Abstract
The filamentous bacterium Streptomyces coelicolor has a complex life cycle involving the formation of hair-like aerial mycelia on the colony surface, which differentiate into chains of spores. Genes required for the initiation of aerial mycelium formation have been termed ‘bld’ (bald), describing the smooth, undifferentiated colonies of mutant strains. We report the identification of a new bld gene designated as sco3099 and biochemical analysis of its encoded enzyme, cytochrome P450 (P450, or CYP) 107U1. Deletion of sco3099 resulted in a mutant defective in aerial hyphae sporulation and sensitive to heat shock, indicating that P450 107U1 plays a key role in growth and development of S. coelicolor. This is the first P450 reported to participate in a sporulation process in Streptomycetes. The substrate and catalytic properties of P450 107U1 were further investigated in mass spectrometry-based metabolomic studies. Glycocholic acid (from the medium) was identified as a substrate of P450 107U1 and was oxidized to glyco-7-oxo-deoxycholic acid. Although this reaction is apparently not relevant to the observed sporulation deficiency, it suggests that P450 107U1 might exert its physiological function by oxidizing other steroid-like molecules.
Keywords: Cytochrome P450, Streptomyces, antibiotic synthesis, sporulation, steroid oxidation, metabolomics
Introduction
The filamentous bacterium Streptomyces coelicolor has a complex life cycle that encompasses numerous developmental stages and morphological changes [1,2]. The process is initiated with spore germination, continues through vegetative growth, and develops to aerial hyphae. Upon nutrient depletion, vegetative growth ceases and spore chains form on the tips of the aerial filaments. Maturation of the spore chains leads to the release of free spores. The morphology and development of Streptomycetes are tightly regulated by a complex regulation system. The first group, the whi genes (named for white colony, which stems from lack of the gray pigment during sporulation) are essential for the maturation of spores, e.g. sigma factor whiG [3] and sigF [4]. The second group of genes is required for the growth of aerial hyphae, which were named bld genes. Mutations in these genes lead to loss of white fuzzy aerial hyphae, resulting in colonies with a smooth, bald appearance. The bld group genes are highly diverse in their products, e.g. a tRNA (bldA) [5], an ABC transporter (bldK) [6], and transcription factors including bldB [7], bldD [8], bldM [9]. Interestingly, the deficiency of bld gene products influences not only the formation of aerial filaments but also the production of antibiotics in S. coelicolor [10]. Research on the relationship between extracellular stimuli and colony appearance implicated signaling molecules such as γ-butyrolactones [11] also being involved in morphological differentiation and antibiotic production.
Cytochrome P450s (P450s, or CYP genes)1 constitute a superfamily of monooxygenases containing a prosthetic heme-iron group found in almost all living organisms. P450s catalyze a great variety of reactions, which are often involved in the metabolism of xenobiotic chemicals or the biosynthesis of endogenous primary and secondary metabolites. In S. coelicolor A3(2), 18 P450s were detected through in silico sequence analysis [12,13], but only a few of them have had functions elucidated and most of the enzyme functions are still elusive.
In this paper, we report that sco3099, a gene encoding P450 107U1, is required for the development of aerial hyphae in S. coelicolor. Disruption of sco3099 abolishes spore formation. This is the first P450 reported to play a critical role in a sporulation process of Streptomycetes. Glycocholic acid (from the medium) was identified as a substrate of P450 107U1, suggesting that P450 107U1 may exert its function in sporulation by oxidizing steroid-like molecules.
Materials and methods
Bacterial strains, plasmids, and media
S. coelicolor A3(2) strain M145 was used as the parent strain for mutant isolation (John Innes Center, Norwich, UK) (Table 1). A traditional gene replacement strategy, using an apramycin resistance gene as a selective marker, was used to generate the mutants of the target genes, as follows (Table 2). The null mutant of gene sco3099 (Δsco3099:: aac(3)IV) was isolated by transforming S. coelicolor M145 to apramycin resistance with pTL1001 derivatives [15] and screening for sensitivity to thiostrepton. In order to bypass the methyl-specific restriction system of S. coelicolor, the Escherichia coli methylation-deficient strain ET12567 was used in the conjugation. Standard procedures for conjugation between E. coli and Streptomyces were used [16]. Yeast extract-malt extract medium (YEME) was used for growth of mycelia (liquid), and rich medium R2YE (agar) and mannitol-soya flour medium (MS) were used as solid media in conjugation and colony screening, respectively. Final concentrations for antibiotic screens were 25 μg apramycin ml−1, 50 μg thiostrepton ml−1, and 200 μg kanamycin ml−1. For E. coli, the concentrations were 100 μg ampicillin ml−1 and 100 μg apramycin ml−1 for Luria-Bertani media.
Table 1.
S. coelicolor A3(2) derivatives used in this work
| Strain | Genotype | Source |
|---|---|---|
| A3(2) | Wild type | This laboratory |
| J1700 | bldA null mutant | John Innes Center |
| J669 | bldB agaA1 cysD18 mthB2 bldB43 | John Innes Center |
| J2166 | bldC null mutant | John Innes Center |
| bldD | bldD null mutant | John Innes Center |
| WC109 | bldH null mutant | John Innes Center |
| NS17 | bldK null mutant | John Innes Center |
| bldM | bldM null mutant | John Innes Center |
| bldP | sco3099::aac(3)IV | This work |
Table 2.
