Abstract
Objective
Traditionally, children are cochlear implant (CI) candidates if bilateral severe to profound hearing loss is present and amplification benefit is limited. The current study investigated abilities of adolescents with asymmetric hearing loss (one ear with severe to profound hearing loss and better hearing contralaterally), where the poorer ear received a CI and the better ear maintained amplification.
Study Design
Within-subject case study
Setting
Pediatric hospital, outpatient clinic
Patients
Participants were five adolescents who had not met traditional CI candidacy due to one better hearing ear, but did have one ear that met criteria and was implanted. All maintained hearing aid (HA) use in the contralateral ear. In the poorer ear pre-implant, three participants had used amplification and the other two had no HA experience.
Main Outcome Measure
Participants were assessed in three listening conditions: HA alone, CI alone, and both devices together (bimodal) for speech recognition in quiet and noise, and sound localization.
Results
Three participants had CI open-set speech recognition and significant bimodal improvement for speech recognition and localization compared with the HA or CI alone. Two participants had no CI speech recognition and limited bimodal improvement.
Conclusions
Some adolescents with asymmetric hearing loss who are not typical CI candidates can benefit from a CI in the poorer ear, compared to a HA in the better ear alone. Additional study is needed to determine outcomes for this population, especially those who have early onset profound hearing loss in one ear and limited HA experience.
Introduction
Traditionally, children are considered candidates for a cochlear implant (CI) if they have bilateral severe to profound sensorineural hearing loss (SPHL). Children with asymmetric hearing between ears, who have SPHL in one ear and less severe hearing loss in the other ear, are not typically recommended for cochlear implantation due to higher speech recognition abilities in the better hearing ear. Often, these children wear either bilateral hearing aids (HAs) with minimal-to-no benefit in the poorer ear, or a unilateral HA in the better ear while leaving the poorer ear unaided. In either case, binaural listening cues required for optimal performance in difficult listening environments are not available.
Children with a unilateral hearing deficit (i.e. hearing is normal in only one ear) show disadvantages in communication and educational settings compared to those who hear well bilaterally (1,2). Understanding speech in noise is challenging (3), sound localization is diminished or often impossible (4) and children with unilateral hearing loss are at risk for social, behavioral, and academic difficulties (5,6,7,8,9). Children with symmetrical hearing loss are similarly disadvantaged if they only use one HA (10,11,12,13) or one CI (14,15,16,17,18) and no device contralaterally.
To improve the potential for binaural hearing benefits, bilateral input is needed. This can be attempted with two HAs, two CIs, or a CI in one ear and a HA in the other (referred to as bimodal device use). The amount of improvement individuals receive from bimodal fittings often depends on the amount of low frequency hearing in the amplified ear (19,20,21,22). When a HA in the contralateral ear cannot provide adequate auditory cues and no bimodal advantage is present (i.e. hearing abilities are similar between the CI-only and bimodal conditions), bilateral cochlear implantation may be advised. Both bimodal device use and bilateral cochlear implantation often provide benefits compared to unilateral CI use for ease of listening, speech recognition in quiet and in background noise, improved sound quality, and sound localization (for reviews: 23,24,25,26). Children with SPHL in one ear and better thresholds contralaterally who wear HAs in each ear, have reduced bilateral benefits due to HA limits for amplifying the poorer ear. A recent study showed that adults with asymmetric hearing loss can benefit from a CI in the poorer ear combined with a HA in the better ear (27), however adults with postlingual SPHL in the implanted ear showed greater benefit than those with prelingual SPHL. The current study investigated speech recognition and localization abilities in five adolescents who were not traditional CI candidates due to asymmetric hearing loss and received a CI in the poorer ear with continued HA use in the better ear.
Materials and Methods
This study followed procedures in accordance with the ethical standards of the Helsinki Declaration and the Human Research Protection Office (HRPO #201102249) at Washington University School of Medicine (WUSM).
Participants were five adolescents with histories of asymmetric HL (one ear had SPHL and met CI candidacy criteria; one ear had better hearing and did not meet CI candidacy). (See Table 1 for demographic information.) Participants had been evaluated clinically for a CI due to communication difficulties, particularly in noise. Pre-implant bilateral HA word recognition scores for P2–P5 were not substantially greater than the aided better ear alone in quiet or noise. For P1, although marginal improvement occurred bilaterally in quiet, there was no improvement and limited ability in noise. (Table 1 provides clinically obtained pre-implant word recognition scores for the better ear, poorer ear and bilaterally). The progressive nature of P1’s hearing loss and reports of a negative impact on social interactions and academics were considered in the evaluation process. After comprehensive evaluations, the clinical CI team at Saint Louis Children’s Hospital recommended a CI for each child’s poorer ear.
Table 1.
