Abstract
Objective
HIV-positive pregnant women are at heightened risk of becoming lost to follow-up (LTFU) from HIV care. We examined LTFU before and after delivery among pregnant women newly-diagnosed with HIV.
Methods
Observational cohort study of all pregnant women ≥18 years (N=300) testing HIV-positive for the first time at their first ANC visit between January–June 2010, at a primary healthcare clinic in Johannesburg, South Africa. Women (n=27) whose delivery date could not be determined were excluded.
Results
Median (IQR) gestation at HIV testing was 26 weeks (21–30). 98.0% received AZT prophylaxis, usually started at the first ANC visit. Of 139 (51.3%) patients who were ART-eligible, 66.9% (95%CI 58.8–74.3%) initiated ART prior to delivery; median (IQR) ART duration pre-delivery was 9.5 weeks (5.1–14.2). Among ART-eligible patients, 40.5% (32.3–49.0%) were cumulatively retained through six months on ART. Of those ART-ineligible at HIV testing, only 22.6% (95%CI 15.9–30.6%) completed CD4 staging and returned for a repeat CD4 test after delivery. LTFU (≥1 month late for last scheduled visit) before delivery was 20.5% (95%CI 16.0–25.6%) and, among those still in care, 47.9% (95%CI 41.2–54.6%) within six months after delivery. Overall, 57.5% (95%CI 51.6–63.3%) were lost between HIV testing and six months post-delivery.
Conclusions
Our findings highlight the challenge of continuity of care among HIV-positive pregnant women attending antenatal services, particularly those ineligible for ART.
Keywords: HIV/AIDS, pregnant, antenatal, loss to follow-up, retention, South Africa
Introduction
South Africa has a national antenatal HIV prevalence of 30.2% (Republic of South Africa Department of Health 2010a) and more people living with HIV than any other country in the world (UNAIDS 2010). In an ongoing effort to improve care for pregnant women with HIV and to prevent mother-to-child transmission (PMTCT), South Africa’s 2010 HIV treatment guidelines called for lifelong ART to be initiated for all pregnant women with a CD4 value ≤350 cells/µl (Republic of South Africa Department of Health 2010b) and PMTCT guidelines mandated AZT prophylaxis from 14 weeks of pregnancy (Republic of South Africa Department of Health 2010c). However, implementation of these guidelines remains inadequate, with pregnant women in South Africa commonly presenting for their first ANC visit well into their second trimester or later, delaying HIV diagnosis, AZT prophylaxis and lifelong ART initiation (Black et al. 2008; Hoffman et al. 2010; Stinson et al. 2010).
In addition to late presentation for ANC services, some studies suggest that pregnant women have poorer retention in HIV care than men and non-pregnant women (Kaplan et al. 2008; Wang et al. 2011). Retention in HIV care is paramount, as HIV-positive patients require routine management and daily adherence to ART once initiated (Republic of South Africa Department of Health 2010d); mortality is high among patients who drop out of ART programs (Brinkhof et al. 2009; Fox et al. 2010), and HIV/AIDS contributes to nearly half (43.7%) of maternal deaths in South Africa, far overshadowing deaths due to other obstetric complications such as hemorrhage or sepsis (National Committee on Confidential Enquiries into Maternal Deaths 2008). An analysis of nearly 30,000 women initiating antiretroviral therapy (ART) in South Africa found loss was 54% higher in pregnant than in non-pregnant women (aHR 1.54; 95%CI 1.38–1.72), despite lower mortality (Myer et al. 2012a). The reasons for these differences are still being identified, and may include lower rates of immunosuppression and a lack of perception of need for treatment (Wang et al. 2011; Solarin & Black 2012), heavier financial burden (Wang et al. 2011), travel or relocation during pregnancy (Hoffman et al. 2010; Wang et al. 2011; Ferguson et al. 2012a), and stigma (Hoffman et al. 2010; Ferguson et al. 2012b; Thorsen et al. 2008). In South Africa, antenatal coverage is widespread and nearly all births occur in healthcare facilities (Shisana et al. 2009), but there is still great difficulty in ensuring an unbroken continuum of HIV care between antenatal care at a primary health clinic (PHC), labor and delivery at a hospital, and postnatal care and ongoing HIV care returning at the PHC.
To date, investigations of retention in HIV care among pregnant women have provided an estimate of loss to follow-up between specific milestones, such as HIV testing to ART initiation (Stinson et al. 2010; Kaplan et al. 2008; Ferguson et al. 2012a), or ART initiation to between 6 months and 3 years on treatment (Kaplan et al. 2008; Wang et al. 2011; Myer et al. 2012a), without evaluating the impact of delivery on loss. While studies from the US demonstrate ART adherence often declines in the postpartum period compared to during pregnancy (Mellins et al. 2008; Ickovics et al. 2002; Tedaldi et al. 2002; Turner et al. 2000), few studies have documented postpartum attendance in HIV care in sub-Saharan Africa in routine clinic settings (Nassali et al. 2009). We hypothesize loss to follow-up may differ before and after delivery given that women’s motivations for seeking care may change during these time periods. This analysis of data from a cohort of newly-diagnosed HIV-positive pregnant women examines loss to follow-up through early stages of HIV care to better understand how loss to follow-up is influenced by delivery.