Plasmids used or constructed in this work
| Name | Genotype |
|---|---|
| pTL1001 | E. coli-Streptomyces shuttle vector, temperature sensitive, thiostrepton resistant [15] |
| pCR@2.1 | Clone vector, Invitrogen |
| pTZ3099L | pCR@2.1 derivative |
| pTZ3099R | pCR@2.1 derivative |
| pTZ3099 | pTL1001 derivative, gene replacement of sco3099 |
| pTGV2 | E. coli-Streptomyces shuttle vector, thiostrepton resistant [14] |
| pTLV2 | Plasmid with insertion of 450 bp ermEp promotor |
| pTZ3099com | pTGV2 derivative, for complementation of sco3099 |
Chemicals and reagents
Glycocholic acid was purchased from Sigma-Aldrich (St. Louis, MO).
Glyco-7-oxo-deoxycholic acid (N-[(3α,5β,12α)-3,12-dihydroxy-7,24-dioxocholan-24-yl]glycine, CAS 78537-20-9) was prepared by the oxidation of glycocholic acid with N-bromosuccinimide [17]. N-Bromosuccinimide (32 mg, 0.18 mmol, 0.84 mol equiv) was added to a solution of glycocholic acid (100 mg, 0.22 mmol) in CH2Cl2/H2O (4 ml, 1:1, v/v) in a screw-cap vial. The reaction was stirred vigorously at 40 °C for 30 min. The reaction mixture was directly purified via flash column chromatography (10% CH3OH in CH2Cl2, v/v) to afford glyco-7-oxo-deoxycholic acid as a mixture with the starting material, and this material was further purified using HPLC with UV detection (210 nm) to afford glyco-7-oxo-deoxycholic acid (2 mg) for characterization by 1H NMR. Rf 0.8 (CH3OH:CH2Cl2, 5:3, v/v, visualized by staining with CAM reagent (Ce(NH4)2(NO3)6 (0.5%, w/v), ammonium molybdate (12%, w/v), and H2SO4 (20%, v/v) in H2O)).
Spinach ferredoxin and NADPH-ferredoxin reductase were purchased from Sigma-Aldrich.
P450 107U1 (sco3099) mutant strain construction
With genomic DNA as a template, a 1.2 kb upstream fragment was amplified by PCR with the primers 5′-ATATAAGCTTGAGCGTCTGACCGGGCAG-3′ and 5′-TATATCTAGAGCTGGCCACGCTGCTGAC-3′, digested by XbaI and HindIII, respectively. In a similar way, a 1.2 kb downstream fragment was amplified with the primers 5′-ATATGAATTCACTAGTCCGCTGGGCAACCGGGTG-3′ and 5′-TATAGGATCCTACGGCGCCTGGGACGTC-3′, digested by EcoRI and BamHI, and then cloned into pCR@2.1 (Table 2, Fig. 1)). After sequence analysis, the fragments were digested and ligated with a 1.5 kb apramycin resistance gene, aac(3)IV, which was linearized by XbaI/EcoRI digestion (Fig. 1). The resultant 2.9 kb fragment was cloned into the HindIII/BamHIII site of the shuttle vector pTL1001 [15] to generate the gene replacement plasmid pTZ3099, in which sco3099 was replaced by the apramycin resistance gene aac(3)IV. The plasmid was introduced into S. coelicolor by conjugation, and the mutant strains were obtained by screening on plates with apramycin or thiostrepton. The correct mutant colonies were apramycin-resistant but thiostrepton-sensitive.
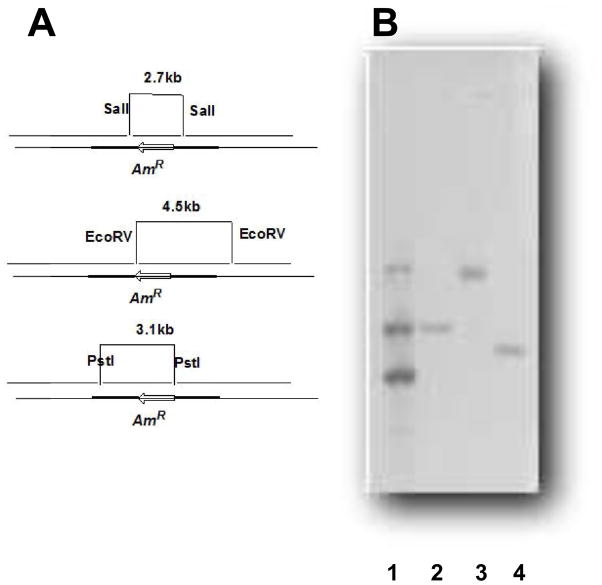
Fig. 1.
Construction of mutant strain and Southern blotting to verify the genotype of the sco3099 knockout. Lane 1, positive control with 6.0, 2.9, and 1.5 kb markers; lanes 2, 3, and 4, DNA of the knockout was digested by SalI, EcoRV, and PstI, and the corresponding 3.1, 5.5, and 2.0 kb fragments were detected, respectively.
Southern hybridization
The 1.5 kb apramycin resistant gene aac(3)IV was random-primer labeled with digoxigenin (DIG)-11-dUTP to prepare a probe (following the instructions for the DIG-High prime kit, Roche CAT. NO. 11745832910). Genomic DNA from S. coelicolor wild type and mutant strains was extracted from liquid media and digested with EcoRV, PstI, and SalI. The DNAs were transferred to and fixed on nitrocellulose paper and then hybridized with DIG-labeled probe. Signals were detected using an anti-DIG/alkaline phosphatase conjugate and the substrates 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium, yielding a light-blue precipitate.
Gene complementation of sco3099
To complement the p450 107U1 mutant Δsco3099, a PCR fragment of sco3099 was amplified by PCR with the following primers: 5′-ATATCATATGACCGGCAGCTCTTCC-3′ and 5′-TATAAAGCTTTCACTTTTGCGTCGGCGAC-3′. The fragment was ligated into pTGV2 using the NdeI and HindIII sites, under the control of the constitutive promoter ErmEp. The plasmid was introduced into the mutant by conjugation, and thiostrepton-resistant colonies were selected.
