Abstract
CMG2 is a transmembrane extracellular matrix binding protein that is also an anthrax toxin receptor. We have shown that high affinity CMG2 binders can inhibit angiogenesis and tumor growth. We recently described a high throughput FRET assay to identify CMG2 inhibitors. We now report the serendipitous discovery that PGG (1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose) is a CMG2 inhibitor with anti-angiogenic activity. PGG is a gallotannin produced by a variety of medicinal plants that exhibits a wide variety of anti-tumor and other activities. We find that PGG inhibits CMG2 with a submicromolar IC50 and it also inhibits the migration of human dermal microvascular endothelial cells at similar concentrations in vitro. Finally, oral or intraperitoneal administration of PGG inhibits angiogenesis in the mouse corneal micropocket assay in vivo. Together, these results suggest that a portion of the in vivo anti-tumor activity of PGG may be the result of antiangiogenic activity mediated by inhibition of CMG2.
Keywords: pentagalloyl glucose, 5GG, angiogenesis, corneal neovascularization, cancer, polyphenol
INTRODUCTION
Angiogenesis is the process by which new blood vessels are generated from existing vessels. It is necessary whenever more than a few mm of additional tissue are generated and often accompanies tissue remodeling. As a result, a wide variety of diseases are angiogenesis-dependent. These include cancer, psoriasis, macular degeneration, and diabetic retinopathy1. Angiogenesis is initiated by pro-angiogenic growth factors such as bFGF and VEGF and begins when endothelial cells partially degrade their basement membrane and migrate into the surrounding stroma using a provisional matrix composed of collagen I, fibrin, vitronectin and fibronectin. Migration through the stroma is dependent on MMPs including MMP2 and MMP14 and is led by endothelial “tip cells”. Following the formation of the nascent vessel, a new basement membrane composed of laminin, collagen IV, nidogen and perlecan is laid down cooperatively by pericytes and endothelial cells and this stabilizes the vessel both mechanically and against additional angiogenic stimulus 2.
Capillary morphogenesis gene 2 (CMG2) was originally identified as a gene up-regulated in endothelial cells undergoing tube formation in collagen gels3. It is composed of an amino-terminal, metal binding von Willibrandt factor (vWF) domain, an Ig-like domain, a transmembrane domain, and a hydrophilic intracellular domain3. It is the primary receptor for anthrax toxin, which binds via the vWF domain, and crystal structures of the complex have been generated4–6. In the context of anthrax intoxication, CMG2 has been well studied7, however its natural function in the absence of toxin is less well established. Its vWF domain has homology with integrins and like integrins binds to extracellular matrix molecules including collagen IV, laminin, and fibronectin8. Certain mutations in CMG2 result in Hyaline Fibromatosis Syndrome which is characterized by the accumulation of hyaline material in the skin and other organs9. In contrast, mouse knock-outs do not display this phenotype and are fertile, but cannot perform MMP14-dependent uterine remodelling essential to parturition. CMG2 is expressed in tumor vasculature10 and modification of CMG2 expression affects the ability of endothelial cells to proliferate and form networks in extracellular matrix10. Modulation of binding also appears to affect the ability of endothelial cells to migrate in vitro11. In vivo, we have observed that anthrax protective antigen and mutants with increased half-life exhibit antiangiogenic activity in the corneal micropocket assay in a CMG2-binding dependent manner11. As expected, these agents also inhibit tumor growth in mice11.
PGG (1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose) is a very common polyphenol plant metabolite. It has been studied in a number of diseases including several models of cancer12, where oral administration leads to a decrease in tumor growth13. It inhibits a wide range of biological processes, including PTP1B14, angiogenesis15, VEGF receptor binding16, and DNA polymerase activity17. However, most of these processes are only inhibited at PGG concentrations in the tens of micromolar. In contrast, recent pharmacokinetic studies demonstrate that intraperitoneal administration of PGG results in single-digit micromolar plasma concentrations and oral administration results in submicromolar plasma concentrations18. These contrasting results indicate that the pharmacologic target responsible for inhibition of tumor growth by PGG may not yet have been identified. Here we report the serendipitous discovery of CMG2 as additional target for PGG action with the potential to explain a portion of the antitumor activity of the molecule.
