Abstract
Objective
Associations between within-person changes in ovarian hormones and dysregulated eating (binge eating, emotional eating) have been observed across the menstrual cycle. However, studies have not examined moderators that may contribute to differential associations between individuals. We investigated body-weight regulation variables (body mass index (BMI), dietary restraint) that have theoretical relevance by virtue of their associations with both phenotypes.
Method
Women (N = 196) provided emotional eating ratings and saliva samples for 45 days. BMI and restraint were assessed at three time-points and averaged.
Results
Results showed significant estradiol × progesterone interactions in the prediction of within-subject changes in emotional eating. Neither BMI nor restraint moderated these relationships, although a trend-level dietary restraint × estradiol interaction was observed where estradiol’s effects were enhanced in high restraint scorers.
Conclusions
Findings confirm a role for hormones in changes in emotional eating and suggest that restraint might enhance hormone effects in severe groups.
Emerging data suggest significant associations between within-person changes in ovarian hormones and changes in dysregulated eating across the menstrual cycle. Binge eating and emotional eating reach their peak during the mid-luteal phase of the cycle (1–4.) and are predicted by within-person changes in estrogen and progesterone (1–3.). Early work suggested that low levels of estradiol, and high levels of progesterone, predict increased binge eating across the menstrual cycle in women with bulimia nervosa (BN) (1; 4.) and from the community (2–3.). However, these studies did not examine interactions between estradiol and progesterone, despite animal studies demonstrating significant interactions between the hormones (with progesterone antagonizing the effects of estrogen) for food intake (5–6.).
In the largest sample of women studied to date, our group found significant interactions between estrogen and progesterone, where emotional eating scores were highest when both estradiol and progesterone levels were elevated (3.). These interactions seemed to account for mid-luteal increases in dysregulated eating, as progesterone reaches its peak during the mid-luteal phase and estrogen exhibits a secondary peak as well. Importantly, we accounted for within-person, menstrual cycle changes in body mass index (BMI) and negative affect in all analyses; although changes in BMI and negative affect significantly predicted changes in emotional eating across the menstrual cycle, changes in hormones remained significant predictors of emotional eating, even after controlling for the effects of these covariates. Finally, the direction of associations was from the changes in hormones to the changes in emotional eating (rather than the reverse) (3.). Data from our studies (2–3.) and those of others (1; 4.) suggest that these within-person hormone/dysregulated eating associations are present across the spectrum of binge eating tendencies in women with BN (1; 4.) and women from the community (2–3.).
Although these studies have been helpful for identifying female-specific, biological risk factors for changes in dysregulated eating across the menstrual cycle, they have not identified specific conditions under which hormonal changes increase risk for dysregulated eating. Identifying potential, between-subject moderators of hormone/dysregulated eating relationships could significantly increase understanding of the multitude of factors contributing to menstrual cycle changes in dysregulated eating.
A particularly important set of moderators to examine are those related to body weight regulation, including body mass index (BMI) and dietary restraint (i.e., behavioral and/or cognitive attempts to restrict food intake). Both risk factors predict the development and maintenance of dysregulated eating (i.e., binge eating, emotional eating). For example, higher BMI and higher dietary restraint scores predict binge eating (7–12.) and emotional eating (13–18.). Although dietary restraint measures were originally conceptualized as assessing actual dieting behaviors (e.g., decreased caloric content), recent research indicates that self-reported dietary restraint may capture an “intent to diet” rather than pure caloric restriction per se, as actual food intake does not always map on to self-reports of dietary restraint (19–20.).
Importantly, although within-subject hormone/dysregulated eating associations cannot be accounted for by changes in BMI across the menstrual cycle (i.e., they are not mediated by BMI changes; see above), it remains unknown whether between-subject differences in BMI and/or dietary restraint change the magnitude of associations between within-person changes in hormones and dysregulated eating. These variables could strengthen associations between within-person changes in hormones and dysregulated eating by increasing the risk for dysregulated eating in the presence of vulnerable hormone environments.
Alternatively, it is possible that these between-subject variables could attenuate hormone/dysregulated eating associations through their effects on ovarian hormone levels. Higher BMI has been associated with elevated hormone levels (particularly estrogen) (21–22.), while lower BMI and sustained dieting can lead to decreased hormone levels (23.). Both types of effects attenuate within-person changes in hormones across the menstrual cycle (23–24.), as they can lead to chronically high or low hormone levels, regardless of menstrual cycle phase. In that case, we also would expect attenuated changes in dysregulated eating and decreased associations between within-person changes in both variables.