Methods
This study was conducted at Witkoppen Health and Welfare Centre (“Witkoppen”), a busy facility (8500 clinic visits per month) providing primary healthcare services to formal and informal settlements in northern Johannesburg, South Africa. Between January and June 2010, 300 pregnant women (≥18 years) tested HIV-positive for the first time at their first ANC visit at Witkoppen. We conducted a retrospective cohort study through file review to assess attrition from pre-ART and on-ART care up to a minimum of 12 months after testing HIV-positive.
HIV Care
At Witkoppen, HIV care is integrated with antenatal care for pregnant women, with assessment for and initiation of ART occurring within ANC, and all documentation contained within a single clinic file per patient. In early 2010, Witkoppen began initiating all adult patients with CD4 ≤350 cells/µl onto ART, one year prior to national guidelines calling for this approach. We confirmed ART eligibility at the time of HIV testing for each subject through file review. During the time when the study was conducted, CD4 testing was initiated at the same facility immediately following an HIV-positive test result, with a visit scheduled two weeks later to receive the CD4 results. For ART eligible patients, an ART initiation visit was scheduled two to four weeks after receiving the CD4 results. Patients who wish to transfer facilities receive a referral letter and the file is noted accordingly.
Antenatal and Postnatal Care
Witkoppen offers antenatal and postnatal care, HIV testing and treatment on-site, as well as other primary healthcare and social welfare services. HIV testing at Witkoppen occurs during a woman’s first ANC visit and all HIV-positive pregnant women are given twice-daily zidovudine (AZT) starting at 14 weeks gestation, as called for in PMTCT guidelines (Republic of South Africa Department of Health 2010c). All women who receive antenatal care at Witkoppen are designated to deliver at Hillbrow Hospital in central Johannesburg (32.5 km from Witkoppen) or, if considered high-risk, at Charlotte Maxeke Johannesburg Academic Hospital (28.9 km). At the hospital, women not yet on lifelong ART are given intrapartum single-dose nevirapine and three-hourly AZT, as well as postpartum single-dose tenofovir with emtricitabine for PMTCT. Newborns also start a six-week course of nevirapine syrup from birth (Republic of South Africa Department of Health 2010c). The hospitals do not routinely report delivery information back to Witkoppen. After delivery, ANC patients return for postnatal care and HIV treatment services at Witkoppen for ten weeks and then resume general adult care, all within the same facility and using the same clinic file. Mothers and infants make three postnatal care visits together: 3–7 days after delivery; six weeks after delivery for an infant polymerase chain reaction (PCR) HIV test; and 10 weeks after delivery for infant HIV PCR results. Women previously ineligible for ART should have a repeat CD4 at the six-week postpartum visit.
Timing of delivery
Data on gestational age at HIV testing based on last menstrual period were available in most (88.3%) patient files, while date of delivery was available in patient files for 39.7% of women. Thus, we determined a delivery date for 91% of women using either the actual date or an estimation assuming 40 weeks gestation. Women with no calculated delivery date (n=27, 9.0%) were excluded, leaving an analytic sample size of 273.
Statistical Methods
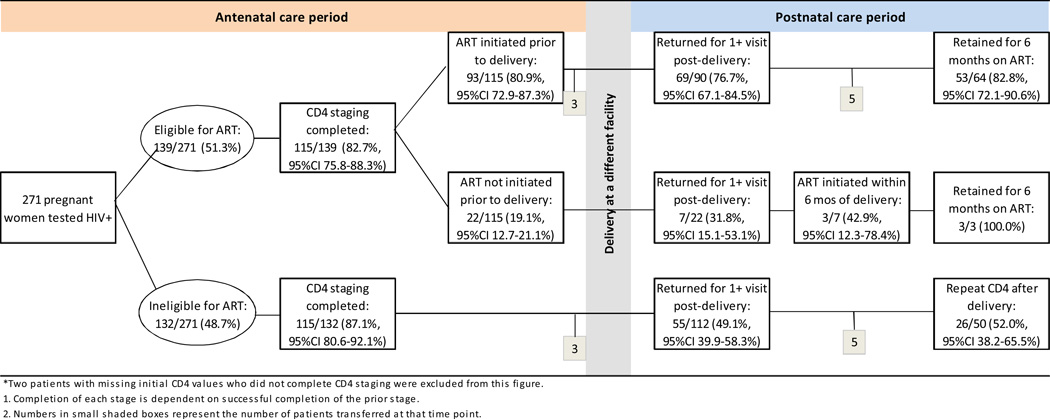
Patient characteristics are described using proportions and 95% confidence intervals (95%CI) for categorical variables, and medians and interquartile ranges (IQR) for continuous variables. We examine retention among pregnant women in two ways. First, we report retention through several stages of pre- and post-ART care within the antenatal and postnatal periods using counts and proportions with 95%CI. The first stage for all patients is completing CD4 staging, including results notification, prior to delivery. For the ART-eligible, subsequent stages include ART initiation before or after delivery, completing a clinic visit after delivery, and six months retention on ART. For ART-ineligible patients, subsequent stages include returning for a clinic visit after delivery and receiving a repeat CD4 test after delivery. A diagram of these stages is presented in Figure 1. Retention is defined as completing each stage at Witkoppen and is determined using clinic visit data. Patients who transfer are noted and removed from the analysis at the start of the stage in which they transferred. Cumulative retention analyses exclude patients who transferred during the antenatal (n=6) and postnatal (n=10) periods.
