Abstract
The use of microchip devices to study cellular systems is a rapidly growing research area. There are numerous advantages of using on-chip integrated electrodes to monitor various cellular processes. The purpose of this review article is to give examples of advancements in microchip-based cellular analysis, specifically where electrochemistry is used for the detection scheme. These examples include on-chip detection of single cell quantal exocytosis, electrochemical analysis of intracellular contents, the ability to integrate cell culture/immobilization with electrochemistry, and the use of integrated electrodes to ensure cell confluency in longer term cell culture experiments. A perspective on future trends in this area is also given.
Keywords: Microchip, Microelectrode, Cell Culture, Single Cell Analysis
Introduction
The use of microchip-based systems for carrying out analytical measurements is now a well-established field. The first demonstration of a micro-total analysis system (μ-TAS) was in 1979 from Terry et al., where a miniature gas chromatograph with a thermal conductivity detector was fabricated on a silicon wafer [1]. In the early 1990’s, a group of scientists at Ciba Geigy in Switzerland introduced the use of microfabricated analysis systems for electrophoresis-based separations [2,3]. Initial publications in this area focused on basic chip operations (such as injection and separation schemes [4,5]) as well as the use of alternate chip materials including polydimethylsiloxane (PDMS) [6,7].
The small channel dimensions (on the micron scale) and the ability to manipulate small sample volumes (less than 1 nL is routine) dictates the use of a sensitive detection technique in microchip devices. Initially, most studies utilized laser-induced fluorescence (LIF) detection, primarily due to the simplicity of constructing these systems, the ease of focusing the laser beam in the channels, and the low limits of detection that are achievable (pM to nM, depending on the analyte) [8,9]. The main disadvantage of the LIF approach is the requirement for derivatization with a fluorophore for most analytes. Another major detection technique that has been utilized for microchip devices is electrochemistry. Many biologically significant compounds (such as catecholamine neurotransmitters) can be detected sensitively and selectively without derivatization so that close to real-time analysis is possible [10,11]. It is possible to fabricate the microelectrodes with many of the same photolithographic procedures that are used to construct the microchannel component of the microchips [12]. In addition, the electrode can be fabricated directly on the chip, leading to a fully integrated system [13]. The use of electrochemical detection in microchip systems has been extensively reviewed [9,12,14–17].
In the 2000’s, many researchers realized that microchip-based systems have many advantages for the analysis of cellular systems. These advantages include fast analysis times (on the order of seconds), high-throughput analysis, the possibility for portability and disposability, the ability to utilize small channel volumes, and the ability to inject small sample volumes. In terms of monitoring biological systems, these characteristics are important in terms of 1) minimizing dilution effects so that small concentrations of analyte can be detected [18,19]; 2) enabling high temporal measurements so that there is little time between the biological event and analysis [20–22]; 3) increasing the throughput so that many samples can be analyzed in a given time [23,24]; 4) integrating multiple functions such as cell manipulation or immobilization and analysis [25,26]; and 5) the ability to fabricate structures that can mimic the 3-dimensional nature of in vivo environments [27]. Initial reports in this area focused on the manipulation [28] or immobilization of various cell lines [29]. Since that time, this research area has exploded, as can be demonstrated by the number of review articles involving “cells-on-chip” [30–40].
Due to the many advantages of using electrochemical detection in microchip devices (given above), there have been many recent reports of using on-chip integrated electrodes to monitor cellular processes. The electroanalytical techniques that are commonly employed for these types of studies are amperometry, voltammetry and resistance measurements, with the analytes usually being electroactive. The purpose of this Trends review article is to update the reader on the advances of on-chip cellular analysis, specifically where electrochemistry is used for the detection scheme. This is not a comprehensive review; rather we focus on several key studies that demonstrate the possibilities of microchip-based electrochemical detection for cellular analysis. If the reader desires a more in-depth literature review in the area of cells-on-chip we refer them to review articles that detail on-chip single cell analysis [30–33], immobilization of cell layers in microchip devices [32,34–39], and chip-based electrochemical detection of cellular systems [40].