In order to address both of these possibilities, we examined the moderating effects of BMI and dietary restraint on associations between within-person changes in hormones and emotional eating across the menstrual cycle in the large, community-based sample of women described in Klump et al. (3.). As in our previous analyses, we controlled for within-subject changes in negative affect in all analyses, as negative affect is a potent predictor of within-person changes in emotional eating (25.) and is also related to both of the moderators examined herein.
Methods
Participants
Participants were 196 (110 monozygotic (MZ), 86 dizygotic (DZ)) female twins ages 16–22 drawn from the Twin Study of Hormones and Behavior across the Menstrual Cycle project from the Michigan State University Twin Registry (MSUTR; N ~ 19,000 twins) (26.). The MSUTR recruits twins through birth records using methods described previously (26.). Prior (27.) and current analyses indicate that MSUTR twins are demographically representative of the recruitment region (89% Caucasian; 11% African American).
Inclusion/exclusion criteria ensured that we captured natural variations in hormones: 1) menstruation every 22–32 days for the past 6 months; 2) no hormonal contraceptive use in the past 3 months; 3) no psychotropic or steroid medications within the past 4 weeks; 3) no pregnancy or lactation within the past 6 months; and 4) no history of genetic or medical conditions known to influence hormone functioning or appetite/weight. Despite these criteria, BMI, emotional eating scores, and restraint scores did not differ between our participants and other MSUTR twins (d‘s = .02–.18).
Procedures
The larger data collection included behavioral and hormone measures that were collected daily across a 45-day study period, as well as repeated measures that were collected during three in-person study visits. The daily measures included ratings of emotional eating, negative affect, and the collection of salivary samples for ovarian hormones. Salivary samples were collected every morning within 30 minutes of waking (see Klump et al., 2008 (2.)) for a detailed description of methods), while daily ratings of emotional eating and negative affect were made each evening (after 5:00 pm). The timing of data collection ensured that a given day’s hormone collection preceded that day’s behavioral ratings.
In addition to the daily measures, three in-person visits occurred at the start of the study (i.e., “intake” session), mid-way through data collection (~day 23, “intermediate” session), and at the end of data collection (~day 45, “final” session). Each in-person visit included assessments of BMI and dietary restraint (see below) as well as a re-assessment of study eligibility and the collection of all samples. Between visits, staff contacted participants 1x/week to answer questions and confirm protocol adherence. These procedures were effective for minimizing drop-outs (3%) and missing data (≤ 6%) and identifying twins who were no longer eligible (3% due to pregnancy or medication use).
Measures
Sample characteristics on all study measures are presented in Table 1. Although we used daily data for emotional eating, hormones, and negative affect, we present average values across the data collection period for all variables in order to characterize the sample and allow for comparisons of sample composition in future work.
Table 1.
Sample Characteristics.
| Variable | Mean (SD) | Range in Sample |
|---|---|---|
| Age | 17.84 (1.58) | 16–22 |
| Body Mass Index (BMI) | 23.98 (5.63) | 16.71–46.78 |
| Dietary Restraint | 0.57 (0.82) | 0–4 |
| Emotional Eating | 0.34 (0.43) | 0–3 |
| Estradiol (pg/ml) | 3.19 (0.03) | 0.95–10.63 |
| Progesterone (pg/ml) | 119.05 (63.21) | 22.41–368.00 |
| Negative Affect | 15.09 (4.06) | 10–35 |
Note. Values for BMI and dietary restraint are average values across the three in-person assessments. Values for emotional eating, estradiol, progesterone, and negative affect are average values across the 45-day data collection period.
Daily Measures of Emotional Eating, Hormones, and Negative Affect
Emotional Eating
Emotional eating was assessed with the Dutch Eating Behavior Questionnaire (DEBQ) (17.). The DEBQ emotional eating subscale assesses the tendency to eat excessive amounts of food for reasons that are typically endorsed by individuals who experience recurrent binge-eating episodes (e.g., “Did you have a desire to eat when you were discouraged?”). This scale was used in the previous community study of ovarian hormones/binge eating (2.) because it exhibits robust fluctuations across the menstrual cycle (2.) and is significantly correlated with traditional binge measures (e.g., r’s = .55 – .69) (28–29.)as well as palatable food intake (e.g., ice cream) in the laboratory (30.). Subscale scores also successfully discriminate among normal controls, overweight women, and bulimic patients (31.). Similar to previous research (2.), instructions were modified with permission to refer to that day (i.e., “true in relation to you TODAY”). Internal consistency of our modified version was excellent (average α = .90).