Demographic information for each participant
| Age at Test (y;m) | Etiology | Age at Dx (B) (y;m) | Age SPHL (P) (y;m) | Age Aided (B/P) | Pre-CI Word Score 60 dB SPL(%) | Age at Implant (y;m) | Length CI Use (y;m) | ||
|---|---|---|---|---|---|---|---|---|---|
| Quiet (B/P/HA+HA) | +8 dB SNR4TB (B/P/HA+HA) | ||||||||
| P1 | 11;0 | EVA | 3;8 | 8;3 | 3;9/3;9 | 80/34/92+ | 40/12/36+ | 8;9 | 3;2 |
| P2 | 10;1 | Unk | 2;3 | 0 | 2;5/2;5 | 80/20/84+ | 28/0/24+ | 5;3 | 5;2 |
| P3 | 19;8 | Unk | 4;2 | 4;10 | 4;4/4;4 | 40/0/36+ | 20/0/16+ | 14;4 | 5;4 |
| P4 | 12;10 | EVA | 3;8 | 0 | 4;0/na | 72/0/76§ | 40/0/40§ | 12;4 | 0;6 |
| P5 | 15;10 | Unk | 3;8 | 0 | 4;0/na | 58/0/50§ | 46/0/42§ | 15;4 | 0;6 |
Note: B = better ear; CI = cochlear implant; dB = decibel; Dx = initial diagnosis of hearing loss; EVA = enlarged vestibular aqueduct; HA+HA= binaural hearing aids; HL = hearing loss; m = months; na = not applicable; P = poorer ear; Prog = progressive; SNR = signal-to-noise ratio; SPHL = severe to profound hearing loss(mid to high frequencies); SPL = sound pressure level; Unk = unknown; y = years; 4TB = four-talker babble;
recorded Phonetically Balanced Kindergarten (PBK) lists;
recorded Consonant-Vowel Nucleus-Consonant (CNC) lists
Figure 1 provides individual ear pre-implant hearing thresholds for each participant. Unaided thresholds were measured with pulsed pure tones using insert earphones and aided thresholds were measured with warbled tones in the soundfield. The pre-implant unaided pure-tone average (PTA of .5, 1, 2 kHz) ranged from 25 to 75 dB HL (mean = 52.7, SD = 21.78) in the better ear and 77 to 120 dB HL (mean = 94.5, SD = 16.6) in the poorer ear. The pre-implant aided PTA range was 15 – 30 dB HL in the better ear (mean = 25.7, SD = 6.3) and 35 – 85 dB HL in the poorer ear (mean = 55.7, SD = 23.6). P5 was unable to detect sound with a HA in the poorer ear at the limits of the audiometer. All participants had their poorer ear implanted.
Figure 1.
Individual audiometric thresholds as a function of frequency are shown for each participant prior to cochlear implantation. Unaided thresholds are displayed with an O for the right ear and an X for the left ear. The R and L connected by dashed lines indicate aided thresholds for the right and left ear, respectively. Arrows indicate no response.
At the time of study enrollment, participants ranged in age from 10 years, 1 month to 19 years, 8 months (mean age 14 years, 10 months). All were bimodal listeners who had used a CI for at least six months with continued HA use contralaterally. Prior to study assessment, audibility was maximized for each participant and each device (HA and CI). For HA fittings, initial gain and Maximum Power Output settings were adjusted until output levels reached Desired Sensation Level targets, as verified by the Audioscan Verifit System with Real-Ear-to-Coupler-Difference measures for soft (50 dB SPL), average (60 dB SPL) and loud (70 dB SPL) speech. Aided thresholds were then obtained and more gain applied as needed until, if possible, the aided soundfield thresholds fell below 35 dB HL from .25 – 6 kHz. For P2–P5, frequency compression was utilized to improve high frequency audibility, ensuring that there was no degradation of speech clarity or sound quality when applied (28). P1’s HA did not have frequency compression technology. For CI fittings, each participant’s speech processor program was optimized so that conversational speech was audible, loud sounds were comfortable, and soft speech cues were available as confirmed by soundfield detection levels below 35 dB HL from .25 – 6 kHz. Participant device information is provided in Table 2.
Table 2.
Devices used by participants
| HA | CI Speech Processor | CI Internal Device | |
|---|---|---|---|
| P1 | Widex Inteo 19 | Harmony | Advanced Bionics 90k(Hifocus 1j) |
| P2 | Phonak Nios | Freedom | Nucleus Contour Advanced(CI24RE) |
| P3 | Phonak Naida UP | Harmony | Advanced Bionics 90k (Hifocus 1j) |
| P4 | Phonak Naida UP | CP810 | Nucleus CI512 |
| P5 | Phonak Naida UP | Harmony | Advanced Bionics 90k (Hifocus 1j) |
Note: HA = hearing aid; CI = cochlear implant; UP = Ultra Power
A within-subject case study design was used in which participants attended two test sessions of approximately 3 hours each. All measures were presented twice and results averaged. Results from one session are presented for P5 who was unable to return for the second test session. Participants were tested in three conditions: HA alone, CI alone, and bimodal. For the CI-only condition the contralateral ear was plugged and muffed. All speech recognition stimuli were calibrated and presented via a loudspeaker at 0° azimuth in a double-walled sound booth. The test battery was selected to avoid ceiling and floor effects when possible and included measures that replicated real-life listening situations.