RESULTS
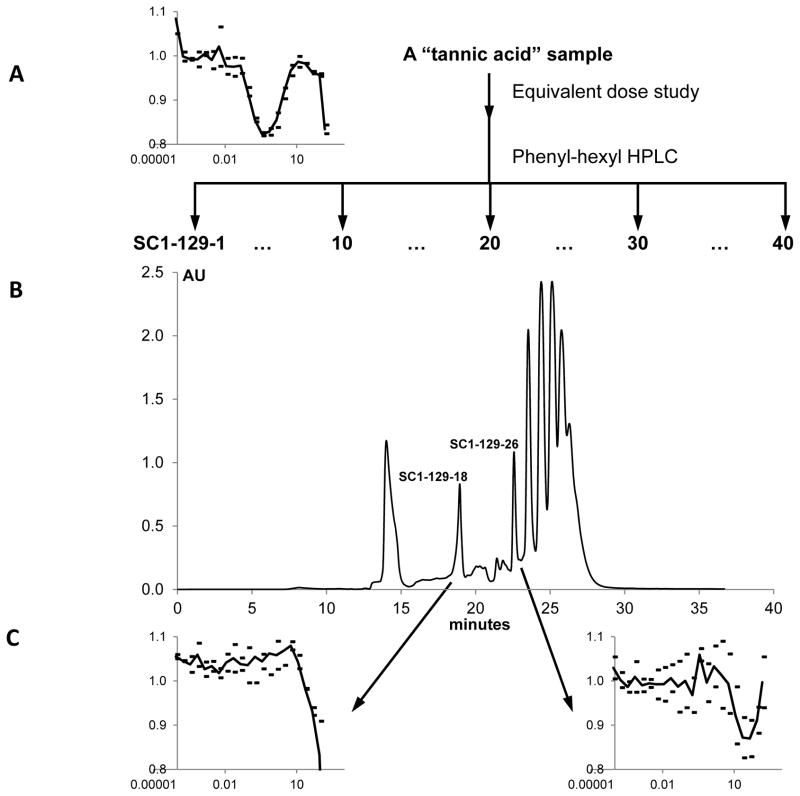
As previously reported19, the screening of a “known bioactives” library for CMG2 inhibitors resulted in a “hit” on tannic acid. Further characterization of this hit demonstrated a complex inhibition curve and toxic effects upon administration in mice19, damping our enthusiasm for further study in the context of angiogenesis. However, during follow-up experiments at Clemson University we discovered that there were dramatic differences in the anti-CMG2 activity of separate lots of tannic acid, even though they were from the same supplier. In order to ascertain why one sample was active, as determined by the CMG2 FRET assay, while another was not, HPLC analysis was carried out. In the HPLC chromatogram of the CMG2 inactive “tannic acid” sample, one broad peak was observed, while many peaks were exhibited in the chromatogram of the CMG2 active “tannic acid” sample (Figure 1A and B). Clearly, the active “tannic acid” sample is a mixture of many compounds, and we hypothesized that one of the contaminants was responsible for the significant anti-CMG2 activity observed with the impure sample.
Figure 1.
Identification of the active compounds in the “tannic acid” sample. The scheme used to purify CMG2-active compounds (A) is shown along with a representative HPLC-trace (B). CMG2 FRET assay results on serial dilutions of the starting material and crucial active fractions are also shown (C).
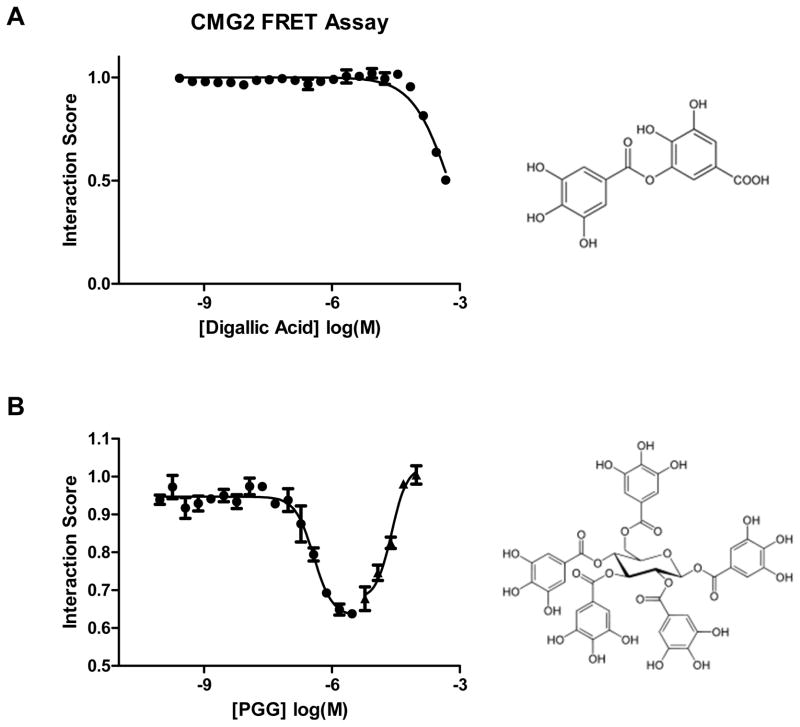
To test this hypothesis, an equivalent dose study was carried out. Forty fractions were collected after a gradient HPLC and tested in the FRET assay. This resulted in the identification of two active peaks. Based on the UV and the polarities of these peaks, the HPLC program was modified, and these two peaks were well separated from the others. One, SC1-129-26 (Retention Time: 22.5 min; Figures 1B), was identified as 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG), and another, SC1-129-18 (Retention Time: 18.5 min; Figures 1B), digallic acid (see Supplementary Figures). Both PGG and digallic acid were active against CMG2 with an IC50 of ~50 μM for digallic acid and a complex inhibition curve for PGG that exhibited 50% of maximal CMG2 inhibition at ~300 nM (Figure 1C). We have also previously observed that CMG2 interaction with PA is inhibited by phenolic natural products20. Given these results, we also assessed methyl gallate, propyl gallate, and a number of additional polyphenols (Table 1) at concentrations up to 300 μM. None of these compounds exhibited any inhibitory activity.
