Abstract
Study Design
This study used retrograde neuronal tracing and immunohistochemistry to identify neurons innervating the C6/C7 facet joint and those expressing calcitonin gene-related peptide (CGRP) in the dorsal root ganglion (DRG) of rats after painful cervical facet joint injury.
Objective
The objective of this study was to characterize the innervation of the C6/C7 facet joint after painful joint injury in the rat.
Summary of Background Data
The cervical facet joint is a source of neck pain, and its loading can initiate persistent pain. CGRP is a nociceptive neurotransmitter; peptidergic afferents have been identified in the facet joint’s capsule. Although studies suggest that facet joint injury alters CGRP expression in joint afferents, the distribution of neurons innervating the C6/C7 facet joint and their expression of CGRP after a painful joint injury have not been investigated.
Methods
Holtzman rats received an intra-articular injection of cholera toxin subunit B (CTb) in the C6/C7 facet joints. After injection, subgroups underwent either a painful joint distraction or sham procedure. Mechanical sensitivity was assessed, and immunohistochemical techniques were utilized to quantify CGRP expression and CTb labeling in the C5-C8 DRGs.
Results
Facet joint distraction induced (p≤0.0002) hypersensitivity. Neurons labeled by the joint injection were identified in the C5-C8 DRGs. Significantly more (p≤0.0001) CTb-positive neurons were identified in the C7 DRG than any other level. At C7, 54.4±15.3% of those neurons were also CGRP-positive, whereas only 41.5±5.4% of all neurons were CGRP-positive; this difference was significant (p=0.0084).
Conclusions
The greatest number of afferents from the C6/C7 facet joint has cell bodies in the C7 DRG, implicating this level as the most relevant for pain from this joint. In addition, peptidergic afferents appear to have an important role in facet joint-mediated pain.
Keywords: facet, joint, pain, innervation, DRG, neuron, CGRP, retrograde labeling
INTRODUCTION
In the United States, neck and back syndromes are the most common cause of job-related disability, with annual costs exceeding $50 billion [1]. Neck injuries are reported in up to one in three rear-end motor vehicle collisions [2], and the cervical facet joint has been identified as the source of pain in as many as 67% of neck pain patients [3]. Anesthetic nerve blocks and radiofrequency neurotomy of the branches of the nerves innervating the facet joint provide pain relief [3,4,5], further demonstrating facet joint innervation to have a direct relationship to pain. The lower cervical facet joints are most commonly symptomatic after neck injury, and biomechanical studies identify the C6/C7 facet joint capsule to undergo the greatest strains in whiplash simulations [6,7,8,9], suggesting that joint to be the most relevant to injury-induced pain. Mechanical facet joint injury is sufficient to activate nociceptors in the joint [10,11] and to induce persistent pain [12,13,14] in animal models. The C5/C6 facet joint in the rat is multi-segmentally innervated, and the expression pattern of neuropeptides is altered in the joint afferents after transection of the capsular ligament [15]. Although the afferents innervating the cervical facet joint are suggested to be crucial to the maintenance of joint-mediated neck pain, the pattern of neurons innervating the C6/C7 facet joint is undefined, and little is known about the effects of injury to this joint.
Many pain mediators are upregulated in the DRG in response to joint inflammation and injury [12,16,17,18,19]. Specifically, the neuropeptide calcitonin gene-related peptide (CGRP), which is normally produced in 40% of the primary afferents [20], has been implicated as a contributor to joint pain and neuronal excitability [15,21,22,23,24] and is commonly used to identify peptidergic neurons [15,25,26]. Recent evidence suggests that some forms of pain may be mediated by specific subpopulations of primary sensory neurons [25,27] or by a change in the phenotype of peptidergic afferents [15,18,28,29]. Despite the association of peptidergic afferents and CGRP expression with joint pain, no study has investigated the relationship between CGRP expression in facet joint afferents and painful mechanical facet injury.
Distraction of the C6/C7 facet joint, as may occur during whiplash and other neck injuries, induces persistent pain, upregulates the neuropeptide substance P in the DRG and induces neuronal hyperexcitability in spinal neurons at day 7 in the rat [12,13,14,30,31]. Specifically, painful joint distraction upregulates substance P in the C7 DRG at day 7 after injury [31], which suggests peptidergic afferents at this spinal level have a particularly important role in joint-mediated pain. Although peptidergic fibers are identified in the human facet joint capsule [32,33], no study has defined the effect of a biomechanical and clinically-relevant painful C6/C7 facet injury on neuropeptide expression in joint afferents. The goal of this study was to identify the distribution of afferents that project to the C6/C7 facet joint after a painful joint distraction using neuronal tracing methods [15,34]. Because of the suspected contribution of peptidergic afferents at the C7 level to injury-induced pain, we also investigated the frequency of peptidergic neurons in that group of joint afferents as compared to all other neurons in the DRGs at the C7 level.