Hormones
Hormone concentrations were measured in saliva. Saliva collection is a non-invasive method for repeated sampling schedules that is associated with higher compliance and more robust hormone-behavior associations than bloodspots (1.).
Salivary assays were conducted by Salimetrics, LLC (State College, PA) using enzyme immunoassay kits that show excellent intra- and inter-assay coefficients of variation (estradiol = 7.1 % and 7.5%; progesterone = 6.2% and 7.6%), assay sensitivity (determined by interpolating the mean optical density minus 2 SDs of 10–20 replicates at the 0 pg/mL level; estradiol = 0.10 pg/mL; progesterone = 5 pg/mL) and method accuracy (determined by spike recovery and linearity; estradiol = 104.2% and 99.4%; progesterone = 99.6% and 91.8%). In order to capture key periods of hormonal change (e.g., mid-late follicular through the premenstrual phase) while maximizing the number of subjects assessed, we assayed samples every other day during menstrual bleeding and the early follicular phase when hormone levels were expected to be low and stable.
Negative Affect
The Negative Affect scale from the Positive and Negative Affect Schedule (32.) was used to measure negative affect (i.e., depression and anxiety) each day. This scale exhibits good convergent and discriminant validity (32.) and excellent internal consistency (average α = .85 in our sample).
BMI and Dietary Restraint
As noted above, BMI and dietary restraint were collected at the intake, intermediate, and final in-person assessment sessions only. Because we were interested in examining how stable, between-subject differences in these variables could moderate within-person associations between hormones and emotional eating, we calculated the average BMI and average dietary restraint score across the three assessment periods and used these trait levels in analyses. This approach was supported by high correlations across the three assessment periods for both variables (BMI r’s > .99; dietary restraint r’s = .70–.81).
Body Mass Index (BMI)
BMI (weight (in kilograms)/height (in meters)2) was calculated using height and weight measured with a wall-mounted ruler and digital scale, respectively.
Dietary Restraint
The Restraint subscale from the Eating Disorders Examination Questionnaire (EDE-Q) (33.) was used to assess dietary restraint in all participants. This subscale assesses attempts to restrain eating, regardless of actual success, and has demonstrated excellent internal consistency (33–35.) and convergent validity with the restraint subscale of the EDE interview (33; 35–36.).
Statistical Analyses
Data Preparation
We standardized our between-subject moderators (i.e., BMI, dietary restraint score) by the overall sample mean and standard deviation prior to analysis to aid in interpretation of moderation effects. Similar to past studies (1–2.) and our previous analysis of this dataset (3.), we calculated five-day rolling averages for the within-subject, daily measures (i.e., hormones, emotional eating, and negative affect) to reduce the influence of hormone pulsatility and increase the signal to noise ratio (37–39.). These averages were then converted into within-person Z scores based upon each participant’s overall mean and standard deviation across data collection. Within-person, standardized values are ideal because they index the extent to which changes in a woman’s hormones, relative to her equilibrium, predict changes away from her equilibrium in emotional eating (1–2; 4; 37.). They also tend to be sensitive to small deviations from equilibria, making them ideal indicators of how within-person changes in hormones predict changes in emotional eating in non-clinical samples of women.
Missing daily data were handled two ways. We prorated raw scores for all behavioral measures (e.g., emotional eating, dietary restraint, and negative affect) when ≤10% of the items were missing, and coded the scores as missing if > 10% of items were missing. For the daily measures data (e.g., hormones, emotional eating, negative affect), we calculated the rolling averages if ≥ 3 days of data were available within the 5-day window. If fewer than 3 days were available, then the average for that day was coded as missing (occurring < 2.6% of the time).
Moderation
We used mixed linear models (MLMs) to examine the extent to which average BMI and dietary restraint moderated associations between within-subject changes in hormones and emotional eating across the menstrual cycle. MLMs are ideal for these analyses as we can examine moderating effects while controlling for negative affect and the non-independence of the repeated measures and twin data. We controlled for the non-independence of the twin data by estimating random intercepts and allowing them to correlate.1 Because most co-twins were assessed across the same 45-day period, we also estimated a time-specific dyadic correlation that allowed the twins’ residual scores for emotional eating to correlate from day-to-day. For each of these random effects, we specified a compound symmetry covariance structure which estimates a single intercept variance or a single residual variance across twins and time. The models also allowed for random slopes for each of the repeated measures predictors (i.e., hormones, negative affect). However, because there was no evidence that these slopes correlated across twins, we fixed the cross-twin correlation to zero for all predictors.