Table 1.
Polyphenols assessed for inhibition in the CMG2 FRET assay.a
| Compound | IC50 (μM) |
|---|---|
| Tannic acid | >300 |
| PGG | 0.3 |
| Digallic Acid | 50 |
| Methyl Gallate | >300 |
| Propyl Gallate | >300 |
| Epigallocatechin | >300 |
| Gallic Acid | >300 |
| Dopamine | >300 |
| Myricetin | >300 |
| Vanillic Acid | >300 |
| Quercetin | >300 |
| 3-(3,4-dihydroxy phenyl) propionic acid | >300 |
| Methyl-3,4-dihydroxybenzoate | >300 |
| (−)-Epigallocatechin | >300 |
| Protocatechillic acid | >300 |
| Curcumin | >300 |
| (−)-Epicatechin | >300 |
| Caffeic acid | >300 |
Compounds were assessed for inhibiton of PA-CMG2 interaction by FRET at a single high concentrations, and then, where appropriate at 2-fold dilutions across a six order of magnitude concentration range, with IC50 determined by nonlinear fitting to the Hill equation.
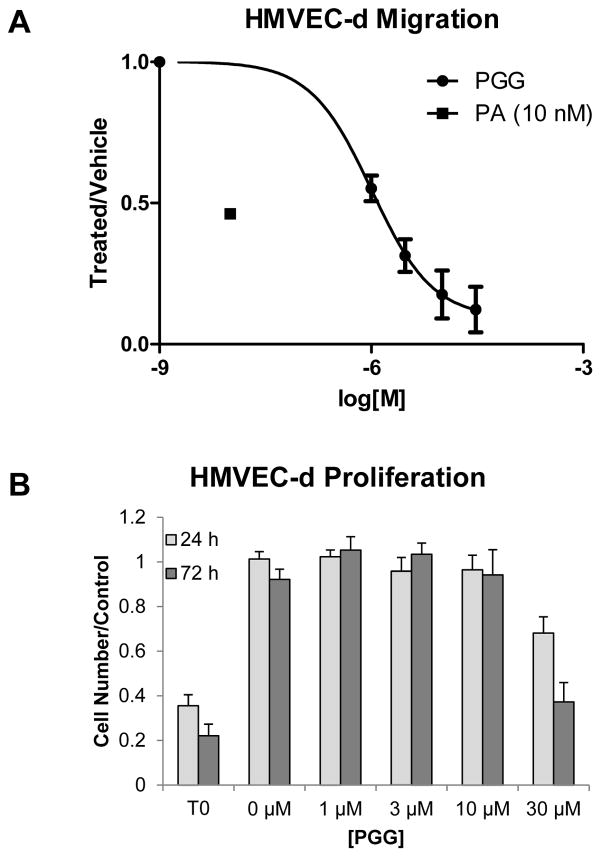
Since PGG was the more potent of the compounds that had been identified, we decided to assess its capacity to inhibit angiogenesis ex vivo and in vivo. We have observed that both native Anthrax Protective Antigen (PA) and PASSSR, a mutated form of PA that spends a prolonged period of time at the receptor surface without being internalized, inhibit the migration of human dermal microvascular endothelial cells (HMVEC-d)11. We assessed PGG’s effect in this same ex vivo assay. As is true of PASSSR, PGG inhibited endothelial cell migration at doses where it exhibited no cytotoxic or cytostatic effects (Figure 3).
Figure 3.
