Abstract
This paper attempts to quantify the social, private, and public-finance values of reducing obesity through pharmaceutical and medical interventions. We find that the total social value of bariatric surgery is large for treated patients, with incremental social cost-effectiveness ratios typically under $10,000 per life-year saved. On the other hand, pharmaceutical interventions against obesity yield much less social value with incremental social cost-effectiveness ratios around $50,000. Our approach accounts for: competing risks to life expectancy; health care costs; and a variety of non-medical economic consequences (pensions, disability insurance, taxes, and earnings), which account for 20% of the total social cost of these treatments. On balance, bariatric surgery generates substantial private value for those treated, in the form of health and other economic consequences. The net public fiscal effects are modest, primarily because the size of the population eligible for treatment is small while the net social effect is large once improvements in life expectancy are taken into account.
Keywords: obesity, health spending, ageing, microsimulation
A. Introduction
Obesity has more than doubled over the past thirty years, with a wide variety of well-documented consequences on health and health care expenditures (Flegal et al, 2012; Flegal et al, 1998; Flegal et al, 2005). Recent evidence has also suggested significant public fiscal impacts of obesity, which worsens morbidity without much offsetting decline in longevity (Goldman et al, 2010). Evidently, obesity is now a problem of health, health care spending, and public finance.
It is thus natural to ask whether any proposed solutions to obesity reduce its social costs. On the one hand, behavioral interventions to reduce weight have proven difficult. Over the twentieth-century, population-wide gains in weight have occurred because of declining physical activity and rising calorie intake (Lakdawalla and Philipson, 2009; Cutler, Glaeser and Shapiro, 2003). Modifying physical activity patterns is quite difficult. Targeting unhealthy food consumption may help, but the availability of many calorically dense substitutes makes it difficult to achieve large net reductions in caloric intake (Chouinard et al., 2007).
On the other hand, recent medical innovations have shown promise. Some modest benefits have been demonstrated by pharmacotherapy. For example, one of the two most commonly prescribed anti-obesity medications -- sibutramine -- has been found to reduce weight and the prevalence of hypertension and diabetes (James et al., 2000; O’Meara et al., 2002).2 But the effects on weight remain modest (around 5%) and much more difficult to sustain without side effects. Much more promising are recent advances in surgical treatments, which have made it possible to alter calorie needs by constricting the stomach. Laparoscopic gastric bypass and adjustable gastric banding are two of the most common bariatric procedures. Such procedures are becoming less invasive. In 2005, 170,000 Americans received this treatment (Cremieux et al., 2008), which is currently reserved for those either with BMI over 40, or with BMI over 35 and the presence of other co-morbidities. Current evidence points to a 20% reduction in BMI for those treated with surgery, and the effects persist up to 10 years after surgery.
In our study, we evaluate the social costs and benefits of surgical intervention against obesity. As a secondary analysis, we also consider the social costs and benefits of pharmacotherapy, although our results largely confirm its modest social benefits.
To our knowledge, few studies have quantified the broader social value of either surgical or pharmaceutical interventions against obesity, including the long-term effects on health, medical costs, earnings, and the social costs of changes to government spending or revenue. Rather, the existing literature has focused its attention on the private benefits and costs to an employer (or insurer) that covers bariatric surgery. Finkelstein and Brown (2005) estimated annual health care costs attributable to obesity and compared those to the cost of the surgery and of work days lost. They calculate that it takes an average of 5 to 10 years before an insurer or employer would recoup its private costs. Similarly, Crémieux et al. (2008) conducted a similar analysis on a sample of patients who were followed before and after surgery. They estimate much larger benefits, and consequently a shorter cost-recovery period, of less than 3 years. A review of cost-effectiveness for surgery by Picot et al. (2009) reveals acceptable cost-effectiveness ratios in terms of net treatment cost (net of lifetime medical expenditures avoided) divided by incremental Quality Adjusted Life Years (QALYs) gained.
Although they clearly demonstrate the potential for health cost savings, these studies exclude important long-term impacts on health care costs and life expectancy, as well as financial effects outside the health care sector. First, most studies follow health care costs for less than three years after surgery. This excludes the long-term cost-savings that may be enjoyed, for example, by the Medicare program. An exception is Craig and Tseng (2002), who extrapolate lifetime cost savings from published data on lifetime medical costs attributable to obesity (Thomson et al., 2009). While useful, this extrapolation is limited by its substantial reliance on two data points – lifetime spending for patients with BMI of 32 and 37.5, respectively – even though the BMI of patients treated with bariatric surgery often exceeds 40. Similar issues arise with the analysis of life expectancy. Most studies focus on the direct effects, but do not take a long-run perspective, which would consider competing risks, reinforcing co-morbid conditions, and other aspects of health dynamics. Finally, little is known about the effects on wages, taxes, productivity, and annuity burdens for the public-sector, along with the associated deadweight costs created or avoided.