Figure 1.
Patient retention before and after delivery among 271* recently-diagnosed HIV-positive ANC patients
Second, we use time-to-event analysis to assess loss to follow-up before and after delivery by estimating person-time in the antenatal and postnatal periods. Loss to follow-up is defined as not returning to the clinic within a minimum of one month after the last scheduled visit. For the antenatal period, person-time began accruing at testing HIV-positive and ended at the first of the following three events: (1) delivery, (2) loss to follow-up or (3) transfer to another facility prior to delivery. For women remaining in care at the beginning of the postnatal period, person-time began accruing at delivery and ended at the earliest of: (1) six months after delivery; (2) loss to follow-up; or (3) transfer after delivery. No deaths were reported during the antenatal and postnatal time periods studied, so these represent all possible known outcomes. After confirming proportional hazards using Schoenfeld residuals, we examined predictors of loss within the antenatal and postnatal periods using Cox proportional hazard regression, adjusting for covariates based on prior knowledge and change-in-estimate evaluation, and report adjusted hazard ratios (aHR) and 95%CI.
Ethical approval for this study was granted by the Human Research Ethics Committee of the University of the Witwatersrand and exemption was given by the Public Health-Nursing IRB at the Office of Human Research Ethics at the University of North Carolina at Chapel Hill.
Results
Clinical and demographic characteristics at HIV testing of the 273 pregnant women are summarized in Table 1. The median (IQR) age at the first ANC visit was 27 years (24–31) and the median CD4 count at the time of HIV testing was 357 cells/µl (238–500). Overall, almost half of the cohort (44.7%) was born outside of South Africa, most notably in Zimbabwe (89.3% of those foreign-born).
Table 1.
Characteristics of the study participants (N=273)
| Patient characteristic | |
|---|---|
| Continuous variables, median (IQR) | |
| Age at HIV testing | 27 (24–31) |
| First CD4 value (cells/µl) | 357 (238–500) |
| Weeks of gestation at first ANC visit | 26 (21–30) |
| Categorical variables, n (%) | |
| Age at HIV testing | |
| 18–24 years | 78 (28.6%) |
| 25–29 years | 98 (35.9%) |
| 30 years and older | 97 (35.5%) |
| First CD4 value | |
| <50 cells/µl | 4 (1.5%) |
| 50–199 cells/µl | 48 (17.6%) |
| 200–350 cells/µl | 83 (30.4%) |
| >350 cells/µl | 136 (49.8%) |
| Missing | 2 (0.7%) |
| Nationality | |
| Born in South Africa | 143 (52.4%) |
| Born outside of South Africa | 122 (44.7%) |
| Missing | 8 (2.9%) |
| Employment status | |
| Employed | 97 (35.5%) |
| Not employed | 167 (61.2%) |
| Missing | 9 (3.3%) |
| Gestation at first ANC visit | |
| ≤20 weeks | 59 (21.6%) |
| >20 weeks | 214 (78.4%) |
IQR, interquartile range
The median (IQR) gestational age at first ANC visit was 26 weeks (21–30) and the median (IQR) time from HIV testing to delivery was 3.1 months (2.1–4.1). AZT monotherapy as part of PMTCT was almost universally implemented. During pregnancy 98.0% received at least one 30-day supply of AZT, all but one of whom (99.7%) started AZT on the day of testing HIV-positive.
Retention during antenatal care
Figure 1 shows retention of pregnant women through early HIV care, contingent on completing earlier stages. Roughly half of all women were ART-eligible at the time of testing; median (IQR) CD4 at HIV testing was 244 cells/µl (171–299) among ART-eligible and 500 cells/µl (420–599) among ART-ineligible women. Completing CD4 staging prior to delivery was high at 84.9% (95%CI: 80.2–88.8%) overall, and varied little by ART eligibility (87.1% vs. 82.7%). Of those who were ART-eligible and who completed CD4 staging prior to delivery (n=115), most (80.9%; 95%CI: 72.9–87.3%) went on to initiate lifelong ART prior to delivery. These women spent a median (IQR) of 27 days (17–41.5) from HIV testing to ART initiation and a median (IQR) 9.5 weeks (5.1–14.2) on ART prior to delivery. Of the remaining 22 ART-eligible women who completed CD4 staging but did not initiate ART prior to delivery, most (n=18, 81.8%) never returned for ART initiation, one returned but left before initiation, one required further counseling, one refused ART, and one completed the initiation visit but was rescheduled to return for initiation a week later and did not return. Of all 139 ART-eligible women, 66.9% (95%CI: 58.8–74.3%) initiated treatment prior to delivery.