Effects of PGG on endothelial cells ex vivo. A) Endothelial cell migration. Cells were allowed to migrate to media containing 5% serum for 4 hours. The average of two independent experiments (n=3 for each experiment), performed on separate days is presented. Data was normalized to the no-treatment control to account for day-to-day variation in number of cells migrated. Curve represents a standard binding isotherm with IC50 of 820 nM. B) Endothelial cell proliferation. Cells were plated in EGM2 or EGM2 with PGG with 2000 cells per well and assessed at 24 and 72 hours. The average of four independent experiments (n=4 wells for each experiment), performed on separate days is presented. Data was normalized to the average of four untreated wells to account for day-to-day variation in number of cells plated. T0 represents the readout from cells at the start of the assay without treatment, where wells were fixed in absolute ethanol on day 0. The point at 0 μM represents a vehicle control. Error bars indicate standard deviation.
We went on to assess the effect of PGG on angiogenesis in the corneal micropocket assay. When delivered at a dose of 10 mg/kg IP, or 20 mg/kg/day by oral gavage, PGG significantly inhibited bFGF-driven angiogenesis. Importantly we observed no obvious signs of toxicity (weight loss, hunched posture, behavioral abnormalities) indicating that the reduction in angiogenic response is a result of a pharmacologic effect of PGG, rather than general animal toxicity. These results are consistent with previous results demonstrating that PGG can be delivered both IP and orally at doses up to 30 mg/kg/day without ill effects12.
DISCUSSION AND CONCLUSION
Here we demonstrate that PGG has anti-CMG2 activity in vitro in a FRET-based binding assay, ex vivo in an endothelial cell migration assay, and that it has antiangiogenic activity in vivo in the corneal micropocket assay. The biphasic inhibition curve that we observe in the FRET assay is unusual and remains to be explained.
In addition to these results, PGG has previously been shown to have a wide variety of effects in both in vitro and in vivo assays12. In vivo, these effects include inhibition of VEGF binding to VEGF receptor16, insulin mimetic activity21, inhibition of tumor growth13, 15, and inhibition of angiogenesis15. Importantly the antiangiogenic activity of PGG has been evoked at doses of 4 mg/kg15. Recent studies demonstrate that even a relatively high oral PGG dose of 80 mg/kg leads to circulating concentrations <1 μM in experimental animals18. This is likely in part a result of degradation as the compound passes the gut22. Thus, any effect induced by oral delivery of PGG must be mediated by an interaction in the submicromolar range.
Among published in vitro PGG targets, however, only a few mammalian proteins have IC50’s in the submicromolar range17. These include inhibition of certain DNA polymerases17, and the H+, K+, ATPase23. Molecular weight and solubility indicate that it is unlikely that PGG can penetrate the cell membrane and this is confirmed by the saturable nature of the transport of PGG in Caco-2 monolayers22. As a result it seems unlikely that the in vivo effects of PGG are mediated by inhibition of DNA polymerase. This notion is confirmed by studies of PGG on a wide variety of cell lines, including our data on HMVEC, none of which show inhibition of proliferation with IC50’s in the nanomolar range. It is possible that polymerase-inhibitory intracellular PGG concentrations are achieved by much higher extracellular concentrations. However, in endothelial cells we observe the cytostatic effects of PGG (which might be mediated by polymerase inhibition) at concentrations at least an order of magnitude above the concentrations at which inhibition of migration is observed. Thus, in this cell type antimigratory effects which can be mediated by CMG2 are observed at much lower concentrations than antiproliferative effects that could be mediated by DNA polymerase. Nevertheless it remains possible that endothelial cells are substantially more permeable to PGG in vivo than ex vivo. In that case, DNA polymerase activity may also play a significant role in the antiangiogegnic activity of PGG. It is also possible that the antiangiogenic effects that we observed in vivo are mediated by the H+, K+, ATPase; however, there are no examples in the literature of angiogenesis modulation by the H+, K+, ATPase. In addition, the H+, K+, ATPase inhibitor omeprazole fails to inhibit angiogenesis at therapeutic concentrations24.
In contrast, our results demonstrate that PGG has a submicromolar IC50 in the in vitro FRET binding assay as well as in the ex vivo endothelial cell migration assay. In addition, both oral and IP administration results in inhibition of angiogenesis in the corneal micropocket assay. Together, these results demonstrate that PGG interacts directly with CMG2 and is a potent inhibitor of angiogenesis. Combined with our observation that an anthrax protective antigen and related CMG2 antagonist can inhibit endothelial cell migration ex vivo and angiogenesis in vivo, it seems likely that at least a portion of the antiangiogenic effects that we and others have observed in vivo are a result of the CMG2 inhibitory activity.