On-chip detection of quantal exocytosis from single cells
Traditionally, electrochemical-based detection of catecholamine secretion (exocytosis) from vesicles in cell lines such as PC 12 cells has been accomplished using a single carbon fiber microelectrode (typically 7 μm in diameter) placed over a single cell. Upon stimulation (such as with an elevated K+ solution), the vesicles undergo exocytosis where they fuse to the plasma membrane and release their content into the extracellular space, which is detected at the microelectrode. Several groups have measured exocytotic events in this manner [41,42]. The recent trend is to adapt this approach to a microchip format. Various research groups have developed microdevices to monitor quantal exocytosis, with the microchip enabling higher-throughput experiments [43–46]. One of the disadvantages of the traditional carbon fiber approach is the need to locate a single cell and precisely position the microelectrode over the cell. It has been shown that with a microchip approach one can isolate and direct single cells to individually addressed thin-layer electrodes so that single quantal events can be electrochemically detected. Most recently, Gillis’ group has developed a microchip platform with ITO (indium-tin-oxide) 40-microelectrode arrays and selective patterning to direct individual chromaffin cells to each microelectrode (Figure 1A) [47]. Targeting of single cells is achieved by using cell-sized microwell traps fabricated in SU-8 photoresist together with pattering of a poly(L-lysine) layer on the electrodes to promote cell adhesion. The surface between electrodes is made resistant to cell adhesion using a different polymer (polyethylene glycol). The authors demonstrated that the microelectrode array yields consistent and reproducible measurements of single cell exocytosis [47]. It was also shown that media exchange can be carried out without displacing cells from the microelectrode array. These microwell devices could be cleaned and reused with a different batch of cells without degradation of the electrode performance. Certainly this new approach will continue to be utilized for high-throughput studies involving the electrochemical detection of quantal exocytosis on single cells.
Figure 1.
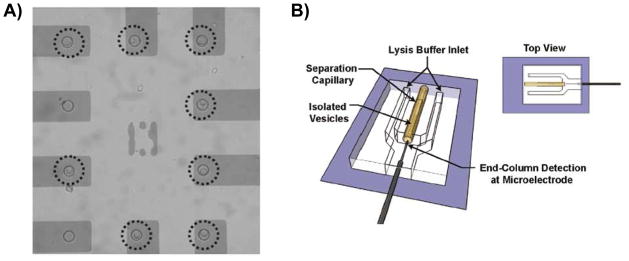
Microchip designs used to measure catecholamine release from single cells and vesicles with electrochemical detection. A) Using a microwell/surface pattering approach, the authors target individual chromaffin cells to microelectrodes (Reprinted with permission from Anal Chem. 2011, 83, 2445–2451. Copyright 2011 American Chemical Society); B) Schematic of device used for measuring content of single vesicles. Vesicles are isolated from cells and electrokinetically injected onto the capillary that is interfaced with a PDMS-based microchip. Once the vesicles exit the capillary, the vesicles are lysed and the contents are amperometrically detected at a carbon fiber microelectrode. (Reprinted with permission from ACS Chem. Neurosci. 2010, 1, 234–245. Copyright 2010 American Chemical Society).
Electrochemical analysis of intracellular contents
The ability to integrate multiple processes while minimizing dilution makes the microchip format uniquely suited for intracellular analysis [30,31]. In terms of using integrated electrodes to measure intracellular contents, our group described a microchip device with integrated injection, on-chip cell lysis, and thin-layer mercury/gold amalgam microelectrodes for the selective detection of intracellular glutathione (GSH) in red blood cells (RBCs) [48]. The thin-layer gold microelectrodes were amalgamated by electrodeposition of mercury and used to determine the GSH content in rabbit erythrocyte samples (2% hematocrit). A 40 nL plug of RBCs was injected, lysed on-chip, and the intracellular GSH amperometrically detected, all in 5 seconds. The amount of GSH detected corresponded to 312 amol/cell, which was in agreement with previously reported values that used fluorescence detection after a 30 min incubation period [48].
In addition to measuring release of neurotransmitters from intact single cells (described in preceding section), there have also been demonstrations of microchip devices with integrated electrodes that can measure intracellular contents of single cells including the contents of single vesicles. Ewing’s group developed a hybrid capillary-microfluidic device to separate, lyse, and electrochemically detect contents of single vesicles [49]. Vesicles from PC 12 cells were first isolated using a selective lysis and differential centrifugation procedure so that the vesicles remained intact in the supernatant. Vesicles were then electrokinetically injected onto a capillary that was interfaced with a PDMS-based microchip (which contained lysis channels and electrodes). The vesicles were separated using capillary electrophoresis and as the separated vesicles exited the capillary into the microchip, it was lysed with a buffer using a sheath-flow approach. The sheath flow was important in this device in order to minimize dispersion of the vesicle contents before they reached the detection electrode. The contents of each vesicle was then amperometrically detected at a carbon fiber microelectrode that was integrated into the PDMS microchip (in an end column configuration, see Figure 1B) so that there was little time between lysis and detection. Prior to this novel approach, the catecholamine content of single vesicles was determined using single carbon fiber electrodes and intact cells (as described in the beginning of the previous section). In this study, the authors used the carbon fiber approach to quantitate the amount of catecholamines released during exocytosis and this new approach to determine the amount of catecholamine each vesicle contains. Using this approach it was found that PC 12 cell-based vesicles only release about 40% of their total catecholamine content during exocytosis [50]. This was a key finding because prior to this study, it was thought that vesicle catecholamine release was all-or-none. The microchip approach enabled the researchers to integrate hydrodynamic focusing of the vesicles, vesicle lysis, and amperometric detection of the vesicle content to provide many new insights into the mechanism of exocytosis.