Speech perception in quiet was assessed with two lists of 50 Consonant-Vowel Nucleus-Consonant (CNC) Monosyllabic Words (29) spoken by a male talker at 50 dB SPL, a soft conversational level, and scored as percent correct. This presentation level was chosen due to ceiling effects at 60 dB SPL during pilot testing. Speech perception in noise was assessed with CNC Words presented at 60 dB SPL in the presence of four-talker babble at a +8 dB signal-to-noise ratio (SNR). Also, the Bamford-Kowal-Bench Speech in Noise test (BKB-SIN; 30) was administered using two list pairs per condition. Sentences were presented at 65 dB SPL, with four-talker babble at prerecorded SNRs, decreasing in 3 dB steps from easy to difficult, and ranging from +21 to −6 dB SNR. The test score represents an SNR for 50% key word accuracy. The sentences and noise were presented from a single loudspeaker.
To assess speech recognition in the presence of environmental noise, the Hearing In Noise Test (HINT) (31) was administered in the R-Space (32,33), a sound system designed to reproduce real-world noise conditions. The participant was surrounded by eight loudspeakers from which recorded restaurant noise was presented at a fixed 60 dB SPL level. The long-term spectrum of the restaurant noise was similar to the speech shaped noise from the original HINT (33). Two lists of 20 sentences each were presented at levels that varied adaptively and resulted in a score representing the SNR for 50% sentence accuracy. Specifically, the initial sentence was presented at +12 dB SNR. If the sentence was not repeated correctly, it was re-administered at a higher SNR until it was repeated correctly. If the participant was unable to do so at the highest SNR, a score of +22 dB was assigned. If and when the first sentence was repeated correctly, the SNR was adapted in 4 dB steps for the first 4 sentences and 2 dB steps for the remaining 16 sentences, with the SNR decreased after each correct response and increased after each incorrect response. Based on the response for the final sentence, the SNR that would be used for a 21st sentence was noted, yielding a total of 21 SNR values. The participant’s score was the average of the last 17 SNR values.
To assess localization, monosyllabic words were presented from a 15 loudspeaker array at a roved 60 dB SPL (± 3 dB) level. Loudspeakers were arranged in an arc, 10° apart, with 10 active and 5 inactive speakers and each speaker visibly numbered. The participant faced the array approximately three feet from the center loudspeaker. Ten words were randomly presented from each active loudspeaker and the participant identified the source speaker locations by number. Participants were not asked to repeat the actual word. In addition to the above measures, the participant’s primary caregiver completed a case history and both the parent and participant were invited to comment on adaptation to and experiences with the CI.
Data Analysis
For speech recognition measures, individual participant scores were compared between device conditions. A binomial model (34,35) was used for the CNC tests with significant differences defined as 0.05. For HINT in R-Space a critical difference of 1.4 dB was used based on the 95% confidence interval for this measure (33). A critical difference of 3.1 dB was used to identify significant differences in SNR for the BKB-SIN based on the 95% confidence interval for adult CI users with two list pairs (30). (Normative data are not currently available for pediatric CI recipients.) The slopes of fitted lines for individual localization responses at each loudspeaker for the HA-only and bimodal conditions were compared using Ordinary Least Squares regression with correction of standard errors for unequal variance between conditions.
Results
Implanted Soundfield Thresholds
CI detection thresholds were obtained in the soundfield. The mean three frequency average (.5, 1, 2 kHz) was 26.0 dB HL (SD = 3.7) and was substantially improved compared to pre-implant aided thresholds for the poorer hearing ear for each individual. All participants had good audibility through their cochlear implant, regardless of hearing history. The unaided and aided thresholds for the better ear were stable when compared to pre-implant levels.
CNC Words
Figure 2 displays post-implant results for CNC words at 50 dB SPL in quiet (top panel) and at 60 dB SPL with four-talker babble at +8 dB SNR (bottom panel). The scores in the three listening conditions are indicated by gray bars for CI-only, black bars for bimodal and white bars for HA-only. P1–P3 had open-set speech recognition in quiet and noise with the CI ear alone, with no significant difference between the CI-only and HA-only scores except for P1 in quiet. Bimodal scores were significantly better than either ear alone in quiet for P1–P3 and in noise for P3 (p < 0.05). P4 and P5, who were implanted in an ear with congenital SPHL, had no HA experience and only six months of CI experience, demonstrated no CI-only speech recognition. Their bimodal scores were similar to their HA-only scores in quiet and in noise.
Figure 2.