EXPERIMENTAL SECTION
General Methods
All NMR experiments were carried out on a Varian INOVA 600 MHz spectrometer. Digallic acid and PGG (1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose) were purified from a “tannic acid” sample (Sigma Aldrich, catalog number 403040-100g, lot #: 06817CJ) on an Agilent 1100 series HPLC (Agilent Technologies) using a semi-preparative Phenomenex Luna Phenyl-hexyl column (25 cm × 10 mm, 5 μm particle size) with a flow-rate of 10 mL/min (solvent A: H2O with 0.1% formic acid; solvent B: acetonitrile with 0.1% formic acid). A second tannic acid sample from the same supplier (Sigma Aldrich, catalog number 403040-50G, lot# MKBC5527) produced a much simpler chromatogram suggesting higher purity. Commercially purchased PGG (Toronto Research Chemicals, Supplier’s ID D270450 and AvaChem Scientific, San Antonio, TX, lot #: 100926) was characterized by NMR and LC-MS together with UV, and found to be authentic and >95% pure (Figure S1).
Purification of digallic acid and PGG
The “tannic acid” sample was dissolved in methanol as a 10 mg/mL solution, and filtered for HPLC study. A 1 mL solution was injected into a Phenyl-hexyl column, and the column was eluted with aqueous acetonitrile (10–100% in 30 min, and 100% for 10 min). Forty fractions were collected from 10 to 30 min, 1 fraction per 30 sec. All the forty fractions were tested in the FRET assay and only fraction SC1-129-18 (tR 18.5 min) and SC1-129-26 (tR 22.5 min) were active. The HPLC purification was repeated four times to obtain enough SC1-129-18 (1 mg) and SC1-129-26 (1.3 mg) for structure elucidation. SC1-129-18 and SC1-129-26 were identified as digallic acid and PGG, respectively, by comparison of the spectroscopic data with a commercially purchased digallic acid (Supplier’s ID D445920) and authentic PGG (Supplier’s ID D270450) from Toronto Research Chemicals (See Figures S1 and S2). PGG identification was further achieved by the comparison of the spectroscopic data obtained with those in the literature25, 26.
FRET assay
The CMG2-Protective antigen (PA) FRET assay was performed as described previously19. Briefly, site directed mutagenesis was used to generate plasmids coding for full length PA, with an E733C mutation (PAE733C) and CMG2 residues 40 –217 with C175A and R40C mutations (CMG2R40C C178A). These proteins were produced in E. coli and purified as described previously, using combinations of ion exchange (HP Q-Sepharose; GE Healthcare), affinity (GST Bind Agarose; Novagen), and size exclusion chromatography (Sephacryl 200HR; GE Healthcare)27. PA was labeled with Alexa fluor 488 C5 maleimide and CMG2 was labeled with Alexa fluor 546 C5 maleimide (Invitrogen). For compound characterization, serial dilutions of compound were prepared in a final volume of 10 μl HBST (50 mM HEPES pH 7.4, 150 mM NaCl, 0.1 mM CaCl2, 0.1% Tween-20) in wells of a 384-well black polypropylene plate (Corning). Following compound plate preparation, 20 μl of a solution of 26 nM CMG2R40C C178A*AF546 in HBST was added using a WellMate liquid handling robot (Matrix Technologies) with integrated stacker. Next, 10 μl of a 30 nM PAE733C*AF488 solution in HBS (50 mM HEPES pH 7.4, 150 mM NaCl, 0.1 mM CaCl2) was then added to all wells using the Wellmate and plates were incubated for 3–4 hours. Final CMG2 concentration (13 nM) and PA concentration (7.5 nM) were sufficient to promote quantitative binding of CMG2 in the absence of effective inhibitors, based on the previously reported Kd (≥ 170 pM)27. Following incubation, plates were read on an Envision (PerkinElmer) plate reader using a 485/14 excitation filter, with 535/25 and 595/60 emission filters. For each plate, positive controls were generated by including 16 wells containing 10mM EDTA in HBST and negative controls by including 16 wells containing 10 mM NaCl in HBST.
Mouse corneal micropocket assay
The corneal micropocket assay was performed as described28 using pellets containing 80 ng of bFGF (Peprotech, Rocky Hill, NJ) in C57BL/6J mice. Groups of five mice each received 20 mg/kg PGG or carrier by daily oral gavage in 5% ethanol. Alternatively, PGG (AvaChem) was delivered intraperitoneally at 10 mg/kg/day in a vehicle consisting of 2.5% (v/v) DMSO, 5% (v/v) Cremaphor EL, 5% (v/v) Tween-80, 7.5% (v/v) N-methyl-2-pyrrolidone, and 0.9% (w/v) NaCl, with controls receiving vehicle alone. Treatment was started on the day of pellet implantation. The area of vascular response was assessed on the fifth postoperative day using a slit lamp. Typically 10 eyes per group were measured.