Integrating Cell Culture with Electrochemistry-based Analysis
While much information can be garnered from single cell studies, there is also interest in culturing layers of adherent cells in microfluidic devices. By integrating a method to analyze the chemicals that are released from the cultured cells it could be possible to investigate processes such as how cells act in concert and how populations of cells communicate with each other. In a sense, such a device can be considered an in vitro-based mimic of an in vivo system that also integrates an analysis scheme.
The ability to directly detect molecules as they are released from cells has made electrochemistry a popular method for measuring short-lived analytes such as nitric oxide (NO). The first demonstration of measuring NO release from cultured cells on-chip came from Spence et al. in 2004, where the authors immobilized a confluent layer of bovine pulmonary artery endothelial cells (bPAECs) in microchannels and used flow injection analysis with integrated carbon ink electrodes for NO detection. [51]. In their work, they determined the optimum conditions for immobilizing bPAECs in PDMS-based microchannels and how to modify the microelectrodes to selectively detect NO. They also presented the ability to quantitatively determine the amount of endothelium-derived NO upon stimulation with adenosine triphosphate (ATP). Later, Hulvey and Martin developed a microfluidic device that utilized a reservoir-based approach for endothelial cell immobilization and integrated carbon ink microelectrodes for amperometric detection of extracellular NO release [52]. In this design, the cells are contained in one area of the device and only the cellular release is carried by a continuously flowing buffer stream to interact with the detection electrode.
Previous work has also shown that a microchip-based approach with integrated electrodes can be used to quantitatively monitor the amount of catecholamines released from a layer of immobilized PC 12 cells upon stimulation with an elevated K+ solution [53]. This initial work focused on the development of a simple method for the immobilization of PC 12 cells in PDMS-based microchannels. Collagen was determined to be the best coating for cell adhesion on the PDMS surface, and it was selectively coated onto the PDMS microchannels. The cell-coated microchannel was then reversibly sealed on glass that contained carbon ink electrodes for amperometric detection. The micromolded carbon ink electrodes were coated with a Nafion membrane to eliminate interferences from ascorbic acid. Various concentrations of PC 12 cells within the PDMS-based microchannel led to a catecholamine release ranging from 20–160 μM upon stimulation [53]. While this study demonstrated the quantification of catecholamines released from PC 12 cells, it was not able to differentiate dopamine and norepinephrine.
A more recent study focused on the development of a device that could detect multiple analytes released from stimulated PC 12 cells [54]. The fabricated device integrated multiple processes such as cell immobilization, on-chip valving, microchip electrophoresis, and electrochemical detection (see Figure 2). Microchip electrophoresis was an innovative feature because it allowed for the detection of multiple analytes (dopamine and norepinephrine) released from the immobilized PC 12 cell layer. This study utilized a reservoir-based cell immobilization technique where a PDMS micropallet, onto which PC 12 cells were immobilized, could be placed within a reservoir on the microchip. On-chip peristaltic pumps were used to continuously sample from the cell-coated reservoir and injection valves were used to enable discrete injections into the separation channel. A palladium decoupler was used to provide an electrophoretic ground, absorb hydrogen produced from the reduction of water at the cathode, and allow for in-channel detection. The released catecholamines (upon stimulation with a nicotine solution) were electrophoretically separated and subsequently detected downstream at a carbon ink electrode. This method was modular in that multiple micropallets could be inserted as desired into the microchip and control studies were easy to perform. A key element of this microchip was the ability to isolate the cells from the high voltage associated with the electrophoretic separation channel. This was the first demonstration of simultaneously monitoring the release of dopamine and norepinephrine from immobilized cells on a microchip device. In the future, this device can be expanded to have multiple reservoirs and pumps to look at multiple cell lines and how the cells interact with each other.