Individual participants’ CNC word scores are shown for the conditions CI-only (gray bars), bimodal (black bars) and HA-only (white bars). The top panel is for words presented at a soft level in quiet, the bottom panel is for words presented in noise. Asterisks denote statistically significant differences in scores between conditions for a given participant (p < 0.05).
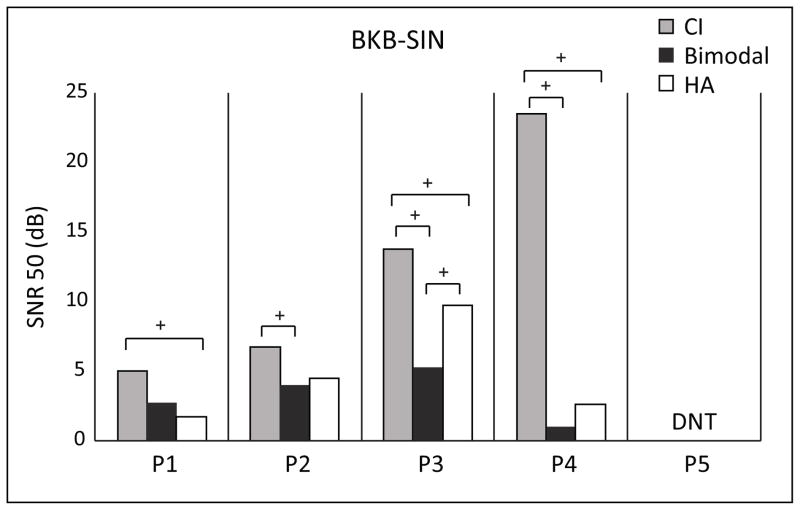
BKB SIN
Figure 3 provides SNR scores (lower scores reflect better performance) for the BKB-SIN at 65 dB SPL with speech and noise from the front. Due to family time constraints P5 was not tested on this measure. Although the bimodal condition resulted in the best score for P2, P3 and P4, only P3 had a significantly better (> 3.1 dB lower) bimodal score compared to either ear alone. P1–P4 had significantly worse scores (> 3.1 dB higher) in the CI only condition compared to the HA-only and/or bimodal conditions. P4 was unable to perform this task with the CI alone. The addition of the CI did not interfere with bimodal performance for any of these four participants; the bimodal score was similar to or better than the HA-only score.
Figure 3.
Individual participants’ BKB-SIN scores are shown for the conditions CI-only (gray bars), bimodal (black bars) and HA-only (white bars). Scores are expressed as an SNR with lower scores indicating better performance. The + symbols denote differences in scores greater than the 95% confidence interval between conditions for a given participant.
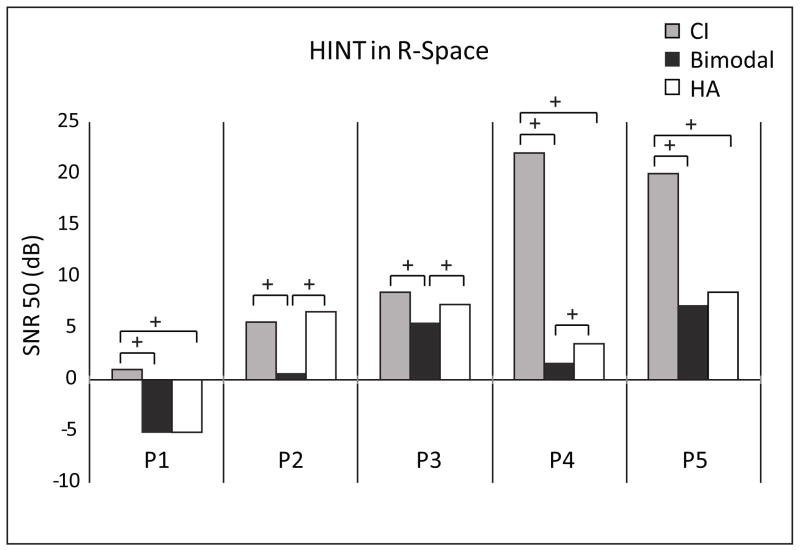
HINT in R-Space
Figure 4 displays SNR results for the HINT in R-Space. P1 had the best performance in noise, with −5.17 and −5.06 dB SNR in the bimodal and the HA-only conditions, respectively. P2, P3 and P4 showed significant improvement bimodally over either ear alone (> 1.4 dB lower). Note that P4 demonstrated bimodal improvement even with poor CI-only performance. P1 and P5 had similar performance in the bimodal and HA-only conditions.
Figure 4.
Individual participants’ HINT in R-Space scores in restaurant noise are shown for the conditions CI-only (gray bars), bimodal (black bars), and HA-only (white bars). Scores are expressed as an SNR. The + symbols denote differences in scores greater than the 95% confidence interval between conditions for a given participant.