To address these diverse gaps in the literature, we estimate the lifetime social costs and benefits of medical interventions to treat obesity. Our study relies on the Future Elderly Model (FEM), an established and well-studied microsimulation model of aging and health. The FEM permits forecasts of lifetime health and economic trajectories for individuals possessing different baseline health characteristics and treated with different health interventions. We combine the machinery of the FEM with current estimates on the long-term effectiveness of surgical and pharmaceutical treatments to estimate long-term impacts on: longevity; competing comorbidities; health care costs (private, Medicare and Medicaid); tax revenue; and Social Security expenditures (disability and old-age pensions). We assess the sensitivity of all our estimates to a range of assumptions about the effectiveness of each intervention and also provide confidence intervals for the net value of each scenario.
The paper is organized as follows. Section B provides an overview of the model and the assumptions used regarding the effectiveness of the interventions. Section C presents results for the eligible population under various scenarios. Section D estimates the long-term aggregate effects of a policy that mandated (or strongly encouraged) the intervention on the eligible population. Finally, we discuss the implications of our results in section E.
B. Methods
Here, we describe both the FEM, which is the underlying engine for our results, as well as the way in which obesity interventions are incorporated.
B.1 The Future Elderly Model
The Future Elderly Model was developed to forecast long-term health and health care costs under different scenarios for medical technology development and utilization (Goldman et al., 2004). Its unique feature is to follow, in a microsimulation framework, the evolution of individual-level health trajectories, rather than the average or aggregate health characteristics of a cohort. It has been used for a number of purposes, including estimating the value of new medical technologies and treatments (Goldman et al., 2005) and estimating the value of prevention (Goldman et al., 2009). It covers the age 50+ population, using data from the Health and Retirement Study (HRS). The current version of the model includes a number of non-health outcomes, including: retirement, earnings, savings, tax payments, and participation in public programs like Social Security and Disability Insurance. This permits analysis of implications for a broad range of markets and institutions. As shown in Goldman et al. (2010), better health usually implies higher Social Security expenditures and tax revenues, which alter the fiscal impacts of various health scenarios.
B.1.1 Health and economic outcomes module
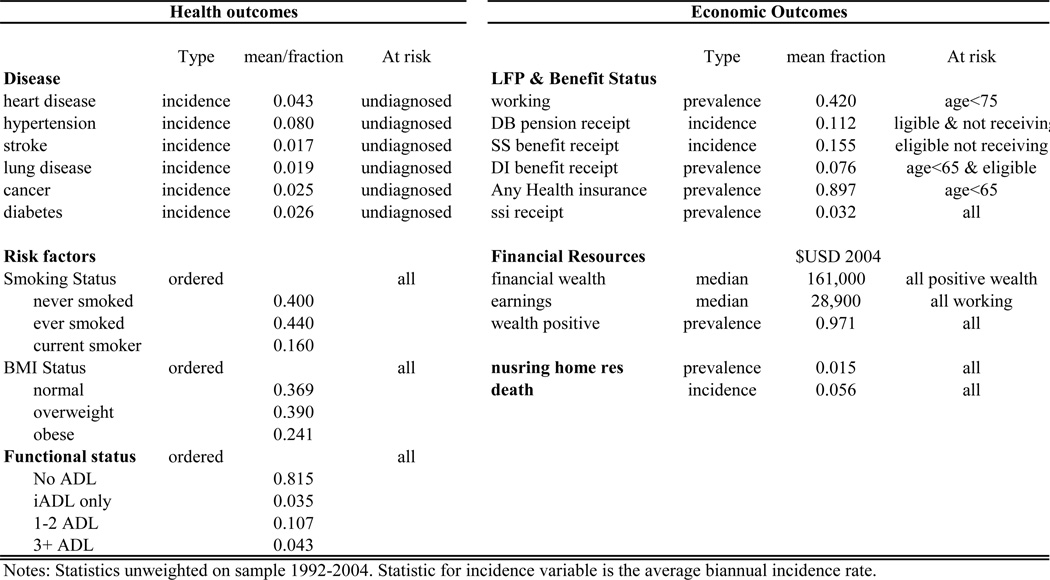
The model has three core components. The first predicts trajectories for health and economic outcomes. Taking as an input a cohort of individuals with particular baseline health and economic characteristics, this component predicts the likely evolution of individuals’ health and economic outcomes. Technically, it consists of 21 nonlinear transition equations from a period t state to a period t+1 state. Each equation depends upon fixed socio-demographic covariates (race, education, and gender), in addition to the health and economic state variables in the model.