MATERIALS AND METHODS
Male Holtzman rats (Harlan Sprague-Dawley, Indianapolis, IN) (414±26g) were housed under USDA- and AAALAC-compliant conditions with a 12–12 hour light-dark cycle and free access to food and water. Experimental procedures were approved by our IACUC and carried out under the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [35].
All surgical procedures were performed under inhalation isoflurane anesthesia (4% induction, 2.5% maintenance). All rats received a bilateral intra-articular C6/C7 facet joint injection of 20μg of the retrograde neuronal tracing molecule cholera toxin subunit B (CTb) conjugated to the fluorescent dye Alexa Fluor 488 (Life Technologies; Carlsbad, CA) and dissolved in sterile PBS. A midline incision was made along the back of the neck, and the C6/C7 facet joints and their capsules were exposed. A 10μL syringe (Hamilton Company; Reno, NV) with a 33G beveled needle was advanced into the facet joint, and 5μL of the CTb solution was slowly injected. Following injection, the exposure was closed in layers using 3–0 polyester suture and surgical staples.
Three days after the CTb injection, a subset of rats underwent either a painful cervical facet joint distraction injury (n=4) or sham procedure (n=5), as described previously [12,16,36]. Under inhalation anesthesia, the surgical staples and suture were removed, and the C6/C7 facet joints were re-exposed. The interspinous ligaments and ligamentum flavum from C5 to T1 were transected, and the C6 and C7 laminae were rigidly attached to a customized loading device via microforceps. For the painful injury group, the bilateral C6/C7 facet joints were distracted by displacing the C6 vertebra rostrally while holding the C7 vertebra fixed [14,16,17,37]. A camera mounted to a surgical dissecting scope tracked markers on the C6 and C7 laminae during injury in order to quantify the distraction. An additional group of rats underwent sham surgical procedures with device attachment but no applied joint distraction. Following surgery, the incision was closed and rats were recovered. The remaining group of rats (normal, n=4) received no surgical procedures after the initial CTb injection.
Bilateral forepaw mechanical hyperalgesia was evaluated in those rats undergoing the painful joint injury or sham control procedure using previously validated methods [14,31,37]. Baseline measurements were recorded for 2 days after the CTb injection. Hyperalgesia was measured on days 1, 3, 5 and 7 after the injury or sham procedure. In each testing session rats were placed in elevated cages with a wire mesh floor and allowed to acclimate to the testing environment. Testing consisted of three rounds of mechanical stimulation to each forepaw using an ascending series of von Frey filaments (Stoelting; Wood Dale, IL). Each filament was applied five times with at least ten minutes separating each round of stimulation. Positive responses, defined as emphatic lifting of the forepaw, were used to determine the mechanical response threshold. A given filament was recorded as the response threshold if the next higher filament also induced a positive response. Because the applied joint distraction is a bilateral injury, response thresholds were averaged between the right and left forepaws for each rat. At each time point, response thresholds were compared between groups and to the respective baseline values using a two-way repeated measures ANOVA with Tukey’s HSD test, with time and group as the factors.
On day 7 after the injury or sham procedures, rats were given an overdose of sodium pentobarbital (65mg/kg) and perfused transcardially with 300mL of PBS and 250mL of 4% paraformaldehyde in PBS (pH7.4). The DRGs on the right side were harvested and post-fixed in the same fixative solution for 2.5 hours at 4°C. DRGs were then transferred to 30% sucrose for five days at 4°C before being embedded in Tissue-Tek® OCT Compound (Sakura Finetek; Torrance, CA). Each DRG was axially sectioned at a 14μm thickness through its entire length, and sections were thaw-mounted onto slides. All sections were washed and blocked with normal donkey serum (Chemicon; Temecula, CA) for two hours before incubation with a polyclonal rabbit anti-CGRP antibody (1:5000; T-4032; Peninsula Laboratories; San Carlos, CA) overnight at 4°C. The following day, sections were washed and incubated with a Cy3-conjugated donkey-anti-rabbit secondary antibody (1:500; Jackson ImmunoResearch; West Grove, PA) for two hours at room temperature and cover-slipped using Fluoro-Gel anti-fade medium (EMS; Hatfield, PA).