Figure 2.
Integrating cell immobilization with electrophoresis-based analysis. A) On-chip peristaltic pumps are used to continuously sample from the cell reservoir and injection valves are used to introduce discrete plugs of releasate into the separation channel. B) Micrograph of immobilized PC 12 cells on a PDMS micropallet. C) Electropherogram demonstrating the release of dopamine (DA) and norepinephrine (NE) released from the immobilized PC 12 cells. A and C reprinted with permission from Electrophoresis 2010, 31, 2534–2540.
Monitoring Cell Confluency with On-Chip Electrodes
To study vascular wall biology with an in vitro model, a confluent layer of endothelial cells is required. The best known vascular wall is the blood brain barrier (BBB), which is a selective membrane composed of a confluent layer of endothelial cells in the cerebral capillaries. It is common to utilize cell lines such as bovine brain microendothelial cells that are cultured on microtitre plates with a polycarbonate membrane insert [55] to mimic a vascular wall. The confluency is typically ensured by measuring the transendothelial electrical resistance (TEER), where a current is passed between the electrodes on either side of the membrane. As the cells grow on the membrane and form tight junctions, the resistivity of the layer increases, resulting in a decrease in the current measured. Thus, a lower resistance is indicative of a cell layer that is not yet completely confluent or, if it was confluent, may suggest layer breakdown. Recent trends in culturing cells on-chip has led to several approaches of transferring the TEER measurement to the microchip format via integrated electrodes. Takayama’s group was the first to fabricate such a device, with embedded Ag/AgCl electrodes within a PDMS-based device to measure TEER [56]. Impedance was utilized in this device to confirm a confluent monolayer of cells with both brain-derived endothelial and epithelial cells. This work was the first report of a microfluidic device to measure TEER in real-time using electrochemistry [56].
A more recent study developed a device to measure TEER (with integrated electrodes) in a manner where cell-to-cell interactions (between flowing red blood cells and an immobilized layer of endothelial cells) can also be investigated [57]. In this device, conductance was also used to monitor cell layer integrity. Bovine pulmonary artery endothelial cells were cultured on a polycarbonate membrane inside a PDMS well. Cell media (or red blood cells) were pumped through a channel on the other side of the membrane (Figure 3A). An integrated aluminum strip (below each well) served as one detection electrode for the TEER measurements, with the top electrode being a copper wire. The top electrode was clamped into place and the microfluidic device was then raised until the copper electrode rested in the wells where a TEER measurement was desired. This device was used to demonstrate that endothelial cells reached confluency at approximately 8 hours, as determined by TEER measurements (Figure 3B). It was also shown that this type of device can be used for TEER measurements on endothelial cells that have been stimulated to produce NO by ATP released from the flowing red blood cells. Fluorescence detection and an intracellular dye were used to show NO production by immobilized endothelial cells that had reached confluence was 34% higher than those cells that had not reached confluence, with the confluency being determined by the integrated TEER measurement.
Figure 3.
Microchip-based TEER measurements. A) Cross-sectional view of device for TEER measurements. Endothelial cells are cultured on the polycarbonate membrane. Cell media or red blood cells are pumped through the underlying channel and resistance is measured across the cell layer. B) Bar and line graph displaying TEER measurements and demonstrating that cells reach confluency at approximately 8 hours. Reprinted with permission from Anal. Chem. 2011, 83, 4296–4301. Copyright 2011 American Chemical Society.
Outlook
These selected works clearly demonstrate the many advantages of using microchip-based systems with integrated electrodes to study cellular systems. In terms of future trends, one area of research is the use of more cell-compatible chip substrates. The vast majority of microchip devices have been fabricated with PDMS. While these devices can be fabricated with rapid prototyping methods [7] and the PDMS is gas permeable (which is important for proper gas exchange in longer-term culture experiments), its use for cell studies does pose several problems. PDMS is a crosslinked polymer composed of hydrophobic dimethylsiloxane oligomers. Residual uncrosslinked monomers can leach from the bulk into the cell culture system and hydrophobic molecules from the media can also partition into the hydrophobic PDMS [58]. Recent work has focused on developing polystyrene-based microchip devices for cell-based assays, with several descriptions of fabricating devices and the resulting improvements in biocompatibility [59–63]. Although polystyrene (PS) devices have mainly been fabricated via hot embossing [59,64], the integration of other components such as electrodes or connections for off-chip functions had not until recently been demonstrated. We recently reported a simple fabrication approach for PS devices that is based upon melting polystyrene (from either a Petri dish or powder form) against PDMS molds or around electrode materials, as shown in Figure 4 [65]. It was shown that the PC 12 cells had better adherence on the PS devices, as compared to use of PDMS. The utility of PS devices with integrated channels and electrodes was demonstrated by electrochemically monitoring the release of catecholamines from PC 12 cells. Potassium stimulation resulted in the release of 114 ± 11 μM catecholamines, a significant increase over the release from cells that had been exposed to an inhibitor (reserpine, 20 ± 2 μM of catecholamines) [66].