Three sets of outcomes or states appear. The first consists of health measures, including: health indicators for physician-diagnosed diseases; activities of daily living (ADL) limitations; and risk factors such as smoking and BMI. For example, log BMI at time t+1 is modeled as a function of lagged log BMI and other covariates at time t. Or, the rate of incidence of diabetes at year t+1 is a function of health conditions already diagnosed at t, including hypertension, BMI and heart disease. Estimation of the relevant transition probabilities is done using longitudinal data from the Health and Retirement Study (1992–2004).
The second set of outcomes consists of economic variables, including: earnings, household wealth, labor force status, disability benefit status, and social security claiming status. These transition probabilities are also estimated from HRS data. The last consists of nursing home entry – a major predictor of health costs – and mortality. Both of these hazards are also estimated from HRS data.
Two important assumptions are maintained in the estimation. The first is that economic transitions do not affect health transitions. There is little evidence in panel data that changes in economic circumstances affect health (Adams et al., 2003; Smith, 2007; Michaud and van Soest, 2008). However, we do allow for baseline economic circumstances at age 50 to affect health trajectories. Second, for reasons of parsimony, we embed expert clinical knowledge about health transitions into the model (Goldman et al., 2004). For example, we allow for causal effects of one health state on another only when there is a clear causal link established in the clinical literature – e.g., the demonstrated effect of hypertension on heart disease. Unsubstantiated causal effects – e.g., of cancer on hypertension – are assumed to be zero by construction. We tested sensitivity to this approach, and found that relaxing these restrictions altered the parameter estimates somewhat, but they did not significantly alter long-term predictions for the scenarios we considered. In the appendix, we give more detail on the estimation of our transition rates and provide statistical justifications for our assumptions.
BMI is considered a risk factor for diabetes, heart disease, stroke and hypertension. We use a spline in log BMI with a knot at a BMI of 30 units, which is the threshold for obesity. The spline above log (30) captures the effects of excess BMI in percentage terms. We omit the spline variable below 30, because there is virtually no effect of BMI on disease incidence between the values of 25 and 30, but large positive effects below 25. We do not allow the obesity interventions to target groups with BMI below 25. Our estimation results show that for those with a BMI over 30, a 25% increase in weight increases the incidence rate of diabetes by 1.7 percentage points, the incidence rate of heart disease by 0.9 percentage points, and the probability of having any ADL limitations by 5.2 percentage points. These numbers encompass only the direct effects on health; the total health effect implied by the dynamic model is more complex – e.g., diabetes affects the incidence of heart disease, which then affects the incidence of ADL limitations, and so on. These feedback mechanisms more accurately capture the complex set of mechanisms that govern the health effects of medical interventions. They are embedded in the model.
B.1.2 Public expenditures and revenue outcomes module
The second component is a policy module that transforms health and economic outcomes into public expenditures and revenues, using tax and expenditure calculators. The “medical expenditure calculator” predicts expected Medicare, Medicaid and private medical expenditures, given a set of health, economic, and demographic states and characteristics. The predictions are based on data from the Medical Expenditure Panel Survey (MEPS) prior to age 65, and the Medicare Current Beneficiary Survey (MCBS) after age 65. We run regressions of total expenditures on health outcomes (physician-diagnosed diseases, ADLs), demographics (age, gender, race and education), and nursing home status. Details on those regressions are provided in the appendix. The model also includes calculators for tax revenue, and Social Security Old-Age and Disability benefits; all these have been validated against administrative totals for these programs.
B.1.3 New cohorts module
A third component of the model is used to “refresh” the cohorts used in the model. Specifically, this draws in a new cohort of 50 year-olds in each simulated year, as the model progresses forward in time. Through manipulation of this cohort’s characteristics, this “refresh” process allows for the incorporation of simulated trends in health, socio-demographic and economic outcomes. For example, we can consider the effects of smoking reductions by gradually decreasing the prevalence of smoking in the incoming cohorts. Long-term forecasting is known to be imprecise, and important assumptions are made concerning these trends in the 50 year-old cohort’s characteristics, so as to enhance the credibility of the long-run predictions. We discuss these assumptions further in Section D, when we implement long-term scenarios for the interventions we consider.
A complete technical appendix containing details on the modeling approach is available online.3
B.2 Interventions Considered
Our primary focus is on interventions involving bariatric surgery, which shows the greatest clinical efficacy. The appendix reports the results of analyzing pharmacotherapy interventions, for the sake of comparison.