Scanning and transmission electron microscopy
Both the mutant and wild type strains were incubated for 5 days in MS media. Individual colonies were excised from the plates and prepared for scanning electron microscopy (SEM) or transmission electron microscopy (TEM). Samples were fixed overnight in 0.10 M sodium cacodylate solution containing 2.5% glutaraldehyde (w/v) and post-fixed in 1% OsO4 (w/v) for 1 h. Samples were then dehydrated using a step-wise ethanol gradient (25%, 50%, 70%, 95%, 100%, all v/v). SEM samples were transferred to a critical point dryer and, when dried, mounted onto SEM stubs and coated with gold before viewing using a Hitachi X-650 scanning electron microscope. TEM samples were subjected to a final dehydration in 100% propylene oxide, infiltrated with Spurr’s resin (SigmaAldrich, Cat No. E0300), and transferred to an embedding mold where they were polymerized in Spurr’s resin at 60 °C overnight. Sections were then cut using a Leica UCT Ultramicrotome and viewed using a Philips CM-12 TEM instrument.
Protein expression and purification
The plasmid for the expression of P450 107U1 was constructed previously [13]. Recombinant proteins were expressed in E. coli BL21(DE3). Transformed E. coli was grown at 37 °C in 2 liters of Terrific Broth containing 100 μg ml−1 ampicillin until the OD600 reached 0.8. After induction with 0.5 mM isopropyl-β-D-thiogalactopyranoside and the addition of δ-aminolevulinic acid to a final concentration of 1.0 mM (for heme synthesis), growth was allowed to continue for an additional 24 h at 24 °C, and the cells were harvested by centrifugation for 10 min at 4,000 rpm in a Sorvall RC-3B Plus centrifuge using an H-6000A/HBB-6 swinging bucket rotor. The pellets were resuspended in 100 ml of lysis buffer (50 mM Tris-Cl, pH 7.5, containing 500 mM NaCl, 0.5 mM EDTA, and 10% glycerol, v/v). The soluble proteins were purified by Ni2+-nitrilotriacetic acid resin (Ni-NTA, Qiagen) chromatography. The Ni-NTA column was washed with 10 volumes of lysis buffer and then 10 volumes of lysis buffer containing 5 mM imidazole. The protein was eluted with elution buffer (20 mM Tris-Cl, pH 7.5, containing 10% glycerol (v/v) and 100 mM imidazole). The fractions were analyzed by sodium dodecyl sulfate-polyacrylamide (7.5%, w/v) gel electrophoresis, and those fractions enriched in P450 107U1 were concentrated to 2.5 ml using an Amicon ultrafiltration device and applied to a desalting column (PD-10, GE Healthcare Life Sciences). The protein was eluted with elution buffer (omitting the imidazole) and then stored at −80 °C (without cleavage of the His-tag). The concentration of P450 was estimated using the spectral method of Omura and Sato [18].
Metabolomic analysis and substrate identification
To search for the substrate of P450 107U1, a previously described metabolomics approach was employed [19]. Cultures of mutants grown on R2YE plates (3- and 7-day) were extracted with ethyl acetate (20 ml/plate). The organic phase was filtered through Whatman filter paper, evaporated in vacuo, and then reconstituted in 200 μl of methanol, with the view that the resulting extract may contain enriched substrates of P450 107U1 because oxidation was blocked. An aliquot of this organic extract was then incubated with recombinant P450 107U1 in the presence of NADPH and spinach ferredoxin and NADPH-ferredoxin reductase. A typical reaction contained 1.0 μM P450 107U1, 2.0 μM spinach NADPH-ferredoxin reductase, and 5 μM spinach ferredoxin in 1.0 ml of 100 mM potassium phosphate buffer (pH 7.4) and an NADPH-generating system (0.5 mM NADP+, 10 mM glucose 6-phosphate, and 1 IU ml−1 yeast glucose 6-phosphate dehydrogenase) and was incubated at 30° C for 2 h. The reaction was extracted twice with 5 ml of ethyl acetate. The organic phases were combined, dried under a N2 stream, and subjected to high-performance liquid chromatograph-mass spectrometry (LC-MS) analysis. LC separation was performed with a Varian Pursuite octadecylsilane (C18) HPLC column (2.1 mm × 100 mm, 3 μm) at 25 °C. Samples (15 μl) were injected, and components were eluted from the column using a linear gradient increasing from 85% mobile phase A (95% H2O, 5% CH3CN, 0.1% HCO2H, v/v) to 95% mobile phase B (5% H2O, 95% CH3CN, 0.1% HCO2H, v/v) over 15 min. Data were collected on a Thermo LTQ linear ion trap mass spectrometer operating in the electrospray ionization (ESI) positive mode, scanning from m/z 100–1000. The metabolic profile obtained after the reaction was compared with a negative control (minus NADPH) using the specialized metabolomics software XCMS [20,21]. Molecules depleted in the enzymatic reaction were considered as possible substrates. High resolution mass spectrometry (HRMS) data on the putative substrate were collected on a Thermo Orbitrap mass spectrometer (ThermoFisher) operated in the ESI positive mode, and the molecular formula was deduced using the manufacturer’s software.