Figure 4.
Polystyrene-based devices with integrated electrodes. A) Polystyrene base with embedded electrodes. Micrograph with embedded 1 mm Pd decoupler and a 100 μm carbon fiber bundle detection electrode (adapted from Analyst, 2012, in press, DOI: 10.1039/c2an36168j, reproduced by permission of The Royal Society of Chemistry); B) Micrograph of PC 12 cells on polystyrene surface and channel (from Analyst, 2012, in press, DOI: 10.1039/c2an36171j, reproduced by permission of The Royal Society of Chemistry).
The advantage of using PS-based microdevices relative to PDMS substrates for improved cellular adhesion is demonstrated in Figure 5. With PDMS devices it is common to coat the device with adhesion factors such as collagen or fibronectin [51,53]; however, when culturing many different cell lines in culture flasks it is common to not use any adhesion factor. The ability to culture cells on uncoated PS devices relative to an uncoated PDMS substrate is demonstrated with recent work from our lab that is shown in Figure 5. In this study, bPAECs (grown to confluency in a T-25 flask with previously described methods [51,52]) were detached (with a trypsin solution), neutralized, spun down, and re-constituted in 1 mL of cell media. PDMS-based reservoirs were placed over either a PDMS microchip (Figure 5A) or a fabricated PS microchip (Figure 5B), both of which were not coated with adhesion factor. An aliquot (200 μL) of the bPAECs were added to each and the cells allowed to attach overnight, after which time the reservoirs were rinsed (via pipetting) with Hank’s Balanced Salt Solution (HBSS). As is evident in the micrographs, only a very small fraction of cells stay adherent on the uncoated PDMS while the majority of the cells remained adherent on the uncoated PS substrate. While confluent layers of bPAECs can be obtained in PDMS if it is pre-coated with fibronectin [51,52], it is our experience that cells adhere more quickly and, with bPAECs in a more elongated morphology, on the PS devices (as compared to use of coated PDMS substrates). We are currently focusing on integrating multiple processes into these more biocompatible PS devices and we expect this is relatively new area of research to continue in growth.
Figure 5.
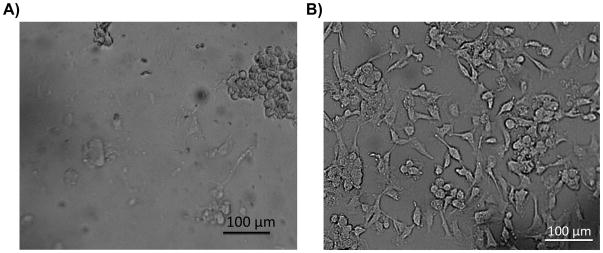
Comparison of cell adhesion on uncoated PDMS and PS-based devices. A) Micrograph of an uncoated PDMS-based microchip with endothelial cells (cultured overnight) after 5 rinses with buffer. The micrograph illustrates the poor adhesion of endothelial cells on uncoated PDMS-based microchips; B) Micrograph of endothelial cells on an uncoated PS-based microchip (cultured overnight) after 5 rinses with buffer, with the same concentration of cells added as in the PDMS experiment. Cells stayed adherent and elongated on the uncoated PS-based microchip after multiple rinses.
There are several other directions of future research in this area. One is making microchips that are compatible with existing lab infrastructure such as a 96-well plate reader so that automated, high-throughput studies can be accomplished. This was recently demonstrated by interfacing a multi-channel device with integrated registration marks to a standard fluorescence plate reader, with the approach being used to fluorescently monitor the release of NO (after derivatization with a DAF-dye) from a flowing stream of RBCs that had been exposed to a hypoxic buffer [67]. Another recent demonstration involves using a PDMS injection block to incorporate a standard micropipette as part of a pumping mechanism, with the final PS device being compatible with a 96-well plate reader [66]. Other areas of promising future work include use of multimodal detection (such as fluorescence and electrochemical detection) to provide more information about biological systems (including simultaneous monitoring of intra- and extra-cellular events), more routine integration with mass spectrometry to provide even more information about the cellular system, and the use of multiple cell types so that cell-to-cell interactions can be probed [68]. Certainly the use of microchip-based electrochemical detection holds a lot of potential for monitoring cellular systems.