Localization
Figure 5 plots individual participants’ localization results by speaker location for CI-only (square symbols), HA-only (triangles) and bimodal (circles). Symbols represent mean responses in degrees azimuth for each of the 10 source speakers and standard deviations are indicated with error bars. The X-axis represents location of the sound source and the Y-axis, the location of the response. Positive numbers reflect loudspeaker locations to the HA side and negative numbers reflect locations to the CI side of the listener. Perfect responses would lie along a diagonal line from the lower left-hand to upper right-hand corners of each plot. An overall root-mean-square (RMS) error score was calculated for each condition, with lower RMS scores indicating more accurate responses. This score is shown in the upper left-hand corner of each plot. A significant improvement in localization was identified in the bimodal compared to HA-only condition for P1, P2, and P3 (p < 0.001). P4 demonstrated a significant decrement in localization (p < 0.01) bimodally compared to HA-only. P5 was unable to localize regardless of condition.
Figure 5.
Individual participants’ localization results are shown for the conditions CI-only (filled squares, left panels), HA-only (filled triangles, center panels) and bimodal (filled circles, right panels). Symbols represent mean responses in degrees azimuth, error bars indicate one standard deviation. X-axis represents the location of the stimuli and the Y-axis, the location of the response. Positive numbers reflect loudspeaker locations to the HA side of the listener and negative numbers reflect those to the CI side. The RMS error for each condition is listed in the corner of each graph. Asterisks denote statistically significant difference in the slope of the line between conditions for a given participant (*** p < 0.001, ** p. < 0.01).
Discussion
All participants had CI-only audibility of 35 dB HL or better from .25 – 6 kHz, and HA-only audibility of 35 dB HL or better through 2 kHz (P5) or through 4 kHz (P1–P4). These findings differ from traditional bimodal users for whom the HA typically provides substantially less audibility than the cochlear implant in the higher pitches (20,36).
Three participants (P1–P3) had more favorable hearing histories in the implanted ear (e.g. sloping and progressive losses (P1 and P3), some aidable hearing across the frequency range (P2), all with HA use from a young age). P1–P3 achieved CI-only open-set speech understanding and demonstrated bimodal benefit on at least one speech recognition measure. Bimodal scores were comparable to or better than HA-only scores on all four speech recognition measures. In addition, these three participants had significantly improved localization bimodally compared to HA-only. Previous studies have investigated the question of whether CI patients would benefit from the addition of a contralateral HA (36,37 in adults, 38 in children). Although, the current study addressed a different question (whether some HA patients would benefit from the addition of a contralateral CI), the findings are similar; there is benefit to using a HA and CI in combination. Using a subset of the current study measures, Potts and colleagues (36) reported on 19 adult bimodal participants who had CI alone open-set word recognition; the results of P1–P3 who also had CI alone open-set word recognition are discussed in comparison.
For CNC words in quiet, the average scores for P1–P3 were considerably higher in the HA-only and bimodal conditions even with a lower presentation level (50 vs. 60 dB SPL). For the HA-only and bimodal conditions respectively, the three asymmetric adolescents had averages of 52% and 73% compared to 12% and 53% for the traditional bimodal adults. Average performance in the CI-only condition was similar, 38% for the three adolescents and 39% for the adults. The localization task in the current study was the same as that of Potts et al. (36), allowing for further comparison. As with speech recognition, the mean CI-only score for P1–P3 (RMS 56°) was similar to that of the adults (RMS 54°). For the HA-only and bimodal conditions the three adolescents’ mean RMS scores were 12° and 10° better than the means for the adults (61° and 39° respectively). Across measures, the better HA-only and bimodal performance for these participants compared to that of the bimodal adults reported by Potts et al. (36) is likely due to better hearing in the HA ear and suggests that audibility across the frequency range for both ears may be advantageous during bimodal device use.
Two participants (P4 and P5) had less favorable hearing histories (e.g., profound congenital hearing loss, had never worn a HA). P4 and P5 had no CI-only open-set speech understanding. Comparison of bimodal and HA-only results indicated that P5 had no bimodal benefit, but also had no bimodal decrement. P4 also had no bimodal benefit or decrement for three measures, with bimodal benefit observed for the HINT in R-Space, yet bimodal decrement noted with localization. These two participants had limited CI experience and it is common for implanted children with congenital SPHL to show progress well past the six-month post-implant interval (39,40,41). However, age at implantation is a significant factor for post-implant progress in the first ear (42,41), and although less defined, is also a factor when adding a second CI sometime after the first (43,44,45,46,47). The long-term prognosis for P4 and P5 is unclear and degree of improvement may differ for speech recognition and localization. Johnstone and colleagues (48) found length of time without auditory stimulation affected sound localization in children with aidable unilateral hearing loss (i.e. one normal hearing ear and one ear in the mild-moderate or moderate-severe hearing impaired range). Results showed that children ages 10–14 years who were fit with a HA by age seven demonstrated bilateral interference for sound localization with a HA in the impaired ear; children ages 6–9 years who were first aided by age five showed bilateral benefit for localization. In the asymmetric hearing loss study by Firszt and colleagues (27), none of the pre/perilingual adults demonstrated detriment (or benefit) with bimodal compared to HA-only localization. A larger longitudinal study of children with asymmetric hearing loss is needed to determine the impact of duration of deafness in this population.