A fluorescent microscope equipped with a digital camera (Olympus; Center Valley, PA) was used to image each DRG section that contained at least one neuron positively labeled with CTb. The total number of neurons that were positive for CTb was counted for each DRG; care was taken to avoid double-counting neurons that appeared in multiple consecutive sections. The total number of CTb-positive neurons was summed for each group at each DRG level. Also, for those CTb-positive neurons, both the cross-sectional area and the intensity of CGRP labeling were quantified using ImageJ software (National Institutes of Health; Bethesda, MD). Each neuron was identified as being either CGRP-positive or CGRP-negative based on its intensity of CGRP labeling. The number of CTb-positive neurons also positively labeled for CGRP was counted at each level for each rat; the total number of those double-labeled neurons at each level was computed for each group. The average percentage of all CTb-positive neurons that were also positive for CGRP also was determined for each group in each DRG. Also, the average cross-sectional area of CTb-positive neurons expressing CGRP was determined. The number of CTb-positive neurons at each level was compared using a two-way ANOVA with Tukey’s HSD test, with group and level as the factors. A two-way ANOVA with Tukey’s HSD test (with group and level as factors) compared the average cross-sectional area of neurons positive for both CGRP and CTb; a separate two-way ANOVA with Tukey’s HSD test compared the ratio of CTb-positive neurons that were CGRP-positive to the total number of CTb-positive neurons. All statistical analyses were performed using JMP 8 (SAS Institute; Cary, NC) software.
To assess the frequency of peptidergic neurons among joint afferents compared to all neurons in the DRG at the C7 level, three sections were chosen from C7 at random from each rat by an evaluator who was blinded to the rat identifications and tissue samples. The cross-sectional area and CGRP labeling intensity of all neurons were quantified. All neurons in the C7 DRG were classified as either CGRP-positive or CGRP-negative. Both the ratio of CGRP-positive neurons to all neurons in each section and the average cross-sectional area of all CGRP-positive neurons in each section were determined. Separate comparisons of the ratio and the average cross-sectional area of CGRP-positive neurons were made between two populations of neurons in the C7 DRG: (1) joint afferents (those identified as CTb-positive neurons) and (2) all other neurons. Comparisons were tested using a two-way ANOVA with Tukey’s HSD and group (injury, sham, normal) and neuron population (CTb-positive neurons, all other neurons) as factors.
RESULTS
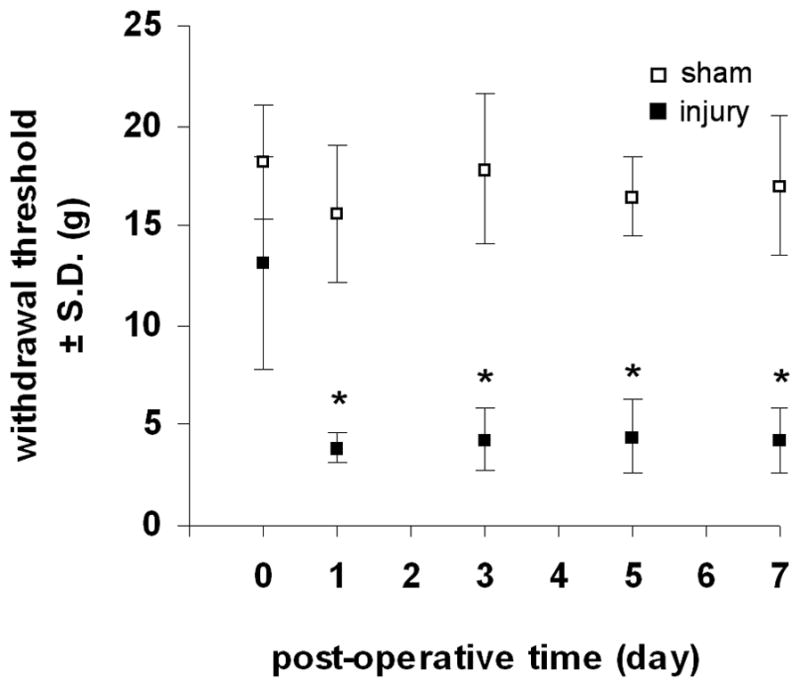
All rats undergoing a facet joint injury received the same magnitude of distraction, and no macroscopic injuries to the facet joint capsular ligament were observed during any of the applied distractions. The average applied distraction was 0.47±0.05mm. There were no differences in baseline withdrawal threshold between the injury and sham groups. Behavioral sensitivity was induced in all rats undergoing a joint distraction (Figure 1). The withdrawal threshold was significantly reduced (p≤0.001) compared to baseline responses in the injury group at all time points after distraction, but sham procedures did not change responses from baseline at any time point (Figure 1). The withdrawal threshold was significantly reduced (p≤0.0002) after injury compared to sham at all time points (Figure 1).