Retention after delivery
The proportion of patients returning for at least one visit after delivery was highest among those who were eligible for ART and initiated treatment prior to delivery (n=90) at 76.7% (95%CI: 67.1–84.5%) with most of these women (82.8%; 95%CI: 72.1–90.6%) retained in ART care for at least six months. Though the numbers of women who completed CD4 staging and were eligible for but did not initiate ART were small (n=22), only 31.8% (95%CI: 15.1–53.1%) returned at any point after delivery, and less than half of those who did (42.9%; 95%CI: 12.3–78.4%) initiated ART within six months of delivery. The picture was much different for those ineligible for ART at the time of testing positive. Among those ART-ineligible who completed CD4 staging (n=112), less than half (49.1%, 95%CI: 39.9–58.3%) returned for any visits after delivery. Of these 50 women, only half (52.0%, 95%CI: 38.2–65.5%) received a repeat CD4 count after delivery.
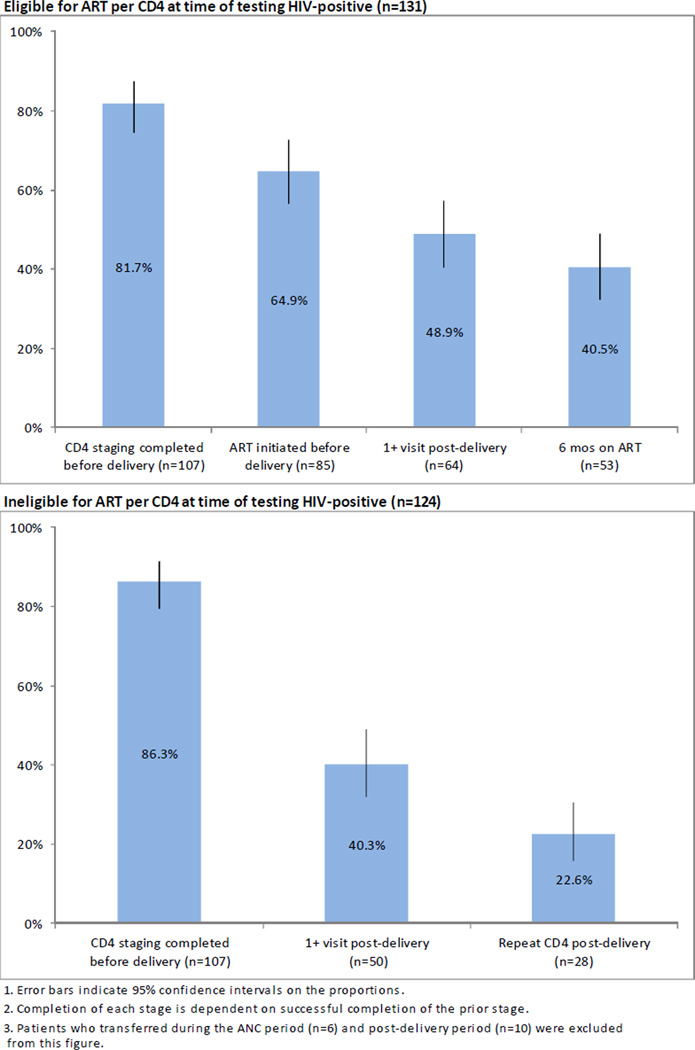
Cumulative retention in HIV care
Figure 2 shows cumulative retention for ART-eligible women from HIV testing through delivery and six months on ART as well as for ART-ineligible women through a post-delivery repeat CD4 count. Cumulatively, less than half (40.5%, 95%CI: 32.3–49.0%) of ART-eligible patients who tested HIV-positive during pregnancy completed CD4 staging, initiated ART prior to delivery, returned after delivery and completed six months on ART. Cumulative retention was even lower among those ineligible for ART at the time of HIV testing: only 22.6% (95%CI: 15.9–30.6%) of patients ineligible for ART continued in care to the point of a repeat CD4 count after delivery.
Figure 2.
Cumulative patient retention from HIV diagnosis through six months on ART for 131 HIV-positive ANC patients eligible for ART, and through repeat CD4 testing after delivery for 123 HIV-positive ANC patients ineligible for ART
The median (IQR) person-time contributed in the antenatal period was 2.8 months (1.7–3.9) and 4.5 months (1.5–6.0) in the postnatal period. Using time-to-event analysis and analyzing all women regardless of ART eligibility at the time of testing, 20.5% (95%CI: 16.0–25.6%) of women were lost to follow-up prior to delivery. Among women in care at delivery, 47.9% (95%CI: 41.2–54.6%) were lost within six months after delivery, for a cumulative loss to follow-up of 57.5% (95%CI: 51.6–63.3%).