Proliferation assay
Human microvascular endothelial cells (Lonza, Walkersville, MD) were maintained in EGM-2 (Lonza, Walkersville, MD) according to the vendor’s instructions and used before passage 7. On day 0, HMVEC-d cells were seeded into 96-well plates, at a concentration of 2000 cells per well. After attachment, media was exchanged for media containing the indicated treatment. Cells were allowed to grow for 1 or 3 days and then quantified using Cyquant (Invitrogen, Carlsbad, CA) according to manufacturer’s directions. The degree of proliferation in culture was measured by comparing wells in each plate fixed in absolute ethanol on day 0 to experimental wells, with fold-proliferation calculated by dividing Cyquant fluorescence readout in experimental wells by that in day 0 wells. Groups were compared using Student’s t-test, with Bonferroni correction where appropriate.
Migration assay
Human microvascular endothelial cells were maintained as above. Polycarbonate transwell inserts, 6.5 mm diameter with 8.0 μm pores, were coated with fibronectin (BD BioScience, San Jose, CA). Cells were harvested and resuspended in EBM (Lonza, Rockland, ME) containing 0.1% BSA (Sigma Aldrich, St Louis, MO). 20,000 cells/well were plated onto the inserts and these were placed into wells of a 24-well plate containing full serum media alone, or media containing the molecule to be tested. Cells were allowed to migrate for 4 hours. Cells on the top of the membrane were removed using cotton-tipped applicators and membranes were fixed and processed using Diff-Quick (Dade diagnostics, Aguada, PR). Membranes were removed from the insert using a scalpel and mounted on slides, and the number of cells in each of 4 10X microscopic fields were counted using a Leica Galen III stereomicroscope.
Supplementary Material
Chromatographic, and spectroscopic characterization of PGG from various sources (A–C) 1H NMR characterization (A) fraction SC-129-26, identified as Penta-O-galloyl-β-D-glucopyranose (PGG), (B) PGG from Toronto Research Chemicals, (C) PGG from AvaChem. Note the similarity of the 1H NMR spectra of these three PGGs: there are five aromatic singlets near 7 ppm, two protons per singlet, from five galloyl moieties; and seven protons between 4 to 6.4 ppm from the sugar moiety (H-6, H-5, H-2, H-4, H-3, and H-1, respectively), with a coupling pattern that indicates a pyranoglucose. (D–F) LC characterization using a Phenomenex Luna C18, 100×4.6 mm column with gradient from 10%–100% acetonitrile (0.1% formic acid) in 20 minutes at a flow rate of 0.7 ml/min. (D) SC-129-26, (E) TRC PGG, (F) AvaChem PGG. (G–I) UV/vis characterization (G) SC-129-26, (H) TRC PGG, (I) AvaChem PGG, (J–L) Mass spectrum of relevant chromatographic peak (MW-H: 939 [M-H]-1) (J) SC-129-26, (K) TRC PGG, (L) AvaChem PGG.
NMR spectrum of SC-129-18 purified from “tannic acid”, identified as digallic acid.
Figure 2.
Effect of digallic acid and PGG in the CMG2 FRET assay. Serial dilutions of pure samples of digallic acid (A) and PGG (B) were included in the CMG2 FRET assay. Curves represent best fit binding isotherms. Error bars indicate standard deviation of mean from at least four independent replicates. Structures of the compounds are indicated on the right.
Figure 4.
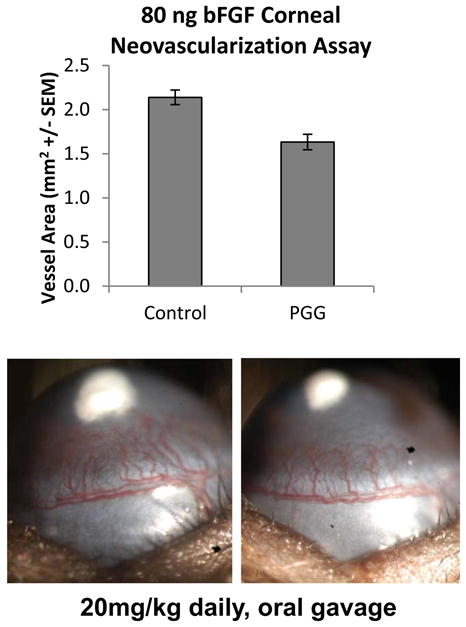
Effect of PGG treatment on corneal angiogenesis in vivo. (Top) Vessel area in response to an 80 ng pellet of bFGF following daily oral treatment with 20 mg/kg PGG or vehicle (5% ethanol) control. The observed difference is statistically significant (P<0.001 by t-test, n=10 eyes for control and 9 eyes for PGG). (Bottom) Picture of a representative eye for each group showing difference in vessel area elicited in control and PGG treated animals.