Figure 1.
Mechanical hyperalgesia in the forepaw as measured by the average±S.D. withdrawal threshold (g) elicited by von Frey filament stimulation. Forepaw hyperalgesia is induced after facet joint injury compared to baseline (p≤0.001) on all days but sham responses are unchanged from baseline. Withdrawal threshold in the injury group is significantly reduced (*p≤0.0002) compared to sham at each post-operative time point.
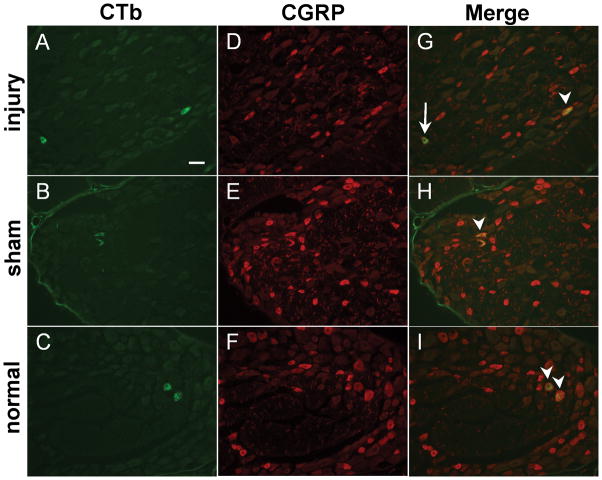
Neurons positive for CTb labeling were detected in all of the DRG levels that were assayed (Figures 2 & 3, Table 1). Significantly more (p≤0.0001) CTb-positive neurons were identified in the C7 DRG than any other DRG (Table 1). The C8 DRG contained significantly more (p≤0.0202) CTb-positive neurons than either of the C6 or C5 DRGs. Although C6 contained more CTb-positive neurons than C5 (Table 1), that difference was not significant. Although these trends were observed within each of the injury, sham, and normal groups, statistical significance was not achieved within each group individually; significance was only achieved when considering all groups together. Further, there were no differences in the number of CTb-positive neurons between groups at any of the cervical levels evaluated (Table 1). There were no significant differences detected in the ratio of CTb-positive neurons that were positive for CGRP to the total number of CTb-positive neurons between any groups at any level (Table 1). Similarly, there were no differences in the average cross-sectional area of the neurons positive for both CTb and CGRP between any groups at any level (Table 2).
Figure 2.
CTb (green) and CGRP (red) labeled neurons in the C7 DRG from injury (A,D,G), sham (B,E,H), and normal (C,F,I). (A–C) CTb-labeled neurons are detected in all groups. (D–F) CGRP-labeling identified peptidergic neurons in the C7 DRG. CTb co-localized with neurons that were both positive for CGRP labeling (arrowheads) and that did not express CGRP (arrow) (G–I). Scale bar in (A) is 50μm and applies to all panels. CTb, cholera toxin subunit B; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion.
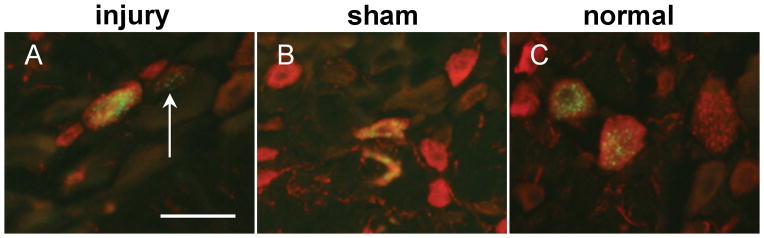
Figure 3.
Magnified view of merged CTb (green) and CGRP (red) labeled neurons from the C7 DRG from injury, sham, and normal rats showing punctate fluorescence of CTb within the cell body. In panel (A), the arrow indicates a CTb-labeled neuron that is not positive for CGRP labeling. Scale bar in (A) is 50μm. CTb, cholera toxin subunit B; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion.
Table 1.