Characterization of oxidation products
A reaction mixture containing 0.5 μM P450 107U1, 2.0 μM spinach NADPH-ferredoxin reductase, 5 μM spinach ferredoxin, an NADPH-generating system (vide supra), and 100 μM glycocholic acid (in 100 mM potassium phosphate buffer, pH 7.4) was incubated at 30 °C for 5 h, followed by extraction with two volumes of ethyl acetate. The organic phase was dried under a stream of N2, and the resulting products were dissolved in 30% aqueous CH3CN (v/v) and separated by HPLC using an octadecylsilane (C18) column (6.2 mm × 80 mm, 3 μm, Agilent Technologies, Palo Alto, CA). Isocratic elution (30% B) was used at a flow rate of 1.0 ml min−1 (isocratic solvent composition, prepared from solvent A (95% H2O, 5% CH3CN, v/v) and solvent B (5% H2O, 95% CH3CN, v/v)). Part of the HPLC eluate (~100 μl min−1) was directed to a LTQ mass spectrometer through a T-sleeve to monitor the elution of oxidized glycocholic acid, due to the lack of a suitable UV signal. The purified oxidation product was analyzed by similar LC-MS methods, except that the mass spectrometer was operated in the MS2 mode to yield a fragmentation signature. The retention and fragmentation patterns of the enzymatic product were compared with those of synthetic glyco-7-oxo-deoxycholic acid (vide supra) to verify its identity.
Results
In silico analysis of sco3099 in S. coelicolor
sco3099, the gene encoding P450 107U1 located from 3394340 to 3395641 in the genome of S. coelicolor A3(2), was designated as one of the three putative P450s in the CYP107 subfamily [13]. P450 107U1 contains the conserved EXXR domain in the K-helix and showed a typical P450 ferrous-CO vs. ferrous difference spectra when expressed in E. coli [13]. P450 107U1 shows homology with other P450s in Streptomyces species, e.g. 95% similarity to PikC (YP006878447.1) in S. venezuelae and 94% similarity to Sav_3536 (NP824713.1) in S. avermitilis MA-4680. However, its function cannot be proposed based on its gene context. Two hydrolases encoded by sco3098 and sco3097 are located upstream (rpfA) [22] and affect germination, vegetative growth, and sporulation by remodeling the cell wall in S. coelicolor. Another gene of note is sco3095, a member of the DivIC superfamily, an indispensable gene in cell division, which initiates septum formation [23]. All of these genes are located in the same strand, suggesting that they might play roles in an operon. In particular, the genes sco3095 and sco3097 to sco3101 are highly conserved in most of the Streptomyces strains with genome sequences available, e.g. S. coelicolor, S. avermitilis, S. griseus, and S. lividans. We propose that P450 107U1 may possess novel functions in the development of Streptomyces other than secondary metabolism, as do its homologs in other strains.
Inactivation of sco3099
In order to characterize the function of the P450 107U1 gene sco3099, we constructed a mutant strain by gene replacement, with sco3099 replaced by the apramycin resistance gene acc(3)IV. The plasmid pTL1001 was used as a shuttle vector in conjugation to introduce the constructed plasmid from E. coli ET12567 to S. coelicolor A3(2). The conjugants with apramycin resistance were selected, followed by thiostrepton screening to obtain the thiostrepton-sensitive mutant strains. The mutant strains were further examined by Southern blotting analysis with a digoxigenin-11-dUDP-labeled apramycin resistance gene as a probe. Digestion with SalI and PstI generated 3.1 and 2.0 kb fragments, respectively, in the upstream region. The genotype of the downstream region was confirmed by digestion by EcoRV to generate a 5.5 kb fragment (Fig. 1).
Phenotypic analysis of the mutant strain
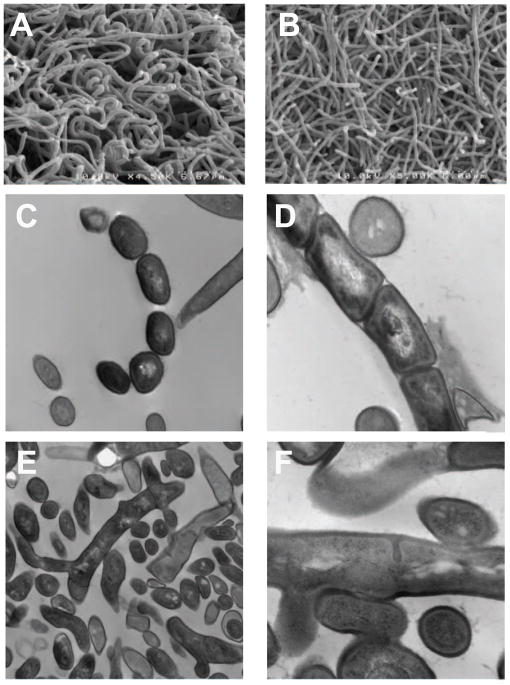
The sco3099 mutant strain showed the same growth rate as wild type S. coelicolor in liquid media, e.g. TSB and YEME. When grown in R2YE agar plates, the mutant strain did not form aerial hyphae after three days of incubation (Fig. 2) or even longer. The deletion of sco3099 also influenced the antibiotic production ability, in that no blue halo (stemming from the loss of the actinorhodin production) appeared around the mutant colonies. We hypothesized that the development of the mutant strain was blocked in the step of aerial hyphae formation. To address this hypothesis, SEM and TEM were used to identify the phenotypes of the mutant strains. SEM showed that coiled spore chains were observed on the tips of the aerial hyphae after 3-day incubation of the wild type strain (Fig. 3A). Neither spore chains nor coils were observed in the mutant strain (Fig. 3B). Further, the diameter of the mycelia was also thinner than with the wild type strain, indicating that the mutant strain is unable to enter the late stage of differentiation. As in the TEM, various developmental styles were observed in the image of the wild type strain (Fig. 3C, 3D); however, for the mutant strain of sco3099, only the vegetative mycelium could be observed (Fig. 3E). In some circumstances, the septum (although initiated) was not developed completely and resulted in abnormal division in the mutant strain (Fig. 3F). These results indicate that deletion of sco3099 disabled the formation of aerial hyphae and the production of antibiotic, as in the phenotype of other bald mutants [23,24]. We therefore assigned sco3099 as a new bld gene in the filamentous bacterium S. coelicolor, and the inactivation of this gene caused the formation of colonies consisting only of vegetative mycelium.