All participants and their parents reported noticeable bimodal benefit, including P4 and P5. The participants described that listening was easier, especially in noise and at a distance. Parents reported greater ease of localization in everyday environments. It is possible that our test measures were not sensitive enough to capture the bimodal advantages reported for P4 and P5. In particular P4 and her mother felt strongly that localization abilities had improved even though the objective test measure showed a decrement. One possible reason for the discrepancy is that P4’s distance hearing may have improved with wear of both devices, and consequently interpreted as improved localization by the family.
During discussions with P1–P3, each reported they initially preferred the sound quality of their HA over the CI, but they adapted without difficulty to the combined signals after the first week. Clinical records indicated CI-only monosyllabic word understanding ≥30% by one month post-activation, with reports from teachers and caregivers that communication, socialization, and attention improved by three months post-CI. P4 and P5 had more difficulty adapting to the CI. For the first three months they reported a feeling sensation rather than hearing and interference from the new signal in certain situations, particularly noisy places (e.g. school cafeteria). By about six months this subsided; they reported the CI signal as sound and noisy environments as more tolerable.
In conclusion, some children with asymmetric hearing loss benefit from cochlear implantation of the poorer hearing ear, compared to listening with a HA in the better ear alone. The current pilot results suggest that later onset of SPHL and/or consistent HA use from a young age in the poorer ear with asymmetric loss results in improved outcomes following implantation, as evidenced by measures of speech recognition in quiet and noise and sound localization. It is encouraging that the two participants implanted in the poorer ear with congenital SPHL reported a positive experience from the addition of a CI, even though speech recognition was not demonstrated at an early interval of six months. While the effect of duration of deafness is better understood for traditional CI candidates (i.e., shorter length of deafness typically results in better outcomes), this relationship needs further study in the case of asymmetric hearing. In addition, a comparison of pre-implant bilateral HA performance to post-implant bimodal performance for a larger group of children is warranted to determine outcomes in this population.
Acknowledgments
We wish to thank the pediatric cochlear implant team at Saint Louis Children’s Hospital for recruitment efforts and coordination of clinical care with research appointments. We also acknowledge Dorina Kallogjeri, MD, MPH for statistical support. This work was supported by NIH/NIDCD R01DC009010 and ARRA Supplement, and Saint Louis Children’s Hospital Collaborative Faculty-Staff Research Grant. Dorina Kallogjeri was supported by the P30 Research Center for Auditory and Vestibular Studies and NIH/NIDCD P30DC04665.
Footnotes
Portions of this manuscript were presented at the 13th Symposium on Cochlear Implants in Children, July 14-16, 2011, Chicago, IL
References
- 1.Bess FH. The unilaterally hearing-impaired child: a final comment. Ear Hear. 1986;7(1):52–54. doi: 10.1097/00003446-198602000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Bovo R, Martini A, Agnoletto M, et al. Auditory and academic performance of children with unilateral hearing loss. Scand Audiol Suppl. 1988;30:71–74. [PubMed] [Google Scholar]
- 3.Ruscetta MN, Arjmand EM, Pratt SR. Speech recognition abilities in noise for children with severe-to-profound unilateral hearing impairment. Int J Pediatr Otorhinolaryngol. 2005 Jun;69(6):771–779. doi: 10.1016/j.ijporl.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Bess FH, Tharpe AM, Gibler AM. Auditory performance of children with unilateral sensorineural hearing loss. Ear Hear. 1986;7(1):20–26. doi: 10.1097/00003446-198602000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Culbertson JL, Gilbert LE. Children with unilateral sensorineural hearing loss: cognitive, academic, and social development. Ear Hear. 1986;7(1):38–42. doi: 10.1097/00003446-198602000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Oyler RF, Oyler AL, Matkin ND. Unilateral Hearing Loss: Demographics and Educational Impact. Lang Speech Hear Serv Sch. 1988 Apr;19(2):201–210. [Google Scholar]
- 7.Bess FH, Tharpe AM. Performance and management of children with unilateral sensorineural hearing loss. Scand Audiol Suppl. 1988;30:75–79. [PubMed] [Google Scholar]