Table 2 presents the results of multivariate analysis of factors associated with loss within the antenatal and postnatal time periods. Presenting late for the first ANC visit (after 20 weeks gestation) was associated with twice the likelihood of loss prior to delivery after adjusting for age, initial CD4 count and nationality (aHR 2.00; 95%CI 1.00, 4.02) but late presentation was not associated with loss during the postnatal period (aHR 1.09; 95%CI 0.65–1.83). ART ineligibility at the time of HIV testing based on a CD4 count >350 cells/µl was strongly associated with loss to follow-up following delivery (aHR 3.30; 95%CI: 1.95–5.58), while pregnant women age 30 years and older were less likely to be lost after delivery (aHR 0.49; 95%CI: 0.30–0.81). Our results suggest that women born outside of South Africa may be more likely to be lost after delivery (aHR 1.36; 95%CI: 0.90–2.06) than women born in South Africa.
Table 2.
Multivariate analysis of factors associated with loss to follow-up before and after delivery among 273 newly-diagnosed HIV-positive antenatal patients
| Antenatal care period (n=273) |
Postnatal care period (n=211) |
|||||
|---|---|---|---|---|---|---|
|
n (%)* |
Crude HR (95% CI) |
Adjusted HR (95% CI) |
n (%)* |
Crude HR (95% CI) |
Adjusted HR (95% CI) |
|
| Age at HIV testing | ||||||
| 18–24 years | 19 (24.4) | 1.26 (0.66, 2.40) | 1.25 (0.64, 2.42) | 31 (54.4) | 0.96 (0.61, 1.52) | 0.91 (0.57, 1.43) |
| 25–29 years | 18 (18.4) | 1 | 1 | 45 (57.7) | 1 | 1 |
| 30 years and older | 19 (19.6) | 0.94 (0.54, 1.63) | 1.08 (0.55, 2.11) | 25 (32.9) | 0.50 (0.31, 0.82) | 0.49 (0.30, 0.81) |
| First CD4 value | ||||||
| <200 cells/mm3 | 10 (19.2) | 1.12 (0.51, 2.47) | 1.24 (0.56, 2.78) | 16 (41.0) | 1.71 (0.87, 3.36) | 1.72 (0.87, 3.42) |
| 200–350 cells/mm3 | 16 (19.5) | 1 | 1 | 18 (27.3) | 1 | 1 |
| >350 cells/mm3 | 28 (20.4) | 0.95 (0.51, 1.75) | 1.03 (0.55, 1.94) | 67 (63.2) | 3.00 (1.78, 5.05) | 3.30 (1.95, 5.58) |
| Nationality | ||||||
| Born in South Africa | 31 (21.7) | 1 | 1 | 48 (44.9) | 1 | 1 |
| Born outside of South Africa | 25 (20.5) | 1.05 (0.62, 1.78) | 0.99 (0.57, 1.72) | 52 (54.2) | 1.33 (0.90, 1.97) | 1.36 (0.90, 2.06) |
| First ANC visit >20 weeks gestation | ||||||
| No | 14 (23.7) | 1 | 1 | 18 (40.9) | 1 | 1 |
| Yes | 42 (19.6) | 2.11 (1.07, 4.19) | 2.00 (1.00, 4.02) | 83 (49.7) | 1.29 (0.77, 2.14) | 1.09 (0.65, 1.83) |
HR, hazard ratio; CI, confidence interval
Loss to follow-up (LTFU) is defined as not returning to the clinic within ≥1 month after the last scheduled visit; antenatal care period is defined as HIV testing date to delivery date; postnatal care period is defined a s delivery date to 6 months after delivery.
Adjusted models are adjusted for all variables listed.
Proportion LTFU during the period for each category listed.
Discussion
Pregnant women diagnosed with HIV during antenatal care require effective care interventions to ensure the prompt initiation of PMTCT and ART, but also must be linked to HIV care that is sustainable beyond the point of delivery. We found that overall loss to follow-up within 13 months of HIV testing among pregnant women recently diagnosed with HIV was staggeringly high at 57.5% (95%CI: 51.6–63.3%). Unlike studies that begin reporting loss to follow-up at ART initiation, our findings span the period from testing HIV-positive through both the pre-ART and early post-ART periods, periods of high attrition. Patients who initiate ART have already successfully been retained through several visits, and thus, may be more inclined to continue in care. Few reports exist of retention of all patients – both ART eligible and ineligible. Our loss to follow-up proportion, which includes this early loss, is much higher than the 12%–32% among pregnant women after six months to three years on ART reported elsewhere which only focuses on the on-treatment period (Kaplan et al. 2008; Wang et al. 2011; Myer et al. 2012a).