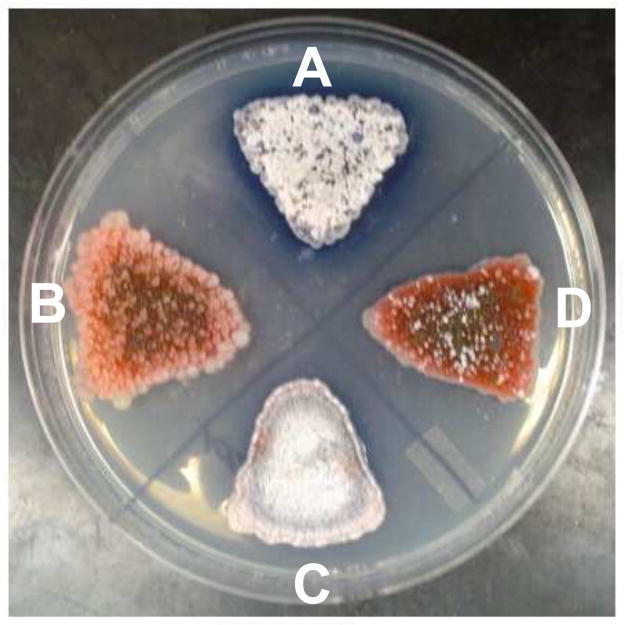
Fig. 2.
(A) Wild type; (B) sco3099 mutant strain; (C) knockout strain rescued with sco3099 cDNA expression; (D) a blank vector was transferred to the mutant strain as a control. All of the strains were incubated on an R2YE agar plate for 3 days.
Fig. 3.
SEM of wild type S. coelicolor (A) and mutant strain sco3099 (B); TEM of wild type (C, D) and mutant strain (E, F). (C) mature spores; (D) premature spores; (E) branch of mycelium; (F) irregular septum.
Gene complementation of sco3099
In order to verify that the phenotype of the mutant strain is related to inactivation of sco3099 and exclude a possible polar effect, the entire gene was introduced back into the mutant strain in pTGV2 under the control of a constitutive promoter, ErmEp. The resulting thiostrepton-resistant colonies produced white aerial hyphae and gray spores on the rich medium R2YE, as did the wild type strain (Fig. 2C), but the strains with the blank vector pTGV2 failed to produce aerial hyphae and spores (Fig. 2D). Meanwhile, the appearance of the blue halo stemming from the production of actinorhodin also indicated the restoration of the antibiotic production ability. These results exclude the possibility of a polar effect and indicate that the bald phenotype is a consequence of the inactivation of sco3099.
Glycocholic acid as a substrate of P450 107U1
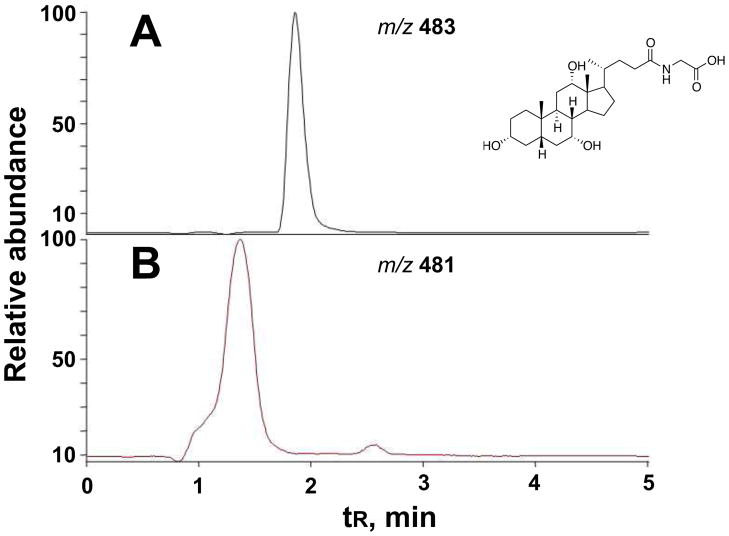
The P450 107U1 protein, heterologously expressed in E. coli, showed typical Fe2+-CO binding spectra with an absorbance maximum at 448 nm (Fig. 4). In order to identify substrates of P450 107U1, a mass spectrometry-based metabolomic method was utilized to identify substrates from the culture, where a substrate might accumulate. An organic extract was prepared from the sco3099-knockout S. coelicolor grown on R2YE agar plates. The resulting extract was incubated with purified P450 107U1 in the presence of spinach ferredoxin and NADPH-ferredoxin reductase and an NADPH-generating system (Fig. 5). Metabolic profiling of the reaction mixtures by LC-MS and subsequent data processing with metabolomics software (XCMS) yielded one putative substrate (m/z 483) and its dehydrogenated product (m/z 481) in the ESI positive ion mode (Fig. 6).
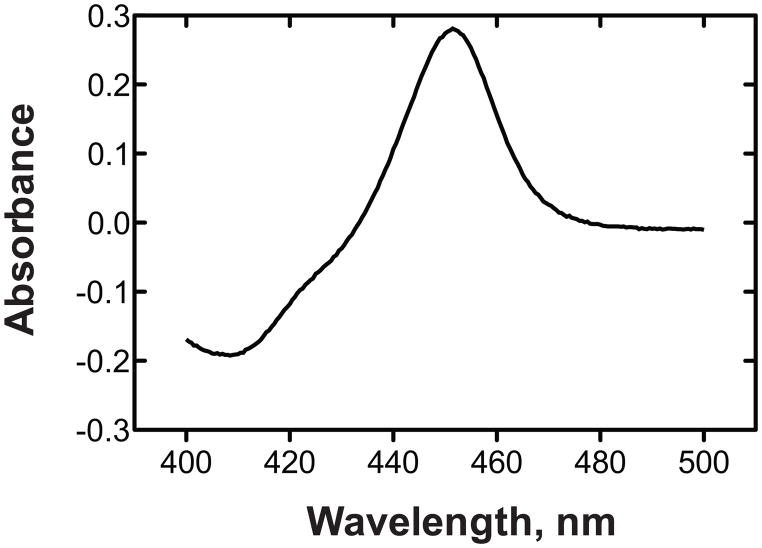
Fig. 4.