- 8.Dancer J, Burl NT, Waters S. Effects of unilateral hearing loss on teacher responses to the SIFTER. Screening Instrument for Targeting Educational Risk. Am Ann Deaf. 1995 Jul;140(3):291–294. doi: 10.1353/aad.2012.0592. [DOI] [PubMed] [Google Scholar]
- 9.Lieu JE. Speech-language and educational consequences of unilateral hearing loss in children. Arch Otolaryngol Head Neck Surg. 2004 May;130(5):524–530. doi: 10.1001/archotol.130.5.524. [DOI] [PubMed] [Google Scholar]
- 10.Byrne D. Clinical issues and options in binaural hearing aid fitting. Ear Hear. 1981 Sep-Oct;2(5):187–193. doi: 10.1097/00003446-198109000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hattori H. Ear dominance for nonsense-syllable recognition ability in sensorineural hearing-impaired children: monaural versus binaural amplification. J Am Acad Audiol. 1993 Sep;4(5):319–330. [PubMed] [Google Scholar]
- 12.Kobler S, Rosenhall U. Horizontal localization and speech intelligibility with bilateral and unilateral hearing aid amplification. International journal of audiology. 2002 Oct;41(7):395–400. doi: 10.3109/14992020209090416. [DOI] [PubMed] [Google Scholar]
- 13.Noble W. Bilateral hearing aids a review of self-reports of benefit in comparison with unilateral fitting. Inter J of Audio. 2006;45(S1):S63–S71. doi: 10.1080/14992020600782873. [DOI] [PubMed] [Google Scholar]
- 14.Verschuur CA, Lutman ME, Ramsden R, Greenham P, O’Driscoll M. Auditory localization abilities in bilateral cochlear implant recipients. Otol Neurotol. 2005;26(5):965–971. doi: 10.1097/01.mao.0000185073.81070.07. [DOI] [PubMed] [Google Scholar]
- 15.Litovsky RY, Johnstone PM, Godar SP. Benefits of bilateral cochlear implants and/or hearing aids in children. Inter J of Audio. 2006;45(S1):S78–91. doi: 10.1080/14992020600782956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe J, Baker S, Caraway T, et al. 1-year postactivation results for sequentially implanted bilateral cochlear implant users. Otol Neurotol. 2007;28(5):589–596. doi: 10.1097/MAO.0b013e318067bd24. [DOI] [PubMed] [Google Scholar]
- 17.Scherf F, Van Deun L, Van Wieringen A, et al. Three-year postimplantation auditory outcomes in children with sequential bilateral cochlear implantation. Annals of Otology, Rhinology and Laryngology. 2009a;118(5):336–344. doi: 10.1177/000348940911800504. [DOI] [PubMed] [Google Scholar]
- 18.Scherf FW, van Deun L, van Wieringen A, et al. Functional outcome of sequential bilateral cochlear implantation in young children: 36 months postoperative results. Int J Pediatr Otorhinolaryngol. 2009b;73(5):723–730. doi: 10.1016/j.ijporl.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Kong YY, Stickney GS, Zeng FG. Speech and melody recognition in binaurally combined acoustic and electric hearing. J Acoust Soc Am. 2005 Mar;117(3 Pt 1):1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- 20.Ching TY, Incerti P, Hill M, van Wanrooy E. An overview of binaural advantages for children and adults who use binaural/bimodal hearing devices. Audiol Neurootol. 2006a;11 (Suppl 1):6–11. doi: 10.1159/000095607. [DOI] [PubMed] [Google Scholar]
- 21.Mok M, Galvin K, Dowell R, McKay C. Speech perception benefit for children with a cochlear implant and a hearing aid in opposite ears and children with bilateral cochlear implants. Audiol Neurotol. 2010;15(1):44–56. doi: 10.1159/000219487. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Spahr AJ, Dorman MF. Frequency overlap between electric and acoustic stimulation and speech-perception benefit in patients with combined electric and acoustic stimulation. Ear Hear. 2010 Apr;31(2):195–201. doi: 10.1097/AUD.0b013e3181c4758d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ching TYC, van Wanrooy E, Dillon H. Binaural-bimodal fitting or bilateral implantation for managing severe to profound deafness: a review. Trends in Amplification. 2007;11(3):161–192. doi: 10.1177/1084713807304357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firszt JB, Reeder RM, Skinner MW. Restoring hearing symmetry with two cochlear implants or one cochlear implant and a contralateral hearing aid. J Rehabil Res Dev. 2008;45:749–68. doi: 10.1682/jrrd.2007.08.0120. [DOI] [PubMed] [Google Scholar]
- 25.Johnston JC, Durieux-Smith A, Angus D, O’Connor A, Fitzpatrick E. Bilateral paediatric cochlear implants: a critical review. Int J Audiol. 2009;48(9):601–17. doi: 10.1080/14992020802665967. [DOI] [PubMed] [Google Scholar]
- 26.Sammeth CA, Bundy SM, Miller DA. Bimodal hearing or bilateral cochlear implants: a review of the research literature. Seminars in Hearing. 2011;32:3–31. [Google Scholar]