According to current guidelines, the first-line treatment for obesity is weight management through exercise and reduced calorie intake. If first-line treatment fails, bariatric surgery may be prescribed, based on weight status and the presence of co-morbidities.
Individuals are classified as obese if their BMI exceeds 30. Within this group, “class 1 obesity” refers to those with a BMI between 30 and 34.9, class 2 obesity to those with a BMI between 35 and 39.9, and finally class 3 (or morbid) obesity to those who have a BMI over 40. The co-morbidities most often considered are hypertension, diabetes and heart disease/stroke (U.S. Department of Health and Human Services, 2001).
There are various ways in which surgery may be used to induce weight loss. The most common procedure entails a "gastric bypass," which consists of partitioning the upper part of the stomach in order to create a pouch connected directly to the intestine. The procedure, also known as Roux-en-Y is commonly done laparoscopically (through a small incision in the abdomen). It usually requires 3 to 5 days of inpatient stay, and the actual operation lasts less than an hour (Dixon et al., 2008). Another common procedure is adjustable gastric banding, which constricts the top of the stomach so as to reduce its size. Vertical banded gastroplastry (stapling) is not used frequently at the present time. Our focus will be on Roux-en-Y gastric bypass, which enjoys the fullest base of evidence on effectiveness and costs.
Patients with BMI over 40, or BMI over 35 with high-risk comorbidities are the target population for surgery as second-line treatment (Picot et al., 2009). Picot et al. (2009) reported on a meta-analysis of such treatments and conclude that gastric bypass surgery reduces weight up to 25% on average, with effects varying according to the target population. In particular, one cohort study (Sjostrom et al., 2004) suggests that these effects are close to permanent with weight loss of 17% relative to baseline after 10 years. Cremieux et al. (2008) report that the average cost of the procedure ranges from $17,000 for laparoscopic gastric bypass to $26,000 for open bypass surgery, which includes additional cost incurred in the month before the surgery, costs incurred in surgery, and additional costs incurred in the first 2 months following surgery. These estimates thus account for the costs for treating post-surgery complications: gastrointestinal symptoms, nutrition and electrolyte abnormalities, bleeding, and reoperation (Shekelle et al., 2004). 30-day mortality risk associated with bariatric surgery ranged from 0.2% to 1.9%, and the more procedures a surgeon performs, the lower is the mortality risk (Shekelle et al., 2004). We do not consider this modestly elevated mortality risk in the bariatric surgery scenarios. This causes us to slightly overestimate the benefits from bariatric surgery. Since most procedures are done using laparoscopic surgery, we use in our scenarios a conservative estimate for treatment costs of $20,000. These serve as our baseline efficacy and cost impact parameters.
C. Cohort Analysis Scenarios
We begin by simulating the effect of treating eligible 50 year-olds, and following the lifetime consequences of the treatment intervention for them. We call these the “cohort analysis scenarios.”
C.1 Scenarios
For our baseline analysis, we define eligibility for the surgery based on two characteristics: BMI, and the presence of “qualifying” co-morbidities. In particular, we consider the presence of diabetes, heart disease, hypertension or ADL limitations, as qualifying comorbidities granting eligibility to those with a BMI between 35 and 40 (Picot et al, 2009).
For bariatric surgery, we will consider two eligibility criteria:
Current Eligibility: those with BMI over 40, or BMI between 35 and 40 with qualifying co-morbidities. This follows current medical guidelines.
Extended Eligibility: those with BMI over 35 or those with BMI between 30 and 35 with qualifying co-morbidities. Compared with the current eligibility scenario, this extends eligibility to those with BMI between 35 and 40 and no pre-existing conditions, and to those with BMI between 30 and 35 and qualifying pre-existing conditions (defined to be any of the following: heart disease, hypertension, diabetes and stroke).
According to these criteria, there are 1.26 million individuals aged 50 eligible for the surgery under the current eligibility criteria, and 2.66 million under the extended eligibility criteria.
In our baseline scenarios, we apply a 25% weight loss to those who get the surgery, based on the clinical evidence discussed above. We consider this weight loss to be permanent. However, in sensitivity analysis, we consider a scenario where patients regain 50% of the lost weight after 10 years. We also consider sensitivity analyses where the surgery reduces weight by 15% and 35%, respectively, rather than by the 25% assumed in the baseline scenario. These analyses are also helpful in illustrating the impact of excluding detailed side effects from our simulations, since the most severe side effects mitigate the positive health benefits of the treatment.