Fe2+-CO vs Fe2+ difference spectrum of purified P450107U1 [18]. The P450 concentration was 3 μM.
Fig. 5.
Metabolomic analysis by XCMS [20,21]. LC-MS of a putative substrate at m/z 483 (A) and its dehydrogenated product at m/z 481 (B). See Materials and methods for LC-MS details.
Fig. 6.
Incubation of commercial glycocholic acid with P450 107U1 results in a dehydrogenated product. (A) Trace for glycocholic acid; (B) trace for product. (glyco-7-oxo-deoxycholic acid).
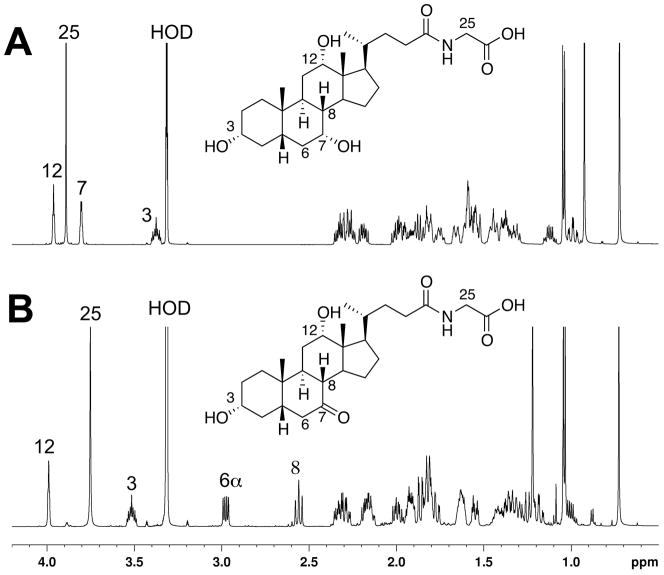
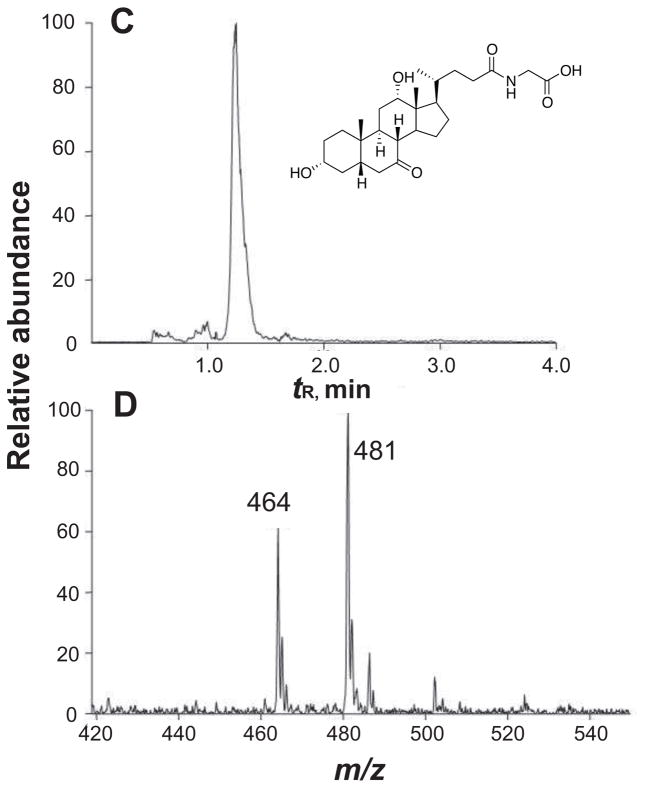
The putative substrate also gave a strong signal at m/z 466, indicating that the m/z 483 peak might be an ammonium adduct. The high affinity for ammonium ions, combined with HRMS data (MH+ 466.3155, calculated 466.3169 for C26H43NO6), suggested that the natural product glycocholic acid, with this elemental formula (corrected for NH4+), might be the substrate. To confirm the identity of the substrate, authentic glycocholic acid was incubated with P450 107U1 and the extracted product was analyzed by LC-MS/MS. The product formed in the incubation with glycocholic acid yielded exactly the same dehydrogenated metabolite that had been identified by metabolomics analysis (Fig. 7), as judged by coincident retention time and MS fragmentation.
Fig. 7.
NMR spectra of (A) glycocholic acid and (B) synthetic glyco-7-oxo-deoxycholic acid (in CDCl3, 600 MHz). Some of the key downfield peaks are identified on the spectra. (C) Co-chromatography of the enzymatic oxidation product with synthetic glyco-7-oxo-deoxycholic acid, monitoring relative abundance at m/z 464; (D) mass spectrum of the enzymatic oxidation product, showing the characteristic ammonium ion [M+NH4]+ (m/z 481) in addition to [M+H]+ (m/z 464). See text for HRMS.
Glycocholic acid conversion to glyco-7-oxo-deoxycholic acid by P450 107U1
Glyco-7-oxo-deoxycholic acid was synthesized by the oxidation of glycocholic acid with N-bromosuccinmide [17], and the structure of the resulting compound was verified by 1H-NMR (Fig. 7). The synthetic glyco-7-oxo-deoxycholic acid had the exactly the same HPLC elution time and MS fragmentation pattern as the dehydrogenated glycocholic acid product generated in the enzymatic reaction. P450 107U1 catalyzed the dehydrogenation of glycocholic acid to glyco-7-oxo-deoxycholic acid at a rate of 0.3 min−1 (measured at a substrate concentration of 100 μM).