Because we were interested in average BMI and dietary restraint as between-subject moderators of hormone/emotional eating associations, our models focused on testing the main effects of estradiol and progesterone, as well as all two-way (i.e., moderator × estradiol; moderator × progesterone, estradiol × progesterone) and three-way (moderator × estradiol × progesterone) interactions. A significant two- or three-way interaction between the moderator and hormone levels would indicate that hormone-behavior associations differ according to the moderator level (e.g., that hormone-behavior associations are stronger in individuals with higher BMIs).
Results
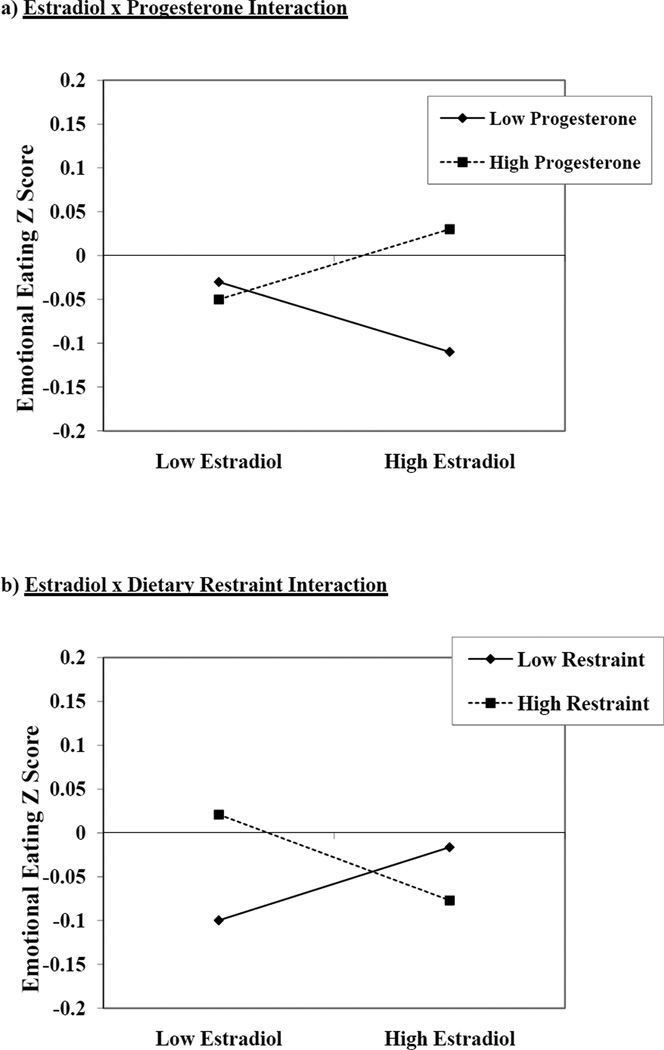
As shown in Table 2, the strongest predictor of emotional eating scores was the estradiol × progesterone interaction. The nature of this interaction in both models (see Figure 1a for an example in the dietary restraint model) was nearly identical to that observed in our prior analysis where higher levels of both estradiol and progesterone (which characterize the mid-luteal phase of the menstrual cycle) were associated with increased emotional eating (3.).
Table 2.
The Moderating Effects of Body Mass Index (BMI) and Dietary Restraint on Within-Person Associations between Ovarian Hormones and Emotional Eating.
| Model | b (SE) | t | df | p |
|---|---|---|---|---|
| Body Max Index (BMI) as a Moderator | ||||
| Intercept | −.04 (.01) | −3.39 | 177 | .001 |
| Estradiol | −.01 (.02) | −0.63 | 215 | .53 |
| Progesterone | .04 (.02) | 1.77 | 213 | .08 |
| Negative Affect | .18 (.03) | 7.17 | 211 | <.001 |
| BMI | .01 (.01) | 0.83 | 176 | .41 |
| BMI × Estradiol | .004 (.02) | 0.16 | 211 | .87 |
| BMI × Progesterone | −.02 (.02) | −1.10 | 210 | .27 |
| Estradiol × Progesterone | .05 (.02) | 2.85 | 202 | .005 |
| BMI × Estradiol × Progesterone | .008 (.02) | 0.44 | 179 | .66 |
| Dietary Restraint as a Moderator | ||||
| Intercept | −.04 (.01) | −3.31 | 161 | .001 |
| Estradiol | −.004 (.02) | −.15 | 193 | .88 |
| Progesterone | .04 (.02) | 1.85 | 193 | .07 |
| Negative Affect | .17 (.02) | 6.54 | 191 | <.001 |
| Restraint | .02 (.01) | 1.18 | 160 | .24 |
| Restraint × Estradiol | −.04 (.02) | −1.88 | 193 | .07 |
| Restraint × Progesterone | .02 (.02) | 1.09 | 185 | .28 |
| Estradiol × Progesterone | .05 (.02) | 2.53 | 185 | .012 |
| Restraint × Estradiol × Progesterone | .005 (.02) | 0.26 | 205 | .79 |
Figure 1.