We use the microsimulation model to project the experience of the 2010 cohort of age 50 individuals under the status-quo, and under each of the four scenarios above. We conduct 1000 simulations and compute the mean outcomes. We use a 3% real discount rate to compute present values from the age of 50. We define healthy life years as years lived without ADL limitations and compute other aggregates in 2010 dollars.
C.2 Results
In the current eligibility scenario, bariatric surgery achieves a permanent reduction in weight of 25% among those with a BMI over 40, or among those with a BMI between 35 and 40 with qualifying co-morbidities. Table 1 gives the simulation results in terms of net present values per capita among the eligible patients. Under the baseline (no intervention) scenario, those eligible have a total life expectancy of 28.8 years at age 50, and a healthy life expectancy of 19.9 years. This implies that they may expect to live close to 8.9 years with ADL limitations. Under the bariatric surgery intervention, they live on average 1.55 years longer and spend 2.91 additional years without ADL limitations. Hence, this implies that their unhealthy life expectancy is reduced by 1.36 years. The present value of their total medical costs is reduced by $4,649: $3,247 of this reduction accrues to Medicare, $276 to Medicaid, and $1,126 to other (private) sources. Because eligible patients live on average 1.15 years longer, the lifetime cost-savings are somewhat muted by their longer life-span.
Table 1.
Bariatric Surgery Cohort Simulation Results
| Current Eligibility | Expanded Eligibility | |||||
|---|---|---|---|---|---|---|
| Baseline | Intervention | Effect | Baseline | Intervention | Effect | |
| Disability free life expectancy | 19.92 | 22.83 | 2.91 | 20.91 | 22.66 | 1.75 |
| Life expectancy | 28.80 | 30.35 | 1.55 | 28.79 | 29.87 | 1.08 |
| Medical costs ($2010) | ||||||
| Medicare | 136 737 | 133 489 | −3 247 | 130 115 | 126 236 | −3 879 |
| Medicaid | 54 749 | 54 474 | −276 | 50 276 | 49 950 | −326 |
| Private Medical Cost | 162 749 | 161 623 | −1 126 | 157 815 | 156 058 | −1 756 |
| Total | 354 234 | 349 585 | −4 649 | 338 205 | 332 244 | −5 961 |
| Other fiscal outcomes ($2010) | ||||||
| Tax Revenue | 82 585 | 84 451 | 1 866 | 81 255 | 82 319 | 1 065 |
| SSI, DI, OASI | 135 931 | 143 381 | 7 450 | 126 074 | 130 960 | 4 886 |
| Earnings | 239 436 | 243 544 | 4 107 | 237 469 | 239 797 | 2 327 |
Notes: averages from 1000 simulations of the projection for a cohort entering at age 50 in 2010. Real discount rate is 3%. All amounts in $2010 dollars. SSI = Supplemental Security Income, DI = Disability insurance, OASI = old-age social insurance. Private medical costs cover other costs not covered by Medicare and Medicaid (e.g. costs covered by employer). Please refer to text for definition of current and expanded eligibility.
This effect is clear when we look at the implications for other government expenditures, most notably Social Security (OASI). There is an increase of $7,450 in spending for programs (SSI, DI, and OASI) following the intervention. Some of that is due to higher per-period earnings, due to a positive effect of weight loss on labor force participation, retirement and wages. Although the present value of earnings increase by $4,107, leading to a tax revenue increase of $1,866, the overall effect on other fiscal outcomes is negative (higher revenue is more than offset by larger increases in other spending).
Since income/payroll taxation involves deadweight loss, more spending from government programs (Medicare, Medicaid, SSI, DI, OASI) based on income/payroll taxation implies more deadweight loss, and vice-versa. Recent literature shows that for every one dollar increase in government revenue in the United States, deadweight loss ranges from 0.62 dollars (Giertz 2009) to 0.76 dollars (Feldstein, 2008).4 We take the average of these estimates and assume that the marginal social cost of an additional dollar of government spending is 0.69 dollars. In our simulations, therefore, the additional deadweight loss from an intervention is calculated as: 0.69*(SSI, DI, $OASI effect + $Medicare Effect + $Medicaid Effect − $Tax Revenue Effect). For the current eligibility scenario in Table 1, the deadweight loss is: 0.69 * (7450 − 3247 − 276 − 1866) = $1,422, indicating an increase in deadweight loss.
We define “total economic cost” as the sum of: additional deadweight loss, additional medical costs, lost earnings, and the treatment cost of bariatric surgery. This turns out to be $12,666 (i.e., cost of treatment + change in medical costs + change in deadweight loss – change in income = $20,000 − $4,649 + $1,422 − $4,107). Medical costs alone amount to $15,351. Accounting for (negative) non-medical costs offsets 20% of the medical costs.