Our analysis also suggests that the focus on cumulative loss to follow-up among pregnant women is missing important trends over time with implications for when to intervene. Loss to follow-up prior to delivery was much lower than after delivery (20.5% vs. 47.9%). The antenatal period is a time of reasonable compliance with HIV care, likely because women are already attending care for another reason for which they have motivation to continue. Our results suggest many women are not returning to the PHC after delivery. Among HIV-positive pregnant women, the challenge is ensuring HIV care extends beyond the period of pregnancy and continues for the lifetime of the mother. In South Africa, infants are to accompany their mothers to the first three postnatal care visits, so failure to attend these visits also suggests a failure to link infants to postnatal care, including PCR testing for HIV and immunizations.
In addition to a high rate of loss to follow-up, we also found that pregnant women presented for their first ANC visit and HIV testing well after the recommended gestational age of 14 weeks for AZT initiation. The median gestational age at HIV testing of 26 weeks in our study matched that found by Stinson et al. (2010), and represents delayed PMTCT efforts. Half of the pregnant patients in our cohort were ART-eligible at their first ANC visit, yet despite the overall trend of late presentation, 66.9% (95%CI: 58.8–74.3%) of those eligible initiated ART prior to delivery. The median time from first visit to ART initiation of 27 days indicates that patients are being initiated promptly after the first visit, but as the median time on ART is 9.5 weeks, they are not presenting early enough to obtain maximal effectiveness of ART for PMTCT, which is reached around 13–15 weeks duration prior to delivery (Chibwesha et al. 2011; Patel et al. 2007). The median duration on ART prior to delivery in our study is consistent with findings of 7.6–10.7 weeks in other studies in South Africa (Black et al. 2008; Hoffman et al. 2010; Stinson et al. 2010; Fitzgerald et al. 2010). Additionally, nearly one-third (31.1%, 95%CI 25.7–41.2%) of ART-eligible women should have initiated before delivery but did not. Recognition of the problem of late presentation to ANC care and its impact on delayed ART initiation, as well as poor linkage to care prior to ART, has spurred interest in rapid or same-day ART initiation among pregnant women using point-of-care CD4 testing (Myer et al. 2012b).
South African ANC patients receive a government-mandated ANC card at their first ANC visit, and must present it at the hospital labor ward prior to delivery. There has been speculation that women only attend one ANC visit to get this card but do not return for additional care. Our data do not support this idea. Among pregnant women in our cohort, 15.1% failed to complete CD4 notification (i.e. attended only one clinic visit), which is half the proportion (30.2%) of non-pregnant adults who did not complete CD4 staging during this same period (Clouse et al. 2013). Our finding of sufficient linkage to care among pregnant women is consistent with that of Kranzer et al. (2010), who found CD4 completion was highest among antenatal care patients in Cape Town when compared to patients of other clinic services. This suggests that many women are making repeat visits while pregnant but many then stop after delivery.
In our study women who presented late for their first ANC visit (>20 weeks gestation) were twice as likely to be lost prior to delivery (aHR 2.00, 95%CI 1.00–4.02), underscoring the difficulty of retaining patients who are initially slow to seek care. Three South Africa-based studies identified fear of HIV testing/diagnosis, confusion over pregnancy status, transport limitations, lack of perceived benefit, and clinic booking delays as common reasons for late presentation among ANC patients (Solarin & Black 2012; Myer & Harrison 2003; Laher et al. 2012), highlighting the multi-level barriers that can prevent timely access to and retention in care among pregnant women. Patients with higher CD4 counts (>350 cells/µl) who were ineligible for ART were more likely to be lost postpartum (aHR 3.30; 95%CI 1.95–5.58), emphasizing the importance of retention during pre-ART care. South Africa’s PMTCT guidelines call for repeat CD4 testing after delivery, but only 22.6% of ART-ineligible women received a repeat CD4 count. Witkoppen has a very high proportion of patients born outside of South Africa, as is now common in PHCs throughout Johannesburg (Faal et al. 2011; City of Johannesburg 2011). These patients may be at greater risk of loss to follow-up after delivery (aHR 1.36; 95%CI 0.90–2.06), likely because of frequent mobility among this group.
Our findings must be considered in light of the study’s limitations. First, we do not know why women ceased care at Witkoppen. Under half (45.2%) of the women who were lost received at least one follow-up phone call. We reviewed the files of 84 pregnant patients who were lost to follow-up whom the clinic had made at least one attempt to contact. Over half (52.3%) were unreachable because their cell phone was off or out of service and 20.2% were found to have moved out of the area, usually outside Johannesburg’s Gauteng Province. This may indicate that women return to rural homes (Hoffman et al. 2010; Wang et al. 2011; Ferguson et al. 2012a) – whether in South Africa or a neighboring country – either immediately prior to or after birth. While migration around delivery is typically short-term, it disrupts the continuity of care, which likely impairs adherence to ART. Further research is needed to better understand pregnancy-associated migration and its impact on maternal and infant health.