Interactions between Estradiol and Progesterone, and Estradiol and Dietary Restraint, in the Dietary Restraint Model. “Emotional Eating Z Score” = 5-day rolling average calculated within subjects, then averaged across participants.
Importantly, the main effects of average BMI and restraint, and the two- and three-way interactions between the moderators and ovarian hormones, were all non-significant. These findings indicate that: 1) unlike within-subject changes in BMI (see Introduction and Klump et al, in press), between-subject differences in BMI and dietary restraint do not significantly predict within-subject changes in emotional eating across the menstrual cycle; and, most importantly 2) between-subject differences in BMI and levels of dietary restraint also do not affect the strength of associations between within-subject changes in ovarian hormones and emotional eating across the menstrual cycle.2
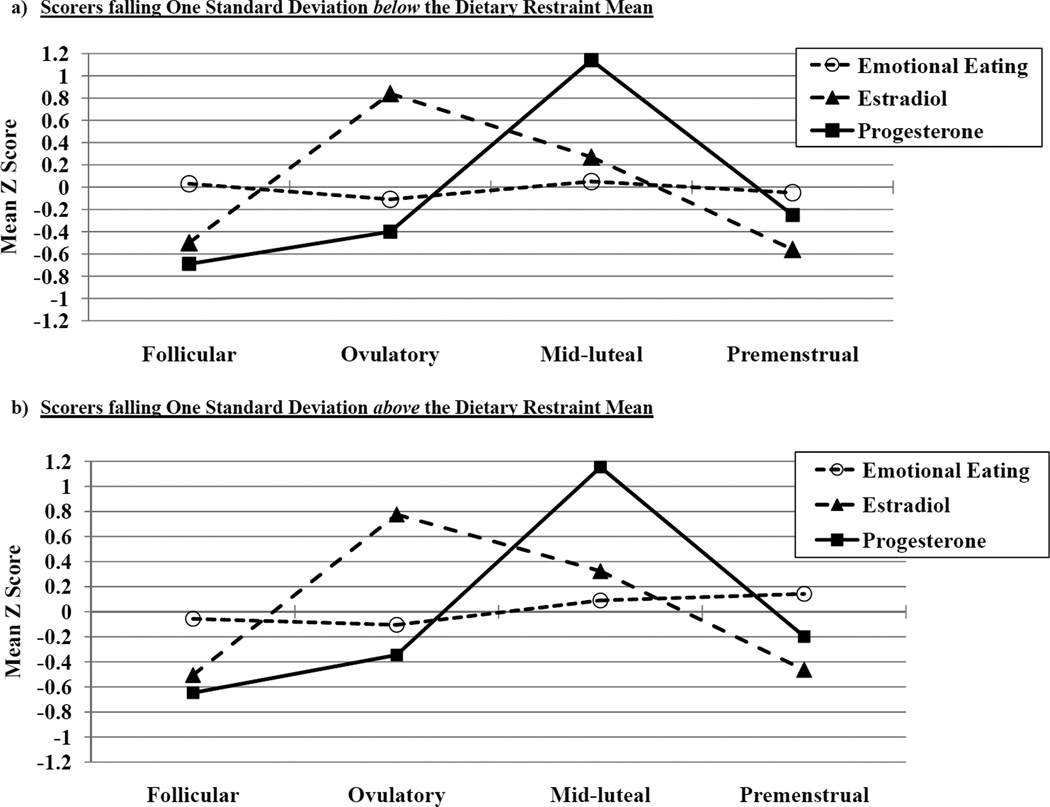
However, there was a trend-level interaction (p = .07) between dietary restraint and estradiol, where the highest levels of emotional eating were observed at low estradiol levels (see Figure 1b) This interaction may seem contradictory or at odds with the significant estradiol × progesterone interaction, as one interaction suggests that emotional eating is highest at low estradiol levels (dependent upon dietary restraint), while the other suggests that emotional eating is highest at high estradiol levels (dependent upon progesterone). These findings can be reconciled when one considers the context for the changes, i.e., the menstrual cycle. Figure 2 includes trajectories of emotional eating, estradiol, and progesterone across menstrual cycle phase (i.e., follicular, ovulatory, mid-luteal, and premenstrual) in women who scored one standard deviation below (N = 51; see Figure 2a) or above (N = 25; see Figure 2b) the mean dietary restraint score in the sample. Similar to previous studies (1–4.), peaks in emotional eating scores were observed in the mid-luteal phase and, to lesser extent, in the follicular phase in low restraint scorers. This pattern maps on well to the estradiol × progesterone interaction in Figure 1a, as the mid-luteal phase is characterized by the highest levels of progesterone and the second highest levels of estradiol of the cycle.