In the extended eligibility scenario, we extend treatment to those patients with a BMI of 35 to 40 but no qualifying co-morbidities, and to patients with a BMI of 30 to 35 and qualifying co-morbidities. Hence, the marginal patient added to this scenario suffers from a less severe form of obesity than in the current eligibility scenario. The results in Table 1 show that the total life expectancy effect is reduced to 1.08 years, or 30% less than before. Healthy life expectancy rises by 1.75 years, rather than 2.91. Expanding eligibility doubles the size of the treated population, which now represents 53% of the total population covered by the HRS cohort. From the fraction treated, we can deduce that the effect on life expectancy in the newly added group is 0.67 years ((1.08 − (1−0.53) * 1.55)/0.53 = 0.67), while the effect on healthy life expectancy is 0.72 years ((1.75 − (1−0.53) * 2.91)/0.53 = 0.72). Hence, the marginal effect is smaller than in the current eligibility scenario, as expected. Because the life expectancy effect is smaller, there is less upward pressure on lifetime medical costs. In terms of medical costs, the average effect is a $5,961 reduction, which is larger than the $4436 reduction obtained under the current eligibility scenario. Since the longevity effect is smaller, there is a larger negative effect on lifetime medical spending. Based on these numbers and the proportion of newly eligible (53%), we can infer that cost savings are $7,124 ((−5,961 − (1−0.53)*(−$4,649))/0.53 = −$7,124) among the newly eligible. Again, because the average life expectancy effect is lower, earnings, taxes and spending rise by less than in the current eligibility scenario: earnings rise by $2,327; taxes rise by $1,065; and other spending by $4,886. The total economic cost, as defined above, is $11,447. For the newly eligible, the total economic cost is $10,366.
These effects do not yet account for the value of life extension. To incorporate this, we value life expectancy gains using the estimates of Viscusi and Aldy (2003), who argue that the best available value of a statistical life-year is $200,000.5 We define the net value of the intervention as the value of life expectancy gains minus the total economic costs (or plus the economic savings). To construct confidence intervals for the net value, we take the 2.5 percentile and 97.5 percentile of the distribution of values drawn from our 1000 simulations. In addition to the net economic value, we also report the incremental cost-effectiveness ratio (ICER, calculated as additional cost divided by additional effectiveness), which is a common metric for the evaluation of medical interventions. Note that, unlike conventional implementations of ICER that consider only direct medical costs, we use total social cost in our calculations.
Table 2 shows those results for the net value estimates and the corresponding confidence intervals. The currently eligible patients enjoy a net value from life expectancy gains of $310,028 ($200,000 times life expectancy gain of 1.55 in Table 1), while those eligible in the extended eligibility scenario enjoy a net value of $216,000 ($200,000 times life expectancy gain of 1.08). These benefits are much larger than the total economic costs, which are $12,666 in the current eligibility scenario and $10,366 for the newly eligible. Thus, treating both types of patients has a net positive economic value. The net value is $297,362 (95% confidence interval (CI): $102,266 to $475, 271) for those in the current eligibility scenario and $204,968 (95% CI: $100,924 to $329,632) for those in the extended eligibility scenario. Hence, both these scenarios provide considerable value. In terms of the social ICER, both scenarios yield a ratio below $12,000, easily meeting the conventional acceptability standards of NICE in the UK (ICER below $30,000–$47,000) (Appleby et al., 2007) or those suggested by Garber and Phelps (1997) (below twice the average income). As shown in the appendix, these social ICER’s remain relatively low, even if we conservatively assume surgery is 10 percentage points less effective ($16,405), or if we conservatively assume a 50% chance of relapse after 10 years ($12,318).
Table 2.
Cost-Effectiveness: Bariatric Surgery
| Current Eligibility | Extended Eligibility | |
|---|---|---|
| Additional Medical Costs | −4 649 | −5 961 |
| Additional Earnings | 4 107 | 2 327 |
| Additional Deadweight Loss | 1 422 | −264 |
| Treatment Cost | 20 000 | 20 000 |
| Total Economic Cost | 12 666 | 11 447 |
| Life Expectancy Gain | 1.55 | 1.08 |
| Value of Life Expectancy Gain | 310 028 | 216 415 |
| Net Value | 297 362 | 204 968 |
| 95% CI | (102 266 to 475 271) | (100 924 to 329 632) |
| Incremental Cost-Effectiveness Ratio | 8 171 | 10 579 |
Notes: averages from 1000 simulations of the projection for the cohort age 50 in 2010. Real discount rate is 3%. All dollar figures are in terms of 2010 dollars. See text for definition of the scenarios. The additional medical costs, earnings and deadweight loss (see text for definition) are added to the treatment cost to define the total economic cost. The value of the life expectancy gain is computed using a value of $200,000 per statistical life year. The net value is defined as the difference between the value of the life expectancy gain minus the total economic cost. A 95% confidence interval is reported for this value by taking the 2.5 and 97.5 percentile of the net values over 1000 simulations. The incremental cost effectiveness ratio is the total economic cost divided by the gain in life years from the intervention.