- 27.Firszt JB, Holden LK, Reeder RM, Cowdrey L, King S. Cochlear implantation in adults with asymmetric hearing loss. Ear Hear. 2012;33:521–533. doi: 10.1097/AUD.0b013e31824b9dfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glista D, Scollie S, Bagatto M, Seewald R, Parsa V, Johnson A. Evaluation of nonlinear frequency compression: Clinical outcomes. Int J of Audio. 2009;48:632–644. doi: 10.1080/14992020902971349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- 30.Etymotic Research. BKB-SIN Speech-in-Noise Test, Version 1.03. Elk Grove illage; IL: 2005. [Google Scholar]
- 31.Nilsson M, Soli SD, Sullivan JA. Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc A. 1994;95:1085–99. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- 32.Revit LJ, Schulein RB, Killion MC, Compton CL, Julstrom SD. Multi-channel sound-field system for assessing the real-world benefit of hearing aids. International Hearing Aid Research Conference; Lake Tahoe, CA. 2002. [Google Scholar]
- 33.Compton-Conley CL, Neuman AC, Killion MC, Levitt H. Performance of directional microphones for hearing aids: real-world versus simulation. J Am Acad Audiol. 2004 Jun;15(6):440–455. doi: 10.3766/jaaa.15.6.5. [DOI] [PubMed] [Google Scholar]
- 34.Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978 Sep;21(3):507–518. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- 35.Carney E, Schlauch RS. Critical Difference Table for Word Recognition Testing Derived Using Computer Simulation. J Speech Lang Hear Res. 2007 Oct;50(5):1203–1209. doi: 10.1044/1092-4388(2007/084). [DOI] [PubMed] [Google Scholar]
- 36.Potts LG, Skinner MW, Litovsky RA, Strube MJ, Kuk F. Recognition and localization of speech by adult cochlear implant recipients wearing a digital hearing aid in the nonimplanted ear (bimodal hearing) J Am Acad Audiol. 2009 Jun;20(6):353–373. doi: 10.3766/jaaa.20.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25(1):9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- 38.Ching T, van Wanrooy E, Hill M, Incerti P. Performance in children with hearing aids or cochlear implants: bilateral stimulation and binaural hearing. Inter J of Audiol. 2006b;45(S1):S108–S112. doi: 10.1080/14992020600783087. [DOI] [PubMed] [Google Scholar]
- 39.Fryauf-Bertschy H, Tyler RS, Kelsay DM, Gantz BJ, Woodworth GG. Cochlear implant use by prelingually deafened children: the influences of age at implant and length of device use. J Speech Lang Hear Res. 1997;40(1):183–199. doi: 10.1044/jslhr.4001.183. [DOI] [PubMed] [Google Scholar]
- 40.Kishon-Rabin L, Taitelbaum R, Muchnik C, Gehtler I, Kronenberg J, Hildesheimer M. Development of speech perception and production in children with cochlear implants. Ann Otol Rhinol Laryngol Suppl. 2002;189:85–90. doi: 10.1177/00034894021110s518. [DOI] [PubMed] [Google Scholar]
- 41.Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear Hear. 2003;24(1 Suppl):24S–35S. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- 42.Kirk KI, Miyamoto RT, Lento CL, Ying E, O’Neill T, Fears B. Effects of age at implantation in young children. Ann Otol Rhinol Laryngol Suppl. 2002;189:69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- 43.Sharma A, Gilley PM, Martin K, Roland P, Bauer P, Dorman M. Simultaneous versus sequential bilateral implantation in young children: Effects on central auditory system development and plasticity. Audiological Medicine. 2007;5(4):218– 223. [Google Scholar]
- 44.Peters BR, Litovsky R, Parkinson A, Lake J. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol. 2007 Aug;28(5):649–657. doi: 10.1097/01.mao.0000281807.89938.60. [DOI] [PubMed] [Google Scholar]
- 45.Gordon KA, Papsin BC. Benefits of short interimplant delays in children receiving bilateral cochlear implants. Otol Neurotol. 2009 Apr;30(3):319–331. doi: 10.1097/MAO.0b013e31819a8f4c. [DOI] [PubMed] [Google Scholar]
- 46.Graham J, Vickers D, Eyles J, et al. Bilateral sequential cochlear implantation in the congenitally deaf child: evidence to support the concept of a ‘critical age’ after which the second ear is less likely to provide an adequate level of speech perception on its own. Cochlear Implants Int. 2009 Sep;10(3):119–141. doi: 10.1179/cim.2009.10.3.119. [DOI] [PubMed] [Google Scholar]
- 47.Van Deun L, Van Wieringen A, Scherf F, et al. Earlier Intervention Leads to Better Sound Localization in Children with Bilateral Cochlear Implants. Audiol Neurootol. 2009;15(1):7–17. doi: 10.1159/000218358. [DOI] [PubMed] [Google Scholar]
- 48.Johnstone PM, Náblek AK, Robertson VS. Sound localization acuity in children with unilateral hearing loss who wear a hearing aid in the impaired ear. J Am Acad Audio. 2010 Sep;21(8):522–534. doi: 10.3766/jaaa.21.8.4. [DOI] [PubMed] [Google Scholar]