Figure 2.
Trajectories of Emotional Eating Scores, Estradiol Levels, and Progesterone Levels across Menstrual Cycle Phases in Scorers falling a) One Standard Deviation below the Dietary Restraint Mean; and b) One Standard Deviation above the Dietary Restraint Mean. Mean Z Score = the mean of the 5-day rolling averages calculated within subjects, then averaged across participants. The number of days included in each phase varied by participant based on their cycle length, but the days roughly corresponded to the following (first day of menstrual bleeding = +1; previous day = −1): Follicular = +3 to +12; Ovulatory = −15 to −12; Mid-luteal = −9 to −5; Premenstrual = −3 to +1.
By contrast, in the high restraint group, emotional eating scores were elevated during both the mid-luteal and the premenstrual phase. The premenstrual phase is characterized by low levels of estradiol and progesterone and has been shown in previous work to be associated with higher levels of binge eating in women with BN (1; 4; 37.). Our findings suggest that elevated levels of dysregulated eating during the premenstrual phase in some (i.e., samples with BN) but not all (e.g., unselected, community samples) samples may be due to increased levels of dietary restraint and the interaction between restraint and low levels of estradiol. Given that progesterone tends to be positively associated with emotional eating scores, it is not surprising that no significant interactions were observed between restraint and progesterone, as progesterone levels are low during the premenstrual phase.
Discussion
Our findings extend previous work by examining moderators of associations between within-person changes in ovarian hormones and emotional eating across the menstrual cycle. Overall, results suggest that hormone/dysregulated eating associations are robust to between-subject differences in trait-levels of BMI and dietary restraint, as analyses showed no significant interactions between these variables and ovarian hormones. Our prior analyses had suggested that hormone/behavior associations were not mediated by changes in body weight across the menstrual cycle (i.e., they were not due to these body weight changes) (3.); our current results extend these findings by showing that between-subject differences in BMI and levels of dietary restraint also do not moderate the strength of hormone/binge eating associations. The fact that the estrogen × progesterone interaction remained significant in the presence of other explanatory variables highlights the importance of ovarian hormones for changes in emotional eating across the menstrual cycle.
There was one minor exception to this pattern of findings – dietary restraint exhibited a trend-level interaction with estradiol, such that emotional eating scores were highest when dietary restraint was high and estradiol levels were low. Importantly, the 3-way interaction between restraint and both hormones was non-significant, suggesting that these effects were independent of significant interactions between estradiol and progesterone. Graphs of hormone/emotional eating interactions (Figure 1) and trajectories across the menstrual cycle (Figure 2) suggested that the estradiol × progesterone interaction contributed to elevated emotional eating in all subjects, whereas the trend-level restraint × estradiol interaction contributed to elevated emotional eating scores during the premenstrual phase in high restraint scorers only.
Overall, our findings add to a growing literature aiming to understand within-person changes in dysregulated eating across the menstrual cycle. In community samples of women, who exhibit the full range of variation in emotional eating, body weight, and dietary restraint, the strongest predictors of within-subject changes in dysregulated eating across the menstrual cycle are changes in ovarian hormones, most specifically, interactions between estradiol and progesterone (2.). In these women, the primary force driving increased emotional eating during the mid-luteal phase appears to be progesterone’s antagonizing effects on estrogen, i.e., progesterone removes the inhibitory effects of estradiol on food intake.
In more disturbed samples (i.e., those with higher dietary restraint scores), we observe broader and potentially stronger (1–3.) hormone effects on dysregulated eating where both estradiol × progesterone interactions and, to a lesser extent, estradiol × restraint interactions contribute to within-subject changes in emotional eating across different menstrual cycle phases. Reasons for the novel effects of estradiol on emotional eating in high restraint scorers remain unclear, but likely relate to processes described earlier, i.e., high dietary restraint (a potent risk factor for dysregulated eating) increases risk for emotional eating in the presence of a vulnerable hormone environment (i.e., low estradiol levels). Because we did not collect food records in this study, it is unknown whether high restraint scores reflect decreased caloric intake and/or “intent to diet”/cognitive restraint. Regardless of the exact processes, however, it appears that emotional eating is more likely to occur in high restraint women when estradiol and progesterone levels are high during the mid-luteal phase and when their cognitive/behavioral control is disrupted by low estradiol levels and associated increased craving/hunger for food (particularly palatable food) (23; 40.) during the premenstrual phase.