Also in the Appendix, we use the same methodology to compute the social value of pharmacotherapy using current guidelines. The net value is $12,834 (95% CI: −19,628 to 60,921) in the current eligibility scenario and $2,710 (−20,250 to 34,691) in the extended eligibility scenario. Hence, the net value calculations do not rule out negative values with 95% confidence. Furthermore, the social ICER estimates are large for both scenarios: $51,066 in the current eligibility scenario and $123,260 in the extended eligibility scenario. Neither of these would meet the NICE threshold. Hence, we conclude that pharmacotherapy is dominated by surgery in the sense of social cost-effectiveness.
D. Long-Term Population Analysis
The earlier section showed that surgical intervention improves lifetime health outcomes for a cohort of 50 year-olds treated, and that these improvements dominate the modest or even negative effects on their lifetime costs. It remains to investigate the population-wide trends implied by these cohort effects.
Under the status quo, Ruhm (2007) has projected large increases in obesity among 50 year-olds, with the largest increases coming for class 2 (BMI between 35 and 40) and class 3 obesity (BMI over 40). Hence, we take Ruhm’s projections up to 2030 and project them out to 2050. This represents the “do-nothing” scenario and leads to a prevalence of obesity of over 50% in 2050, where 15.4% of the population aged 50+ suffers from class 3 obesity (BMI 40 and over). Although this status-quo scenario may be pessimistic, it provides a baseline against which we can measure a range of interventions that would start treating eligible members of new cohorts with surgery and pharmacotherapy.
D.1 Scenarios
We study aggregate trends under several alternative scenarios for obesity treatment. First, the status quo scenario employs obesity trends among incoming cohorts according to Ruhm’s projections. Trends for other conditions are projected using the methodology proposed by Goldman et al. (2004). In addition, the status quo scenario embeds the following assumptions:
The size and demographic composition (race and gender) of the entering 50 year-old cohort is based on Census projections.
Exogenous mortality improvements of 0.88% annually are assumed, as in the Social Security Board of Trustees intermediate scenario. Social Security is more conservative than the Census in its mortality projection.
Real wages rise by 1.1% per year over the long-term. This also comes from the intermediate scenario of Social Security.
-
Real medical costs rise by 1.5% more than earnings in 2004, but this excess growth rate declines linearly to 1% in 2033, 0.4% in 2053, and −0.2% in 2083.
This is similar to the assumptions made by the Centers for Medicare and Medicaid Services.
In sensitivity analyses reported in the appendix, we vary assumptions 2, 3 and 4. We also consider how these results are affected by the introduction of cures for obesity-related conditions, which would lower the marginal benefits of the treatments we consider. A more complete description of the population model is given in the technical appendix.
The second set of scenarios we consider are the “current eligibility” scenarios, in which all patients eligible according to current guidelines are treated. Specifically, we model interventions that apply bariatric surgery according to current guidelines, and separately consider interventions that apply pharmacotherapy according to current guidelines. In addition, we also consider the joint effects of pharmacotherapy and bariatric surgery, since they can be implemented simultaneously. If a patient is eligible for both treatments, she receives only bariatric surgery. This is because surgery delivers better efficacy, and we were unable to find clear evidence that simultaneous use of surgery and pharmacotherapy is indicated for such patients. Given that pharmacotherapy delivers small effects, most of the changes in the long-term simulations occur because of the surgery intervention.
Finally, we consider a set of “extended eligibility” scenarios, based on the extended guidelines for bariatric surgery and pharmacotherapy specified above. Once again, we model the separate application of bariatric surgery and pharmacotherapy, and then consider them jointly (considering the most effective treatment for each patient).
We focus in particular on the net revenue effect and on the net social value. The net social value for a given year considers the life extension benefit from added population minus treatment cost of those newly treated and the deadweight loss calculus involving revenue and expenditures. In the text, we also discuss the present value of this net social value in 2004 for the population that will be treated between 2004 and 2050. We use a discount rate of 3%.