Discussion
In this study we report the identification of a new bld gene, sco3099, which encodes the monooxygenase P450 107U1. Inactivation of sco3099 blocked the formation of aerial hyphae on R2YE agar plates. This is the first P450 reported to be indispensable for sporulation in Streptomycetes. During the development of S. coelicolor, the appearance of the aerial filaments is a significant event because of the morphological changes and the shift to secondary metabolism. By growing the bld mutants in proximity, fuzzy aerial filaments were restored in certain pairings. A hierarchy of the bld genes was thus deduced based upon the ability of each mutant to restore the aerial hyphae development [9,25]. During the incubation of mutants of sco3099 in proximity to other known bld mutants, only the bldA mutant was restored to produce white aerial hyphae on the edge of the colonies (data not shown). bldA (encoding a rare tRNA(Leu) UUA) influenced the development of S. coelicolor at the early stage of vegetative mycelium formation and affected the biosynthesis of the red pigment undecylprodigiosin by regulating the transcription efficiency of the pathway specific regulator redZ, which contains the rare UUA codon [26]. However, no UUA codon is present in the sequence of sco3099 and its flanking regions, thus excluding the possibility of assigning P450 107U1 as a target of the rare leucyl tRNA. We therefore define sco3099 as a new member of the bld gene family, although crosstalk may exist between these bld genes.
Metabolomic analysis was employed to probe the substrate and catalytic activities of P450 107U1. Glycocholic acid, originating from the medium in the plates, was identified as one of the substrates and was oxidized to its 7-keto derivative. The addition of glycocholic acid (10–200 μM) did not affect sporulation of wild type S. coelicolor on R2YE plates (data not shown); the results of Fig. 2A and 2B could be repeated in R2YE plates devoid of glycocholic acid, arguing that a product of glycocholic acid is not necessary for spore formation. A similar molecule, 3,7,12-trihydroxy-24-cholanic acid methyl ester (methyl ester of cholic acid) was reported to be produced by the facultative anaerobe Streptococcus faecium, another soil bacterium, under anaerobic culture, and this molecule has antibacterial activity [27]. Further, several marine bacteria, including member of the genuses Streptomyces, Myoides, and Donghaeana, have been reported to produce cholic acid, deoxycholic acid, glycocholic acid, and glycodeoxycholic acid [28,29]. Although the biotransformation of exogenous glycocholic acid is not considered directly relevant to the observed phenotype, given the evidence above, this finding nevertheless indicates that P450 107U1 might exert its function in sporulation by oxidizing steroid or steroid-like molecules. Hopanoids are pentacyclic sterol-like triterpenes found in many microbial organisms [30]. Several hopanoids had been identified during the development and differentiation from substrate to aerial hyphae in S. coelicolor A3(2) [31]. A recent study demonstrates that hopanoids have sterol-like properties in terms of membrane ordering [32]. Further studies on the biotransformation of hopanoids and efforts with new hopanoids may shed light on the mechanism of sporulation processes in Streptomycetes.
Highlights.
Deletion of the P450 gene of Streptomyces coelicolor blocked sporulation and antibiotic synthesis.
Purified recombinant P450 107U was used in a metabolomic screen, and glycocholic acid was identified as a substrate.
P450 107U1 catalyzed the oxidation of the 7α-hydroxy group of glycocholic acid to a ketone.
Acknowledgments
This project was supported by grant R01 GM069970 from the National Institutes of Health (F.P.G.). We thank Dr. K. F. Chater for providing the bld mutant strains and K. Trisler for assistance in preparation of the manuscript.
Footnotes
Abbreviations used: DIG, digoxigenin; ESI, electrospray ionization; HRMS, high resolution mass spectrometry; LC-MS, liquid chromatography-mass spectrometry; MS, mannitol-soy flour; NTA, nitrilotriacetic acid; P450, cytochrome P450 (also CYP); PCR, polymerase chain reaction; SEM, scanning electron microscopy; TEM, transmission electron microscopy; YEME, yeast extract-malt extract.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wildermuth H, Hopwood DA. Septation during sporulation in Streptomyces coelicolor. J Gen Microbiol. 1970;60:51–59. doi: 10.1099/00221287-60-1-51. [DOI] [PubMed] [Google Scholar]
- 2.Kalakoutskii LV, Agre NS. Comparative aspects of development and differentiation in actinomycetes. Bacteriol Rev. 1976;40:469–524. doi: 10.1128/br.40.2.469-524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chater KF. Taking a genetic scalpel to the Streptomyces colony. Microbiology. 1998;144:1465–1478. doi: 10.1099/00221287-144-6-1465. [DOI] [PubMed] [Google Scholar]