Ratio and average percentages of CGRP-positive neurons that are also CTb-positive compared to the number of CTb-positive neurons.
| Ratio of peptidergic CTb-positive neurons* | |||||
|---|---|---|---|---|---|
| C5 | C6 | C7 | C8 | total | |
| injury (n=4 rats) | 2/3 | 6/11 | 35/64 | 19/31 | 62/109 |
| sham (n=5 rats) | 1/4 | 16/21 | 38/61 | 30/41 | 85/127 |
| normal (n=4 rats) | 2/6 | 15/27 | 37/83 | 33/45 | 87/161 |
| total (n=13 rats) | 5/13 | 37/59 | 110/208 | 82/117 | 234/397 |
| Average % of peptidergic CTb-positive neurons* | |||||
| C5 | C6 | C7 | C8 | total | |
| injury (n=4 rats) | 66.7±57.7% | 45.0±33.2% | 56.2±18.4% | 61.1±13.0% | 56.4±9.4% |
| sham (n=5 rats) | 25.0±35.4% | 78.4±25.5% | 59.8±14.7% | 69.4±43.3% | 66.9±18.3% |
| normal (n=4 rats) | 41.7±52.0% | 47.8±32.6% | 45.9±12.6% | 75.1±19.8% | 53.1±13.7% |
| total (n=13 rats) | 46.9±47.1% | 57.1±31.9% | 54.4±15.3%‡ | 68.6±28.3% | 59.4±14.8% |
Note: The denominator is the total number of CTb-positive neurons from all rats in each group for each DRG level; the numerator is the corresponding total number of neurons from all rats in each group that are positive for both CTb and CGRP. The percent values represent the average percent of CTb-positive neurons that are also CGRP-positive for each group and DRG level.
p=0.0084 compared to the percentage of CGRP-positive afferents of all neurons at C7.
CGRP, calcitonin gene-related peptide; CTb, cholera toxin subunit b.
Table 2.
Average cross-sectional area of neurons positive for both CTb and CGRP.
| Cross-Sectional Area (μm ± S.D.) | ||||
|---|---|---|---|---|
| C5 | C6 | C7 | C8 | |
| injury | 660±353 | 840±264 | 757±142 | 825±71 |
| sham | 741‡ | 732±220 | 688±73 | 802±189 |
| normal | 599±206 | 864±139 | 738±79 | 754±134 |
| total | 652±212 | 804±200 | 724±133* | 794±130 |
Only one neuron found.
p=0.0022 compared to all CGRP-positive neurons in the C7 DRG.
CGRP, calcitonin gene-related peptide; CTb, cholera toxin subunit b.
In the C7 DRG, 41.5±5.4% of all of the neurons were CGRP-positive. However, 54.4±15.3% of CTb-positive neurons at that level expressed CGRP (Table 1), and this difference in the ratios of CGRP-positive neurons between these two populations of neurons was significant (p=0.0084). This trend was also observed in each of the groups but was not significant for any of the groups. Interestingly, the average cross-sectional area of neurons positive for both CTb and CGRP at the C7 level (724±133μm2) was significantly smaller (p=0.0022) than the average area of all the CGRP-positive neurons in the C7 DRG (892±116μm2). Although this relationship was consistent for all of the experimental groups, the differences within each group were not significant.
DISCUSSION
These data characterize a multi-segmental innervation of the C6/C7 facet joint in the rat and demonstrate that the joint innervation is unchanged at day 7 after painful mechanical joint loading (Tables 1 & 2). The applied distraction of 0.47±0.05mm in the current study is in close agreement with a previously identified distraction magnitude (0.49±0.09mm) that was found to be sufficient to induce sustained behavioral sensitivity, while a lower magnitude of distraction (0.19±0.03mm) does not induce even transient mechanical sensitivity [16]. In that context, it is not likely that the joint distractions used in this study (~ 0.5mm) are induced by the normal head movements in the rat, though this has not been studied explicitly. Of the spinal levels analyzed, the greatest number of neurons with projections to the C6/C7 joint had cell bodies in the C7 DRG, followed by the C8, C6, and C5 DRGs (Table 1). This trend in the segmental joint innervation is maintained despite an injury-induced increase in sensitivity to mechanical stimulation of the forepaw (Table 1, Figure 1). Although painful injury does not alter the percentage of joint afferents expressing CGRP in the C7 DRG, greater than one-half of the joint afferents are peptidergic (Table 1), but only slightly more than 40% of all neurons in the C7 DRG are peptidergic. Further, the average cell body is smaller for the peptidergic joint afferents (724±133μm2, Table 2) than for all of the peptidergic neurons (892±116μm2) in the C7 DRG. Although a previous study defined the C5/C6 facet innervation with or without a complete disruption of its capsule [15], that study did not quantify pain. This is the first study to characterize the innervation of the C6/C7 facet joint in the context of injury-induced pain.