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health, the Department of Defense, and the Susan G. Komen Foundation.
The author’s thank the ICCB-Longwood screening facility for access to their libraries, equipment, screening supplies, and technical advice. This work was supported by the National Institutes of Health 1R01EY018829-01 (M.S.R.), the Department of Defense W81XWH-08-1-0710 (M.S.R), W81XWH-08-1-0711 (J.C.) and the Susan G. Komen Foundation KG101356 (L.M.C fellowship).
NON-STANDARD ABBREVIATIONS USED
- CMG2
Capillary Morphogenesis Gene 2
- ANTXR2
anthrax toxin receptor 2
- EGM2
Endothelial growth medium 2
- bFGF
basic fibroblast growth factor, FGF2
- HMVEC-d
human dermal microvascular endothelial cells
- PTP1B
protein-tyrosine phosphatase 1B
- PA
protective antigen
- PASSSR
a protective antigen mutant in which 3 amino acids at the furin cleavage site are mutated to serine
- PGG
1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose, pentagalloyl glucose, 5GG
- vWF
von Willebrand factor
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Figures S1 and S2, NMR spectra of purified compounds and characterization by NMR and LC-MS together with UV of purchased PGG. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3:a005090. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 4.Lacy DB, Wigelsworth DJ, Melnyk RA, Harrison SC, Collier RJ. Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc Natl Acad Sci U S A. 2004;101:13147–13151. doi: 10.1073/pnas.0405405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacy DB, Wigelsworth DJ, Scobie HM, Young JA, Collier RJ. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2004;101:6367–6372. doi: 10.1073/pnas.0401506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santelli E, Bankston LA, Leppla SH, Liddington RC. Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature. 2004;430:905–908. [Google Scholar]
- 7.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 8.Cryan LM, Rogers MS. Targeting the anthrax receptors, TEM-8 and CMG-2, for anti-angiogenic therapy. Front Biosci. 2011;16:1574–1588. doi: 10.2741/3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deuquet J, Lausch E, Superti-Furga A, van der Goot FG. The dark sides of capillary morphogenesis gene 2. EMBO J. 2012;31:3–13. doi: 10.1038/emboj.2011.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves CV, Dufraine J, Young JA, Kitajewski J. Anthrax toxin receptor 2 is expressed in murine and tumor vasculature and functions in endothelial proliferation and morphogenesis. Oncogene. 2010;29:789–801. doi: 10.1038/onc.2009.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers MS, Christensen KA, Birsner AE, Short SM, Wigelsworth DJ, Collier RJ, D’Amato RJ. Mutant anthrax toxin B moiety (protective antigen) inhibits angiogenesis and tumor growth. Cancer Res. 2007;67:9980–9985. doi: 10.1158/0008-5472.CAN-07-0829. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Li L, Kim SH, Hagerman AE, Lu J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res. 2009;26:2066–2080. doi: 10.1007/s11095-009-9932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HJ, Seo NJ, Jeong SJ, Park Y, Jung DB, Koh W, Lee EO, Ahn KS, Lu J, Kim SH. Oral administration of Penta-O-galloyl-{beta}-D-glucose Suppresses Triple-negative Breast Cancer Xenograft Growth and Metastasis in Strong Association with JAK1-STAT3 Inhibition. Carcinogenesis. 2011;32:804–811. doi: 10.1093/carcin/bgr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiss A, Becsi B, Kolozsvari B, Komaromi I, Kover KE, Erdodi F. Epigallocatechin-3-gallate and penta-O-galloyl-beta-d-glucose inhibit protein phosphatase-1. FEBS J. doi: 10.1111/j.1742–4658.2012.08498.x. [Online early access] Published Online: February 13: 2012. [DOI] [PubMed] [Google Scholar]