- 4.Potuckova L, Kelemen GH, Findlay KC, Lonetto MA, Buttner MJ, Kormanec J. A new RNA-polymerase sigma-factor, sigma(f), is required for the late stages of morphological-differentiation in Streptomyces spp. Mol Microbiol. 1995;17:37–48. doi: 10.1111/j.1365-2958.1995.mmi_17010037.x. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor EJ, Baylis HA, Chater KF. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a transfer RNA-like product in Streptomyces coelicolor A3(2) Genes Develop. 1987;1:1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- 6.Nodwell JR, Losick R. Purification of an extracellular signaling molecule involved in production of aerial mycelium by Streptomyces coelicolor. J Bacteriol. 1998;180:1334–1337. doi: 10.1128/jb.180.5.1334-1337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope MK, Green B, Westpheling J. The bldB gene encodes a small protein required for morphogenesis, antibiotic production, and catabolite control in Streptomyces coelicolor. J Bacteriol. 1998;180:1556–1562. doi: 10.1128/jb.180.6.1556-1562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliot MA, Leskiw BK. The BldD protein from Streptomyces coelicolor is a DNA-binding protein. J Bacteriol. 1999;181:6832–6835. doi: 10.1128/jb.181.21.6832-6835.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molle V, Buttner MJ. Different alleles of the response regulator gene bldM arrest Streptomyces coelicolor development at distinct stages. Mol Microbiol. 2000;36:1265–1278. doi: 10.1046/j.1365-2958.2000.01977.x. [DOI] [PubMed] [Google Scholar]
- 10.Susstrunk U, Pidoux J, Taubert S, Ullmann A, Thompson CJ. Pleiotropic effects of cAMP on germination, antibiotic biosynthesis and morphological development in Streptomyces coelicolor. Mol Microbiol. 1998;30:33–46. doi: 10.1046/j.1365-2958.1998.01033.x. [DOI] [PubMed] [Google Scholar]
- 11.Takano E. Gamma-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr Opin Microbiol. 2006;9:287–294. doi: 10.1016/j.mib.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2) Nature. 2002;417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- 13.Lamb DC, Skaug T, Song HL, Jackson CJ, Podust LM, Waterman MR, Kell DB, Kelly DE, Kelly SL. The cytochrome P450 complement (CYPome) of Streptomyces coelicolor A3(2) J Biol Chem. 2002;277:24000–24005. doi: 10.1074/jbc.M111109200. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Q, He Q, Ding W, Tang M, Kang Q, Yu Y, Deng W, Zhang Q, Fang J, Tang G, Liu W. Characterization of the azinomycin B biosynthetic gene cluster revealing a different iterative type I polyketide synthase for naphthoate biosynthesis. Chem Biol. 2008;15:693–705. doi: 10.1016/j.chembiol.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Jia XY, Tian ZH, Shao L, Qu XD, Zhao QF, Tang J, Tang GL, Liu W. Genetic characterization of the chlorothricin gene cluster as a model for spirotetronate antibiotic biosynthesis. Chem Biol. 2006;13:575–585. doi: 10.1016/j.chembiol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Tobias Kieser MJB, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces Genetics. John Innes Foundation, John Innes Centre; Colney, Norwich NR4 7UH, UK: 2001. [Google Scholar]
- 17.Fieser LF, Rajagopalan S. Selective oxidation with N-bromosuccinimide. I. Cholic acid. J Am Chem Soc. 1949;71:3935–3938. [Google Scholar]
- 18.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2379–2385. [PubMed] [Google Scholar]
- 19.Cheng Q, Lamb DC, Kelly SL, Lei L, Guengerich FP. Cyclization of a cellular dipentaenone by Streptomyces coelicolor cytochrome P450 154A1 without oxidation/reduction. J Am Chem Soc. 2010;132:15173–15175. doi: 10.1021/ja107801v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 21.Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem. 2012;84:5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haiser HJ, Yousef MR, Elliot MA. Cell wall hydrolases affect germination, vegetative growth, and sporulation in Streptomyces coelicolor. J Bacteriol. 2009;191:6501–6512. doi: 10.1128/JB.00767-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett JA, Aimino RM, McCormick JR. Streptomyces coelicolor genes ftsL and divIC play a role in cell division but are dispensable for colony formation. J Bacteriol. 2007;189:8982–8992. doi: 10.1128/JB.01303-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Champness WC. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J Bacteriol. 1988;170:1168–1174. doi: 10.1128/jb.170.3.1168-1174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willey J, Schwedock J, Losick R. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Develop. 1993;7:895–903. doi: 10.1101/gad.7.5.895. [DOI] [PubMed] [Google Scholar]
- 26.White J, Bibb M. bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J Bacteriol. 1997;179:627–633. doi: 10.1128/jb.179.3.627-633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SC, Kim CJ, Uramoto M, Yun HI, Yoon KH, Oh TK. Antibacterial substance produced by Streptococcus faecium under anaerobic culture. Biosci Biotech Biochem. 1995;59:1966–1967. doi: 10.1271/bbb.59.1966. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Lee JS, Kim J, Kang SJ, Yoon JH, Kim WG, Lee CH. Biosynthesis of bile acids in a variety of marine bcterial taxa. J Microbiol Biotechnol. 2007;17:403–407. [PubMed] [Google Scholar]
- 29.Maneerat S, Nitoda T, Kanazaki H, Kawai F. Bile acids are new products of a marine bacterium, Myroides sp. strain SM1. App Microbiol Biotechnol. 2005;67:679–683. doi: 10.1007/s00253-004-1777-1. [DOI] [PubMed] [Google Scholar]
- 30.Sahm H, Rohmer M, Bringer-Meyer S, Sprenger GA, Welle R. Biochemistry and physiology of hopanoids in bacteria. Adv Microb Physiol. 1993;35:247–273. doi: 10.1016/s0065-2911(08)60100-9. [DOI] [PubMed] [Google Scholar]
- 31.Poralla K, Muth G, Härtner T. Hopanoids are formed during transition from substrate to aerial hyphae in Streptomyces coelicolor A3(2) FEMS Microbiol Lett. 2000;189:93–95. doi: 10.1111/j.1574-6968.2000.tb09212.x. [DOI] [PubMed] [Google Scholar]
- 32.Saenz JP, Sezgin E, Schwille P, Simons K. Functional convergence of hopanoids and sterols in membrane ordering. Proc Natl Acad Sci U S A. 2012;109:14236–14240. doi: 10.1073/pnas.1212141109. [DOI] [PMC free article] [PubMed] [Google Scholar]