The distribution pattern of neurons innervating the C6/C7 facet joint identified here is consistent with studies characterizing innervation of other cervical facet joints in that joint afferents originate from multiple spinal levels with one level having a dominant number of neurons [15,38]. Indeed, multi-segmental innervation of facet joints is also evident in humans in which the lower cervical facets receive fibers from the medial branches of the dorsal rami above and below the joint [39]. The finding that the most C6/C7 joint afferents originate in C7 and C8 (Table 1) supports the observation of forepaw hypersensitivity (Figure 1) since the C7 and C8 dermatomes in the rat extend from the neck to the forepaw [40]. Further, neurons innervating lumbar facet joints have been identified with dichotomizing axons projecting to peripheral targets [34,41], suggesting that some neurons innervating the C6/C7 facet joint may also possess dichotomizing axons extending into the forelimb and contributing to referred pain. Studies using multiple retrograde tracers are necessary to determine the incidence of dichotomizing axons projecting to the C6/C7 facet joint and forepaw. Nevertheless, these data indicate that the C7 spinal level is likely a major contributor to C6/C7 facet joint-mediated pain.
Surprisingly, both the ratio of CGRP-positive joint afferents and their phenotype are unchanged by injury (Tables 1 & 2, Figure 2). This is surprising since several studies have identified a shift in the phenotypic expression of pain-associated proteins like CGRP and brain-derived neurotrophic factor towards larger-diameter afferents in response to facet inflammation or traumatic injury [15,18]. Despite the lack of change in the phenotype of joint afferents, injury-induced behavioral sensitivity may still result from afferent sensitization. While it is unlikely that the discs and other spinal ligaments contribute to pain in this model, previous work with this same injury model demonstrated that intra-articular injection of an NSAID abolishes facet joint injury-induced pain [16]. Combining that observation with the findings of the current study supports the contribution of joint afferents in pain after facet joint distraction. Yet, the subpopulations of joint afferents contributing to injury-induced pain still remain unknown. CGRP and substance P containing fibers have been identified in human cervical facet capsular ligaments [32,33], supporting the assertion that peptidergic afferents likely mediate pain in this joint. At C7 in the rat, CGRP-positive neurons account for a greater percentage of neurons innervating the C6/C7 joint than they do among all neurons in the C7 DRG. Taken together, these data indicate that peptidergic joint afferents may make a greater contribution to facet joint pain than other neuronal subpopulations. Future studies specifically investigating the roles of these and other populations of joint afferents in joint injury would determine their relative contributions to facet-mediated pain.
Although these data provide insight into the innervation of the C6/C7 facet joint in the rat from C5 to C8, additional spinal levels also may contain joint afferents. In fact, Ohtori et al. found that the C5/C6 facet joint in the rat contains fibers originating in the DRGs from C3-T3, although the vast majority originates in the cervical DRGs [15]. Nevertheless, the C6/C7 facet joint is likely innervated by additional neurons with cell bodies in the upper thoracic DRGs. Only the right DRGs were analyzed in this study, despite the application of a bilateral joint distraction; there is not expected to be differences based on sides since this injury is symmetric. Of note, CTb may not label all joint afferents because not all sensory neurons express the ganglioside GM1, to which CTb binds. Since the majority of sensory neurons (85% of small, 45% of medium, and 60% of large diameter neurons) do express GM1 [42] these findings likely represent the majority of neurons innervating the C6/C7 facet joint. However, it is possible that some neurons, especially among the larger myelinated neurons, may not be able to be labeled by CTb because GM1 is not universally expressed. The use of additional and distinct retrograde neuronal tracing agents would provide a more robust characterization of the full extent of the facet joint’s innervation. However, the majority of the nociceptive afferents are likely captured using the current technique. Further, although no visible leakage of the CTb solution from the facet joint was observed immediately after injection, a small amount may have leaked from the joint into the surrounding soft tissues. Nonetheless, any such leakage likely had only a minimal impact on the neuronal counts since the number of labeled neurons innervating the facet joint in our study is consistent with those reported in a study without joint injury in which cyanoacrylate was applied as a joint sealant [15].
This study identified no differences in the ratio or cross-sectional area of CGRP-positive joint afferents after injury; however, other peptides such as substance P may be differentially upregulated in these neurons. Previous work using this model identified increased substance P and the prostaglandin E2 receptor EP2 in the DRG after painful joint injury [31,36], supporting that additional targets may be upregulated by afferents after injury. The lack of change in the ratio and cross-sectional area of CGRP-positive joint afferents observed in this study after injury may be due to the small sample sizes. Indeed, a previous study by Ohtori et al. required nearly twice as many rats in each group to identify changes in the ratio and size of joint afferents expressing CGRP after a joint capsule transection compared to controls [15]. Additional studies including larger group sizes are necessary to verify our pilot studies finding that the ratio and cross-sectional area of the peptidergic joint afferents are unchanged by painful facet joint distraction. Despite these known injury-induced changes in the DRG, the specific roles of joint afferents in the generation and maintenance of facet-mediated pain are unknown. Regardless, by characterizing the segmental innervation of the C6/C7 facet joint, this study has identified those spinal levels most likely contributing to facet joint pain and provides direction for future studies investigating the cellular mechanisms underlying joint injury-induced pain.