Second, we do not know if patients who drop out of care at Witkoppen continue in care at other facilities, perhaps in a rural facility or outside South Africa, except for those who request a formal transfer. While this is a limitation shared by most studies of loss to follow-up, it is important to note that loss to follow-up in this study reflects ceasing care at one clinic only, but one that was selected for study due to close integration of antenatal care, postnatal care and HIV services. We also cannot link to hospital data to confirm delivery, nor do we have data on infant clinic visits or outcomes. More research is needed into ways to improve linkages across treatment sites and ways to improve patient movement between sites (e.g. providing sufficient supply of antiretrovirals).
Finally, our study data, which were collected retrospectively, were limited to those routinely collected in the patient files, but with very few missing data. Our findings regarding timing of first ANC visit and linkage to care among ANC patients are consistent with previously reported studies from other regions of South Africa (Stinson et al. 2010; Kranzer et al. 2010), suggesting that our results are generalizable to other clinics throughout South Africa.
Further research that examines pregnant women’s reasons for dropping out of care, interventions to reduce ante- and postnatal attrition, and follow-up studies to confirm linkages to care among new mothers, is needed urgently. Case management interventions to improve linkages to HIV care have demonstrated success in the US (Craw et al. 2008; Gardner et al. 2005) and research on implementation of this type of intervention in the southern African setting is warranted.
Our study highlights much room for improvement in the provision of HIV care of pregnant women. We found that pregnant women presented late for their first ANC visit, initiated ART too late to achieve maximal PMTCT effectiveness, and had high rates of drop out around the time of delivery. Pregnant women likely have different motivation for attending healthcare services than non-pregnant adults, and likewise, different reasons why they may become lost. Efforts must be increased to better understand the intentions of pregnant women for seeking healthcare after delivery, understanding their motivations for ceasing HIV care, and improve linkages to care after delivery.
Acknowledgments
Data were collected in collaboration with staff from Witkoppen Health and Welfare Centre, and we are grateful for their cooperation and assistance. This study was made possible by the generous support of the American people through the United States Agency for International Development (USAID), grant number 674-A-12-00029. Matthew Fox was supported by an award from the National Institute of Allergy and Infectious Diseases (NIAID). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID, the United States government, NIH, NIAID or Witkoppen Health and Welfare Centre.
References
- Black V, Hoffman RM, Sugar CA, et al. Safety and efficacy of initiating highly active antiretroviral therapy in an integrated antenatal and HIV clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;49(3):276–281. doi: 10.1097/QAI.0b013e318189a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PloS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibwesha CJ, Giganti MJ, Putta N, et al. Optimal Time on HAART for Prevention of Mother-to-Child Transmission of HIV. J Acquir Immune Defic Syndr. 2011;58(2):224–228. doi: 10.1097/QAI.0b013e318229147e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- City of Johannesburg. [Accessed 10 October 2012];Refugee nurses help at clinic. 2011 Available at: http://www.joburg.org.za/index.php?option=com_content&view=article&id=6711&catid=88&Itemid=266.
- Clouse K, Pettifor AE, Maskew M, et al. Patient retention at key milestones after HIV diagnosis at a primary healthcare clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2013;62(2):e39–e46. doi: 10.1097/QAI.0b013e318273ac48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- Faal M, Naidoo N, Glencross DK, Venter WD, Osih R. Providing Immediate CD4 Count Results at HIV Testing Improves ART Initiation. J Acquir Immune Defic Syndr. 2011;58(3):e54–e59. doi: 10.1097/QAI.0b013e3182303921. [DOI] [PubMed] [Google Scholar]
- Ferguson L, Lewis J, Grant AD, et al. Patient attrition between diagnosis with HIV in pregnancy-related services and long-term HIV care and treatment services in Kenya: A retrospective study. J Acquir Immune Defic Syndr. 2012;60(3):e90–e97. doi: 10.1097/QAI.0b013e318253258a. [DOI] [PubMed] [Google Scholar]
- Ferguson L, Grant AD, Watson-Jones D, et al. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17(5):564–580. doi: 10.1111/j.1365-3156.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald FC, Bekker L-G, Kaplan R, et al. Mother-to-child transmission of HIV in a community-based antiretroviral clinic in South Africa. S Afr Med J. 2010;100(12):827–831. doi: 10.7196/samj.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15(4):405–413. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Black V, Technau K, et al. Effects of highly active antiretroviral therapy duration and regimen on risk for mother-to-child transmission of HIV in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2010;54(1):35–41. doi: 10.1097/QAI.0b013e3181cf9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Wilson TE, Royce RA, et al. Prenatal and postpartum zidovudine adherence among pregnant women with HIV: results of a MEMS substudy from the Perinatal Guidelines Evaluation Project. J Acquir Immune Defic Syndr. 2002;30(3):311–315. doi: 10.1097/00126334-200207010-00007. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva: 2010. [Accessed 10 October 2012]. Available at: http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf. [Google Scholar]