- 15.Huh JE, Lee EO, Kim MS, Kang KS, Kim CH, Cha BC, Surh YJ, Kim SH. Penta-O-galloyl-beta-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis. 2005;26:1436–1445. doi: 10.1093/carcin/bgi097. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Lee HM, Ji ST, Lee SR, Mar W, Gho YS. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose blocks endothelial cell growth and tube formation through inhibition of VEGF binding to VEGF receptor. Cancer Lett. 2004;208:89–94. doi: 10.1016/j.canlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Mizushina Y, Zhang J, Pugliese A, Kim SH, Lu J. Anti-cancer gallotannin penta-O-galloyl-beta-D-glucose is a nanomolar inhibitor of select mammalian DNA polymerases. Biochem Pharmacol. 2010;80:1125–1132. doi: 10.1016/j.bcp.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Shaik AA, Zhang J, Nhkata K, Wang L, Zhang Y, Xing C, Kim SH, Lu J. Preparation of penta-O-galloyl-beta-D-glucose from tannic acid and plasma pharmacokinetic analyses by liquid-liquid extraction and reverse-phase HPLC. J Pharm Biomed Anal. 2011;54:545–550. doi: 10.1016/j.jpba.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers MS, Cryan LM, Habeshian KA, Bazinet L, Caldwell TP, Ackroyd PC, Christensen KA. A FRET-based high throughput screening assay to identify inhibitors of anthrax protective antigen binding to capillary morphogenesis gene 2 protein. PLoS One. 2012;7:e39911. doi: 10.1371/journal.pone.0039911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao S, Cryan L, Habeshian KA, Murillo C, Tamayo-Castillo G, Rogers MS, Clardy J. Phenolic compounds as antiangiogenic CMG2 inhibitors from costa rican endophytic fungi. Bioorg Med Chem Lett. 2012;22:5885–5888. doi: 10.1016/j.bmcl.2012.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Kim J, Li J, Liu F, Liu X, Himmeldirk K, Ren Y, Wagner TE, Chen X. Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem Biophys Res Commun. 2005;336:430–437. doi: 10.1016/j.bbrc.2005.08.103. [DOI] [PubMed] [Google Scholar]
- 22.Cai K, Hagerman AE, Minto RE, Bennick A. Decreased polyphenol transport across cultured intestinal cells by a salivary proline-rich protein. Biochem Pharmacol. 2006;71:1570–1580. doi: 10.1016/j.bcp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Ono K, Sawada T, Murata Y, Saito E, Iwasaki A, Arakawa Y, Kurokawa K, Hashimoto Y. Pentagalloylglucose, an antisecretory component of Paeoniae radix, inhibits gastric H+, K(+)-ATPase. Clin Chim Acta. 2000;290:159–167. doi: 10.1016/s0009-8981(99)00184-9. [DOI] [PubMed] [Google Scholar]
- 24.Sartippour MR, De Leon E, Rubio R, Brooks MN. The effect of commonly used drugs on angiogenesis. Anticancer Res. 2003;23:231–234. [PubMed] [Google Scholar]
- 25.Haddock EA, Gupta RK, Haslam E. The metabolism of gallic acid and hexahydroxydiphenic acid in plants. Part 3. Esters of (R)- and (S)-hexahydroxydiphenic acid and dehydrohexahydroxydiphenic acid with D-glucopyranose (1C4 and related conformations) J Chemical Soc Perkin Trans. 1982;1:2535–2545. [Google Scholar]
- 26.Beretta G, Artali R, Caneva E, Maffei Facino R. Conformation of the tridimensional structure of 1,2,3,4,6-pentagalloyl-beta-D-glucopyranose (PGG) by (1)H NMR, NOESY and theoretical study and membrane interaction in a simulated phospholipid bilayer: a first insight. Magn Reson Chem. 2011;49:132–136. doi: 10.1002/mrc.2718. [DOI] [PubMed] [Google Scholar]
- 27.Wigelsworth DJ, Krantz BA, Christensen KA, Lacy DB, Juris SJ, Collier RJ. Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J Biol Chem. 2004;279:23349–23356. doi: 10.1074/jbc.M401292200. [DOI] [PubMed] [Google Scholar]
- 28.Rogers MS, Birsner AE, D’Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2:2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chromatographic, and spectroscopic characterization of PGG from various sources (A–C) 1H NMR characterization (A) fraction SC-129-26, identified as Penta-O-galloyl-β-D-glucopyranose (PGG), (B) PGG from Toronto Research Chemicals, (C) PGG from AvaChem. Note the similarity of the 1H NMR spectra of these three PGGs: there are five aromatic singlets near 7 ppm, two protons per singlet, from five galloyl moieties; and seven protons between 4 to 6.4 ppm from the sugar moiety (H-6, H-5, H-2, H-4, H-3, and H-1, respectively), with a coupling pattern that indicates a pyranoglucose. (D–F) LC characterization using a Phenomenex Luna C18, 100×4.6 mm column with gradient from 10%–100% acetonitrile (0.1% formic acid) in 20 minutes at a flow rate of 0.7 ml/min. (D) SC-129-26, (E) TRC PGG, (F) AvaChem PGG. (G–I) UV/vis characterization (G) SC-129-26, (H) TRC PGG, (I) AvaChem PGG, (J–L) Mass spectrum of relevant chromatographic peak (MW-H: 939 [M-H]-1) (J) SC-129-26, (K) TRC PGG, (L) AvaChem PGG.
NMR spectrum of SC-129-18 purified from “tannic acid”, identified as digallic acid.