KEY POINTS.
The C6/C7 facet joint in the rat is innervated by neurons from the C5-C8 DRGs.
The greatest number of neurons innervating the C6/C7 facet joint has cell bodies in the C7 DRG, followed by the C8, C6, and C5 DRGs, and this distribution is unchanged by painful facet joint injury.
At C7, the ratio of joint afferents that were peptidergic was significantly greater than the ratio of all neurons that were peptidergic at this level.
These findings suggest that peptidergic afferents in the C7 DRG play a major role in pain from the C6/C7 facet joint.
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). This project was supported by research funding by the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (#AR056288). No relevant financial activities outside the submitted work.
References
- 1.Strine TW, Hootman JM. US national prevalence and correlates of low back and neck pain among adults. Arthritis Rheum. 2007;57:656–65. doi: 10.1002/art.22684. [DOI] [PubMed] [Google Scholar]
- 2.Zuby DS, Lund AK. Preventing minor neck injuries in rear crashes – forty years of progress. J Occup Environ Med. 2010;52:428–33. doi: 10.1097/JOM.0b013e3181bb777c. [DOI] [PubMed] [Google Scholar]
- 3.Bogduk N. On cervical zygapophysial joint pain after whiplash. Spine. 2011;36:S194–9. doi: 10.1097/BRS.0b013e3182387f1d. [DOI] [PubMed] [Google Scholar]
- 4.Barnsley L, Lord S, Bogduk N. Whiplash injury. Pain. 1994;58:283–307. doi: 10.1016/0304-3959(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 5.Rambaransingh B, Stanford G, Burnham R. The effect of repeated zygapophysial joint radiofrequency neurotomy on pain, disability, and improvement duration. Pain Med. 2010;11:1343–7. doi: 10.1111/j.1526-4637.2010.00923.x. [DOI] [PubMed] [Google Scholar]
- 6.Barnsley L, Lord SM, Wallis BJ, et al. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20:20–5. doi: 10.1097/00007632-199501000-00004. discussion 26. [DOI] [PubMed] [Google Scholar]
- 7.Bogduk N, Aprill C. On the nature of neck pain, discography and cervical zygapophysial joint blocks. Pain. 1993;54:213–7. doi: 10.1016/0304-3959(93)90211-7. [DOI] [PubMed] [Google Scholar]
- 8.Panjabi MM, Cholewicki J, Nibu K, et al. Capsular ligament stretches during in vitro whiplash simulations. J Spinal Disord. 1998;11:227–32. [PubMed] [Google Scholar]
- 9.Pearson AM, Ivancic PC, Ito S, et al. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine. 2004;29:390–7. doi: 10.1097/01.brs.0000090836.50508.f7. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Lu Y, Kallakuri S, et al. Distribution of A-δ and C-fiber receptors in the cervical facet joint capsule and their response to stretch. J Bone Joint Surg Am. 2006;88:1807–16. doi: 10.2106/JBJS.E.00880. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Chen C, Kallakuri S, et al. Neurophysiological and biomechanical characterization of goat cervical facet joint capsules. J Orthop Res. 2005;23:779–87. doi: 10.1016/j.orthres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Dong L, Odeleye AO, Jordan-Sciutto KL, et al. Painful facet joint injury induces neuronal stress activation in the DRG: implications for cellular mechanisms of pain. Neurosci Lett. 2008;443:90–4. doi: 10.1016/j.neulet.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KE, Davis MB, Mejilla RM, et al. In vivo cervical facet capsule distraction: mechanical implications for whiplash & neck pain. Stapp Car Crash J. 2004;48:373–95. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- 14.Quinn KP, Dong L, Golder FJ, et al. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–21. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtori S, Takahashi K, Moriya H. Calcitonin gene-related peptide immunoreactive DRG neurons innervating the cervical facet joints show phenotypic switch in cervical facet injury in rats. Eur Spine J. 2003;12:211–5. doi: 10.1007/s00586-002-0506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong L, Guarino BB, Jordan-Sciutto KL, et al. Activating transcription factor 4, a mediator of the integrated stress response, is increased in the dorsal root ganglia following painful facet joint distraction. Neuroscience. 2011;193:377–86. doi: 10.1016/j.neuroscience.2011.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25:1383–93. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 18.Ohtori S, Takahashi K, Moriya H. Inflammatory pain mediated by a phenotypic switch in brain-derived neurotrophic factor-immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints in rats. Neurosci Lett. 2002;323:129–32. doi: 10.1016/s0304-3940(02)00120-9. [DOI] [PubMed] [Google Scholar]