Although we feel that our study had many strengths (e.g., large sample size, daily measurements of emotional eating and hormones), there are two key limitations. First, we examined emotional eating rather than binge eating and thus, it remains unclear whether our results generalize to full-blown binge eating. Although emotional eating scores are highly correlated with traditional measures of binge eating (30; 41.), it will be important to replicate our findings for binge eating episodes.
Second, although we view our use of a community sample of women as a significant strength that simultaneously increased samples sizes/power as well as generalizability to the population at large, it limited our ability to examine moderating associations in women with diagnosable eating disorders. Our use of a community sample also likely resulted in smaller effect sizes for hormones and dietary restraint, as stronger associations between ovarian hormones and dysregulated eating have been found in studies of women with BN (1; 4.) and those with higher levels of emotional eating (3.). Additional research investigating moderators of hormone/emotional eating associations in clinical samples is needed to replicate our findings and test hypotheses of differential effects of restraint on hormone/behavior associations in more disturbed groups. The need for these studies is underscored by the fact that the dietary restraint × estradiol interaction was only of trend-level significance in our community sample. Future clinical studies should assess a large number of subjects and directly measure daily food intake to determine effect sizes (i.e., trend-level or significant effects?) and provide objective measures of dietary restraint and binge eating episodes.
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (NIMH) (R01 MH082054) (KLK, PKK, SAB, MN, CLS, SB) and the Canadian Institute for Health Research (MDR-96630) (SER). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
Footnotes
To further ensure that our use of twins did not unduly influence results, we conducted analyses in samples of MZ and DZ twins only. The pattern of results was identical to that in the full sample (data not shown).
Because BMI and dietary restraint were correlated (r = .23), we also ran the model for BMI with restraint as a covariate, and the model for restraint with BMI as a covariate. Results were identical to those presented herein, with significant estradiol × progesterone interactions but no significant 2- or 3-way interactions between the moderators and hormones.
None of the authors have financial conflicts of interest.
References
- 1.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- 2.Klump KL, Culbert KM, Edler C, Keel PK. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klump KL, Keel PK, Racine SE, Burt SA, Sisk CL, Neale M, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. doi: 10.1037/a0029524. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: Relation to menstrual-cycle phase and cortisol levels. Psychological Medicine. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- 5.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiology and Behavior. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- 7.Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological Bulletin. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Stice E, Agras WS. Predicting onset and cessation of bulimic behaviors during adolescence: A longitudinal grouping analysis. Behavior Therapy. 1998;29:257–276. [Google Scholar]
- 9.Polivy J, Zeitlin SB, Herman CP, Beal AL. Food restriction and binge eating: A study of former prisoners of war. Journal of Abnormal Psychology. 1994;103:409–411. doi: 10.1037//0021-843x.103.2.409. [DOI] [PubMed] [Google Scholar]
- 10.Herman CP, Polivy J. From dietary restraint to binge eating: Attaching causes to effects. Appetite. 1990;14:123–125. doi: 10.1016/0195-6663(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 11.Zocca JM, Shomaker LB, Tanofsky-Kraff M. Links between mothers' and children's disinhibited eating and children's adiposity. Appetite. 2011;56:324–331. doi: 10.1016/j.appet.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanofsky-Kraff M, Faden D, Yanovski SZ, Wilfley DE, Yanovski JA. The perceived onset of dieting and loss of control eating behaviors in overweight children. International Journal of Eating Disorders. 2005;38:112–122. doi: 10.1002/eat.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angle S, Engblom J, Eriksson T, Kautiainen S, Saha MT, Lindfors P, et al. Three Factor Eating Questionnaire - R18 as a measure of cognitive restraint, uncontrolled eating, and emotional eatign in a sample of young Finnish females. The International Journal of Behavioral Nutrition and Physical Activity. 2009;6:41. doi: 10.1186/1479-5868-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindeman M, Stark K. Emotional eating and eating disorder psychopathology. Eating Disorders. 2001;9:251–259. doi: 10.1080/10640260127552. [DOI] [PubMed] [Google Scholar]