Acknowledgments
Support from the National Institute of General Medical Sciences (Award Number R15GM084470-03) is acknowledged.
References
- 1.Terry SC, Jerman JH, Angell JB. IEEE Trans Electron Devices ED-26. 12. 1979. p. 1880. [Google Scholar]
- 2.Harrison DJ, Manz A, Fan Z, Luedi H, Widmer HM. Anal Chem. 1992;64 (17):1926–1932. [Google Scholar]
- 3.Manz A, Graber N, Widmer HM. Sens Actuators B. 1990;1 (1–6):244–248. [Google Scholar]
- 4.Jacobson SC, Hergenroder R, Koutny LB, Ramsey JM. Anal Chem. 1994;66 (7):1114–1118. [Google Scholar]
- 5.Jacobson SC, Hergenroder R, Koutny LB, Warmack RJ, Ramsey JM. Anal Chem. 1994;66 (7):1107–1113. [Google Scholar]
- 6.Becker H, Gartner C. Electrophoresis. 2000;21 (1):12–26. doi: 10.1002/(SICI)1522-2683(20000101)21:1<12::AID-ELPS12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal Chem. 1998;70 (23):4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 8.Ocvirk G, Tang T, Jed Harrison D. Analyst. 1998;123 (7):1429–1434. [Google Scholar]
- 9.Pasas SA, Fogarty BA, Huynh BH, Lacher NA, Carlson B, Martin RS, Vandeveer WR, IV, Lunte SM. Detection on Microchips: Principles, Challenges, Hyphenation, and Integration. In: Kutter JP, Fintschenko Y, editors. Separation Methods in Microanalytical Systems. CRC Press; Boca Raton: 2006. [Google Scholar]
- 10.Martin RS, Gawron AJ, Lunte SM, Henry CS. Anal Chem. 2000;72 (14):3196–3202. doi: 10.1021/ac000160t. [DOI] [PubMed] [Google Scholar]
- 11.Hulvey MK, Frankenfeld CN, Lunte SM. Anal Chem. 2010;82 (5):1608–1611. doi: 10.1021/ac902821v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin RS. Interfacing Amperometric Detection with Microchip Capillary Electrophoresis. In: Henry CS, editor. Methods in Molecular Biology, vol. 339: Microchip Capillary Electrophoresis: Methods and Protocols. Humana Press; Towtowa, NJ: 2006. [DOI] [PubMed] [Google Scholar]
- 13.Keynton RS, Roussel TJ, Crain MM, Jackson DJ, Franco DB, Naber JF, Walsh KM, Baldwin RP. Anal Chim Acta. 2004;507 (1):95–105. [Google Scholar]
- 14.Kuban P, Hauser PC. Electrophoresis. 2009;30 (19):3305–3314. doi: 10.1002/elps.200900217. [DOI] [PubMed] [Google Scholar]
- 15.Vandaveer WR, Pasas SA, Martin RS, Lunte SM. Electrophoresis. 2002;23 (21):3667–3677. doi: 10.1002/1522-2683(200211)23:21<3667::AID-ELPS3667>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Vandaveer WR, Pasas-Farmer SA, Fischer DJ, Frankenfeld CN, Lunte SM. Electrophoresis. 2004;25 (21–22):3528–3549. doi: 10.1002/elps.200406115. [DOI] [PubMed] [Google Scholar]
- 17.Xu J-J, Wang A-J, Chen H-Y. TrAC. 2007;26 (2):125–132. [Google Scholar]
- 18.Easley CJ, Karlinsey JM, Bienvenue JM, Legendre LA, Roper MG, Feldman SH, Hughes MA, Hewlett EL, Merkel TJ, Ferrance JP, Landers JP. Proc Natl Acad Sci USA. 2006;103 (51):19272–19277. doi: 10.1073/pnas.0604663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li MW, Martin RS. Analyst. 2008;133 (10):1358–1366. doi: 10.1039/b807093h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filla LA, Kirkpatrick DC, Martin RS. Anal Chem. 2011;83 (15):5996–6003. doi: 10.1021/ac201007s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Roman GT, Perry ML, Kennedy RT. Anal Chem. 2009;81 (21):9072–9078. doi: 10.1021/ac901731v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Roman GT, Schultz K, Jennings C, Kennedy RT. Anal Chem. 2008;80 (14):5607–5615. doi: 10.1021/ac800622s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dishinger JF, Reid KR, Kennedy RT. Anal Chem. 2009;81 (8):3119–3127. doi: 10.1021/ac900109t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolan NV, Genes LI, Subasinghe W, Raththagala M, Spence DM. Anal Chem. 2009;81 (8):3102–3108. doi: 10.1021/ac900084g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genes LI, Tolan NV, Hulvey MK, Martin RS, Spence DM. Lab Chip. 2007;7 (10):1256–1259. doi: 10.1039/b712619k. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Daou A, Truong TM, Bertram R, Roper MG. Am J Physiol-Endoc M. 2011;301 (4):E742–747. doi: 10.1152/ajpendo.00248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh D, Hamilton GA, Ingber DE. Trends Cell Biol. 2011;21 (12):745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li PCH, Harrison DJ. Anal Chem. 1997;69 (8):1564–1568. doi: 10.1021/ac9606564. [DOI] [PubMed] [Google Scholar]
- 29.Chiu DT, Jeon NL, Huang S, Kane RS, Wargo CJ, Choi IS, Ingber DE, Whitesides GM. Proc Natl Acad Sci. 2000;97 (6):2408–2413. doi: 10.1073/pnas.040562297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price AK, Culbertson CT. Anal Chem. 2007;79 (7):2614–2621. doi: 10.1021/ac071891x. [DOI] [PubMed] [Google Scholar]
- 31.Sims CE, Allbritton NL. Lab Chip. 2007;7 (4):423–440. doi: 10.1039/b615235j. [DOI] [PubMed] [Google Scholar]
- 32.Kovarik ML, Gach PC, Ornoff DM, Wang Y, Balowski J, Farrag L, Allbritton NL. Anal Chem. 2012;84 (2):516–540. doi: 10.1021/ac202611x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salieb-Beugelaar GB, Simone G, Arora A, Philippi A, Manz A. Anal Chem. 2010;82 (12):4848–4864. doi: 10.1021/ac1009707. [DOI] [PubMed] [Google Scholar]
- 34.El-Ali J, Sorger PK, Jensen KF. Nature. 2006;442 (7101):403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 35.Martin RS, Root PD, Spence DM. Analyst. 2006;131 (11):1197–1206. doi: 10.1039/b611041j. [DOI] [PubMed] [Google Scholar]
- 36.Sato K, Mawatari K, Kitamori T. Lab Chip. 2008;8 (12):1992–1998. doi: 10.1039/b814098g. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Tanaka Y, Renberg B, Kitamori T. Anal Bioanal Chem. 2009;393 (1):23–29. doi: 10.1007/s00216-008-2450-9. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Jang K, Yamashita T, Tanaka Y, Mawatari K, Kitamori T. Anal Bioanal Chem. 2012;402 (1):99–107. doi: 10.1007/s00216-011-5296-5. [DOI] [PubMed] [Google Scholar]
- 39.Young EWK, Beebe DJ. Chemical Society Reviews. 2010;39 (3):1036–1048. doi: 10.1039/b909900j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spegel C, Heiskanen A, Skjolding LHD, Emneus J. Electroanalysis. 2008;20 (6):680–702. [Google Scholar]
- 41.Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Lewczyszyn DJ, Near JA, Diliberto EJJ, Viveros OH. Proc Natl Acad Sci. 1991;88:10754. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sombers LA, Ewing AE. Electrochemical monitoring of exocytosis from individual PC12 cells. In: Brajter-Toth A, Chambers JQ, editors. Electroanalytical Methods for Biological Methods. Brajter-Toth; New York: 2002. pp. 279–327. [Google Scholar]
- 43.Amatore C, Arbault S, Chen Y, Crozatier C, Lemaitre F, Verchier Y. Angew Chem Int Ed Engl. 2006;45 (24):4000–4003. doi: 10.1002/anie.200600510. [DOI] [PubMed] [Google Scholar]
- 44.Amatore C, Arbault S, Bouret Y, Guille M, Lemaitre F, Verchier Y. Anal Chem. 2009;81 (8):3087–3093. doi: 10.1021/ac900059s. [DOI] [PubMed] [Google Scholar]
- 45.Gao Y, Bhattacharya S, Chen X, Barizuddin S, Gangopadhyay S, Gillis KD. Lab on a chip. 2009;9 (23):3442–3446. doi: 10.1039/b913216c. [DOI] [PubMed] [Google Scholar]