- 19.Tachihara H, Kikuchi S, Konno S, et al. Does facet joint inflammation induce radiculopathy?: an investigation using a rat model of lumbar facet joint inflammation. Spine. 2007;32:406–12. doi: 10.1097/01.brs.0000255094.08805.2f. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y, Takami K, Kawai Y, et al. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985;15:1227–37. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- 21.Iwakura N, Ohtori S, Orita S, et al. Role of low-affinity nerve growth factor receptor inhibitory antibody in reducing pain behavior and calcitonin gene-related peptide expression in a rat model of wrist joint inflammatory pain. J Hand Surg Am. 2010;35:267–73. doi: 10.1016/j.jhsa.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Orita S, Ishikawa T, Miyagi M, et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011;12:134. doi: 10.1186/1471-2474-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staton PC, Wilson AW, Bountra C, et al. Changes in dorsal root ganglion CGRP expression in a chronic inflammatory model of rat knee joint: differential modulation by rofecoxib and paracetamol. Eur J Pain. 2007;11:283–9. doi: 10.1016/j.ejpain.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Sun R, Tu Y, Lawand NB, et al. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–66. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165:896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–26. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michael GJ, Averill S, Shortland PJ, et al. Axotomy results in major changes in BDNF expression by dorsal root ganglion cells: BDNF expression in large trkB and trkC cells, in pericellular baskets, and in projections to deep dorsal horn and dorsal column nuclei. Eur J Neurosci. 1999;11:3539–51. doi: 10.1046/j.1460-9568.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou XF, Chie ET, Deng YS, et al. Injured primary sensory neurons switch phenotype for brain-derived neurotrophic factor in the rat. Neuroscience. 1999;92:841–53. doi: 10.1016/s0306-4522(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 30.Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J Neurotrauma. 2010;27:163–74. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10:436–45. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Kallakuri S, Singh A, Chen C, et al. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9. 5 containing nerve fibers in human cervical facet joint capsules. Spine. 2004;29:1182–6. doi: 10.1097/00007632-200406010-00005. [DOI] [PubMed] [Google Scholar]
- 33.Kallakuri S, Li Y, Chen C, et al. Innervation of cervical ventral facet joint capsule: histological evidence. World J Orthop. 2012;3:10–4. doi: 10.5312/wjo.v3.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sameda H, Takahashi Y, Takahashi K, et al. Primary sensory neurons with dichotomizing axons projecting to the facet joint and the sciatic nerve in rats. Spine. 2001;26:1105–9. doi: 10.1097/00007632-200105150-00003. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 36.Kras JV, Dong L, Winkelstein BA. The prostaglandin E2 receptor, EP2, is upregulated in the DRG after painful cervical facet joint injury in the rat. Spine. 2012 doi: 10.1097/BRS.0b013e3182685ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisshaar CL, Dong L, Bowman AS, et al. Metabotropic glutamate receptor-5 and protein kinase C-epsilon increase in dorsal root ganglion neurons and spinal glia activation in an adolescent rat model of painful neck injury. J Neurotrauma. 2010;27:2261–71. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- 38.Ohtori S, Takahashi K, Chiba T, et al. Sensory innervation of the cervical facet joints in rats. Spine. 2001;26:147–50. doi: 10.1097/00007632-200101150-00007. [DOI] [PubMed] [Google Scholar]
- 39.Barnsley L, Bogduk N. Medial branch blocks are specific for the diagnosis of cervical zygapophyseal joint pain. Reg Anesth. 1993;18:343–50. [PubMed] [Google Scholar]
- 40.Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67:197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- 41.Umimura T, Miyagi M, Ishikawa T, et al. Investigation of dichotomizing sensory nerve fibers projecting to the lumbar multifidus muscles and intervertebral disc or facet joint or sacroiliac joint in rats. Spine. 2012;37:557–62. doi: 10.1097/BRS.0b013e3182293178. [DOI] [PubMed] [Google Scholar]
- 42.Gong Y, Tagawa Y, Lunn MPT, et al. Localization of major gangliosides in the PNS: implications for immune neuropathies. Brain. 2002;125:2491–506. doi: 10.1093/brain/awf258. [DOI] [PubMed] [Google Scholar]