMed] [Google Scholar]
- 4.Suarez GA, et al. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52(3):523–8. doi: 10.1212/wnl.52.3.523. [DOI] [PubMed] [Google Scholar]
- 5.Yelle MD, et al. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci. 2009;29(33):10264–71. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]