- 46.Barizuddin S, Liu X, Mathai JC, Hossain M, Gillis KD, Gangopadhyay S. ACS Chem Neurosci. 2010;1 (9):590–597. doi: 10.1021/cn1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Barizuddin S, Shin W, Mathai CJ, Gangopadhyay S, Gillis KD. Analytical chemistry. 2011;83 (7):2445–2451. doi: 10.1021/ac1033616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batz NG, Martin RS. Analyst. 2009;34 (2):372– 379. doi: 10.1039/b813898b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omiatek DM, Santillo MF, Heien ML, Ewing A. Anal Chem. 2009;81 (6):2294–2302. doi: 10.1021/ac802466g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omiatek DM, Dong Y, Heien ML, Ewing A. ACS Chem Neurosci. 2010;1 (3):234–245. doi: 10.1021/cn900040e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spence DM, Torrence NJ, Kovarik ML, Martin RS. Analyst. 2004;129 (11):995–1000. doi: 10.1039/b410547h. [DOI] [PubMed] [Google Scholar]
- 52.Hulvey MK, Martin RS. Anal Bioanal Chem. 2009;393 (2):599–605. doi: 10.1007/s00216-008-2468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li MW, Spence DM, Martin RS. Electroanalysis. 2005;17 (13):1171–1180. [Google Scholar]
- 54.Bowen AL, Martin RS. Electrophoresis. 2010;31 (15):2534–2540. doi: 10.1002/elps.201000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gumbleton M, Audus KL. J Pharm Sci. 2001;90 (11):1681–1698. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- 56.Douville NJ, Tung YC, Li RB, Wang JD, El-Sayed M, Takayama S. Anal Chem. 2010;82 (6):2505–2511. doi: 10.1021/ac9029345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel PA, Halpin ST, Martin RS, Spence DM. Anal Chem. 2011;83 (11):4296–4301. doi: 10.1021/ac2004746. [DOI] [PubMed] [Google Scholar]
- 58.Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SE, Murphy WL, Schuler LA, Alarid ET, Beebe DJ. Lab Chip. 2009;9 (15):2132–2139. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young EW, Berthier E, Guckenberger DJ, Sackmann E, Lamers C, Meyvantsson I, Huttenlocher A, Beebe DJ. Anal Chem. 2011;83 (4):1408–1417. doi: 10.1021/ac102897h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Detwiler DA, Dobes NC, Sims CE, Kornegay JN, Allbritton NL. Anal Bioanal Chem. 2012;402 (3):1083–1091. doi: 10.1007/s00216-011-5596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta G, Lee J, Cha W, Tung Y-C, Linderman JJ, Takayama S. Anal Chem. 2009;81 (10):3714–3722. doi: 10.1021/ac802178u. [DOI] [PubMed] [Google Scholar]
- 62.Chen A, Lieu DK, Freschauf L, Lew V, Sharma H, Wang J, Nguyen D, Karakikes I, Hajjar RJ, Gopinathan A, Botvinick E, Fowlkes CC, Li RA, Khine M. Advanced Materials. 2011;23 (48):5785–5791. doi: 10.1002/adma.201103463. [DOI] [PubMed] [Google Scholar]
- 63.Goral VN, Hsiesh YC, Petzold ON, Faris RA, Yuen PK. J Micromech Microen. 2011;21(1):017002. (017008pp) [Google Scholar]
- 64.van Midwoud PM, Janse A, Merema MT, Groothuis GMM, Verpoorte E. Anal Chem. 2012;84 (9):3938–3944. doi: 10.1021/ac300771z. [DOI] [PubMed] [Google Scholar]
- 65.Johnson AS, Halpin ST, Anderson KB, Kirkpatrick DC, Spence DM, Martin RS. Analyst. 2012 doi: 10.1039/c2an36168j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson KB, Halpin ST, Johnson AS, Martin RS, Spence DM. Analyst. 2012 doi: 10.1039/c2an36171j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halpin ST, Spence DM. Anal Chem. 2010;82 (17):7492–7497. doi: 10.1021/ac101130s. [DOI] [PubMed] [Google Scholar]
- 68.Ku C-J, D’Amico Oblak T, Spence DM. Analytical Chemistry. 2008;80 (19):7543–7548. doi: 10.1021/ac801114j. [DOI] [PMC free article] [PubMed] [Google Scholar]