- Kaplan R, Orrell C, Zwane E, Bekker L-G, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22(13):1679–1681. doi: 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzer K, Zeinecker J, Ginsberg P, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PloS One. 2010;5(11):e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laher F, Cescon A, Lazarus E, et al. Conversations with mothers: exploring reasons for prevention of mother-to-child transmission (PMTCT) failures in the era of programmatic scale-up in Soweto, South Africa. AIDS Behav. 2012;16(1):91–98. doi: 10.1007/s10461-010-9875-9. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Chu C, Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20(8):958–968. doi: 10.1080/09540120701767208. [DOI] [PubMed] [Google Scholar]
- Myer L, Cornell M, Fox MP, et al. Loss to follow-up and mortality among pregnant and non-pregnant women initiating ART: South Africa. Presented at 19th Conference on Retroviruses and Opportunistic Infections. Seattle, WA: 2012. Paper 22. [Google Scholar]
- Myer L, Zulliger R, Black S, Pienaar D, Bekker L-G. Pilot programme for the rapid initiation of antiretroviral therapy in pregnancy in Cape Town, South Africa. AIDS Care. 2012;24(8):986–992. doi: 10.1080/09540121.2012.668173. [DOI] [PubMed] [Google Scholar]
- Myer L, Harrison A. Why do women seek antenatal care late? Perspectives from rural South Africa. J Midwifery Womens Health. 2003;48(4):268–272. doi: 10.1016/s1526-9523(02)00421-x. [DOI] [PubMed] [Google Scholar]
- Nassali M, Nakanjako D, Kyabayinze D, et al. Access to HIV/AIDS care for mothers and children in sub-Saharan Africa: adherence to the postnatal PMTCT program. AIDS Care. 2009;21(9):1124–1131. doi: 10.1080/09540120802707467. [DOI] [PubMed] [Google Scholar]
- National Committee on Confidential Enquiries into Maternal Deaths. [Accessed 10 October 2012];Saving Mothers 2005-2007: Fourth Report on Confidential Enquiries into Maternal Deaths in South Africa. 2008 Available at: www.doh.gov.za/docs/reports/2007/savingmothers.pdf.
- Patel D, Cortina-Borja M, Thorne C, Newell M-L. Time to undetectable viral load after highly active antiretroviral therapy initiation among HIV-infected pregnant women. Clin Infect Dis. 2007;44(12):1647–1656. doi: 10.1086/518284. [DOI] [PubMed] [Google Scholar]
- Republic of South Africa Department of Health. National Antenatal Sentinel HIV and Syphilis Prevalence Survey in South Africa. Pretoria: 2010. [Accessed 10 October 2012]. 2011. Available at: www.doh.gov.za/docs/reports/2011/hiv_aids_survey.pdf. [Google Scholar]
- Republic of South Africa Department of Health. [Accessed 10 October 2012];The South African Antiretroviral Treatment Guidelines 2010. 2010 Available at: http://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf.
- Republic of South Africa Department of Health, South African National AIDS Council. [Accessed 10 October 2012];Clinical Guidelines: PMTCT (Prevention of Mother-to-Child Transmission) 2010 Available at: http://www.info.gov.za/view/DownloadFileAction?id=77877.
- Republic of South Africa Department of Health. [Accessed 10 October 2012];Clinical Guidelines for the Management of HIV & AIDS in Adults and Adolescents. 2010 Available at: http://www.sahivsoc.org/upload/documents/Clinical_Guidelines_for_the_Management_of_HIV_AIDS_in_Adults_Adolescents_2010.pdf.
- Shisana O, Rehle T, Simbayi L, et al. South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenagers? Cape Town: 2009. [Accessed 10 October 2012]. Available at: www.hsrcpress.ac.za/product.php?productid=2264. [Google Scholar]
- Solarin I, Black V. “They Told Me to Come Back”: Women’s Antenatal Care Booking Experience in Inner-City Johannesburg. Matern Child Health J. 2012 doi: 10.1007/s10995-012-1019-6. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson K, Boulle A, Coetzee D, Abrams EJ, Myer L. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. 2010;15(7):825–832. doi: 10.1111/j.1365-3156.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- Tedaldi E, Willard S, Gilmore J, et al. Continuation of postpartum antiretroviral therapy in a cohort of women infected with human immunodeficiency virus. TJ Assoc Nurses AIDS Care. 2002;13(1):60–65. doi: 10.1016/S1055-3290(06)60241-0. [DOI] [PubMed] [Google Scholar]
- Thorsen VC, Sundby J, Martinson F. Potential initiators of HIV-related stigmatization: ethical and programmatic challenges for PMTCT programs. Dev World Bioeth. 2008;8(1):43–50. doi: 10.1111/j.1471-8847.2008.00227.x. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Newschaffer CJ, Zhang D, Cosler L, Hauck WW. Antiretroviral use and pharmacy-based measurement of adherence in postpartum HIV-infected women. Med care. 2000;38(9):911–925. doi: 10.1097/00005650-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Wang B, Losina E, Stark R, et al. Loss to follow-up in a community clinic in South Africa--roles of gender, pregnancy and CD4 count. S Afr Med J. 2011;101(4):253–257. doi: 10.7196/samj.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]