- 15.Nolan LJ, Halperin LB, Geliebter A. Emotional Appetite Questionnaire: Construct validity and relationship with BMI. Appetite. 2010;54:314–319. doi: 10.1016/j.appet.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanofsky-Kraff M, Theim KR, Yanovski SZ, Bassett AM, Burns NP, Ranzenhofer LM, et al. Validation of the Emotional Eating Scale adapted for use in children and adolescents (EES-C) International Journal of Eating Disorders. 2007;40:232–240. doi: 10.1002/eat.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- 18.Van Strien T, Herman PC, Verheijden MW. Eating style, overeating, and overweight in a representative Dutch sample: Does external eating play a role? Appetite. 2009;52:380–387. doi: 10.1016/j.appet.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Stice E, Cooper JA, Schoeller DA, Tappe K, Lowe MR. Are dietary restraint scales valid measures of modertate- to long-term dietary restriction? Objective biological and behavioral data suggest not. Psychological Assessment. 2007;19:449–458. doi: 10.1037/1040-3590.19.4.449. [DOI] [PubMed] [Google Scholar]
- 20.Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychological Assessments. 2004;16:51–59. doi: 10.1037/1040-3590.16.1.51. [DOI] [PubMed] [Google Scholar]
- 21.Iverson A, Thune I, McTiernan A, Emaus A, Finstad SE, Flote V, et al. Ovarian hormones and reproductive risk factors for breast cancer in premenopausal women: The Norwegian EBBA-I study. Human Reproduction. 2011;26:1519–1529. doi: 10.1093/humrep/der081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokkoris P, Pi-Sunyer FX. Obesity and endocrine disease. Endocrinology and Metabolism Clinics of North America. 2003;32:895–914. doi: 10.1016/s0889-8529(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 23.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: A retrospective analysis, with implications for appetite research. Physiology and Behavior. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 24.Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140:347–364. doi: 10.1530/REP-09-0568. [DOI] [PubMed] [Google Scholar]
- 25.Haedt-Matt AA, Keel PK, Racine SE, Burt SA, Hu JY, boker S, et al. Does emotional eating regulate negative affect? Concurrent and prospective associations and implicatinos for risk models of binge eating. doi: 10.1002/eat.22247. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- 27.Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite- and same-sex twins. Archives of General Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Strien T. Ice-cream consumption, tendency toward overeating, and personality. Appetite. 2000;52:380–387. doi: 10.1002/1098-108x(200012)28:4<460::aid-eat16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Racine SE, Culbert KM, Larson CL, Klump KL. The possible influences of impulsivity and dietary restraint on associations between serotonin genes and binge eating. Journal of Psychiatric Research. 2009;43:1278–1286. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Strien T. Ice cream consumption, tendency toward overeating, and personality. International Journal of Eating Disorders. 2000;28:460–464. doi: 10.1002/1098-108x(200012)28:4<460::aid-eat16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Wardle J. Eating style: A validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. Journal of Psychosomatic Research. 1987;31:161–169. doi: 10.1016/0022-3999(87)90072-9. [DOI] [PubMed] [Google Scholar]
- 32.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 33.Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. [PubMed] [Google Scholar]
- 34.Luce KH, Crowther JH. The reliability of the Eating Disorder Examination-Self-Report Questionnaire Version (EDE-Q) International Journal of Eating Disorders. 1999;25:349–351. doi: 10.1002/(sici)1098-108x(199904)25:3<349::aid-eat15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 35.Mond JM, Hay PJ, rodgers B, Owen C, Beumont PJV. Temporal stability of the eating disorder examination questionnaire. International Journal of Eating Disorders. 2004;36:195–203. doi: 10.1002/eat.20017. [DOI] [PubMed] [Google Scholar]
- 36.Black CMD, Wilson GT. Assessment of eating disorders: Interview versus questionnaire. International Journal of Eating Disorders. 1996;20:43–50. doi: 10.1002/(SICI)1098-108X(199607)20:1<43::AID-EAT5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. American Journal of Psychiatry. 1987;144:1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- 38.Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ration algorithm. Environmental Health Perspectives. 1996;104:408–413. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. American Journal of Epidemiology. 1998;147:1071–1080. doi: 10.1093/oxfordjournals.aje.a009401. [DOI] [PubMed] [Google Scholar]
- 40.Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Hormones and Behavior. 2011;59:585–593. doi: 10.1016/j.yhbeh.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racine SE, Culbert KM, Burt SA, Sisk CL, Neale MC, Boker S, et al. Exploring the relationship between negative urgency and binge eating: Etiologic associations and the role of negative affect. doi: 10.1037/a0031250. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]