D.2 Results
In Table 3, we present the results of the status quo along with confidence intervals. As discussed in the online technical appendix, the model forecasts the age 65+ population to within 2 million of Social Security’s forecasts. It also predicts that, due to worsening health for incoming cohorts, future elderly cohorts will face greater prevalence of chronic conditions like diabetes and heart disease. Obesity is projected to afflict one in two Americans and the prevalence of class 3 obesity is projected to grow four-fold. The prevalence of diabetes is projected to double over the next 40 years, and the size of the Medicare program grows to $1.5 trillion dollars by 2050. In general, the confidence intervals are quite tight around the average estimates.
Table 3.
Status Quo Population Estimates 2004–2050
| Year | |||||
|---|---|---|---|---|---|
| 2004 | 2030 | 2050 | |||
| mean | (95% CI) | mean | (95% CI) | ||
| Population size (Million) | 80.8 | 121.5 | (120.5 to 122.2) | 146.8 | (145.9 to 147.8) |
| Population 65+ (Million) | 36.3 | 65.5 | (64.5 to 66.3) | 80.2 | (79.4 to 81.2) |
| Prevalence of selected conditions | |||||
| Obese 3 (BMI >=40) (%) | 3.9% | 10.4% | (10.0% to 10.9%) | 15.4% | (14.9% to 15.8%) |
| Obese 2 (35 <= BMI < 40)(%) | 6.3% | 12.9% | (12.3% to 13.4%) | 15.3% | (14.7% to 15.8%) |
| Obese 1 (30 <= BMI < 35)(%) | 17.9% | 24.8% | (24.2% to 25.6%) | 24.8% | (24.3% to 25.5%) |
| Overweight (25<=BMI<30) (%) | 38.2% | 30.7% | (29.8% to 31.4%) | 26.9% | (26.1% to 27.5%) |
| Diabetes | 17.1% | 28.2% | (27.6% to 28.9%) | 33.0% | (32.4% to 33.7%) |
| Heart disease | 23.3% | 30.0% | (29.2% to 30.7%) | 32.2% | (31.4% to 32.9%) |
| Hypertension | 51.2% | 62.9% | (62.2% to 3.6%) | 66.5% | (65.9% to 67.1%) |
| Public revenues from age 51+ ($bn) | |||||
| Federal personal income taxes | 237.5 | 293.7 | (282.2 to 304.8) | 367 | (358.2 to 377.5) |
| Social security payroll taxes | 81.2 | 103.4 | (100.7 to 105.9) | 126.8 | (124.6 to 129.3) |
| Medicare payroll taxes | 20.9 | 24.8 | (24.2 to 25.4) | 30 | (29.4 to 30.6) |
| Total Revenue | 339.6 | 421.9 | (407.1 to 435.8) | 523.7 | (512.5 to 536.6) |
| Public expenses from age 51+ ($bn) | |||||
| Old Age and Survivors Insurance benefits (OASI) | 448.8 | 1215.6 | (1195.3 to 1242.6) | 1698 | (1670.9 to 1727.2) |
| Disability Insurance benefits (DI) | 42.3 | 53 | (46.5 to 59.4) | 79.4 | (67.2 to 89.7) |
| Supplementary Security Income (SSI) | 14.7 | 36.3 | (34.5 to 38.3) | 59.6 | (57.1 to 62.2) |
| Medicare costs | 304.3 | 974.1 | (949.7 to 996.4) | 1511.4 | (1481.3 to 1542.7) |
| Medicaid costs | 127.3 | 328.4 | (309.3 to 346.0) | 593.3 | (568.5 to 625.2) |
| Medicare + Medicaid | 431.6 | 1302.5 | (1265.9 to 1332.5) | 2104.6 | (2058.9 to 2157.5) |
| Total Expenditures | 937.3 | 2607.3 | (2558.1 to 2651.2) | 3941.6 | (3878.5 to 4005.6) |
| Net Revenue | −597.8 | −2185.5 | (−2232.0 to −2137.7) | −3417.9 | (−3482.8 to −3354.7) |
| Total medical cost for age 51+ ($bn) | 986.4 | 2426.8 | (2381.1 to 2465.5) | 3707.2 | (3647.1 to 3772.0) |
Notes: Table reports the average of 100 simulations for population scenarios in the years 2004, 2030 and 2050. 95% confidence intervals based on simulations is reported for 2030 and 2050. Confidence intervals do not change much as we increase the number of replications.
Table 4 presents results of the current eligibility scenario. Because of the higher life expectancy experienced by those treated, the age 65+ population grows by 0.4 million in 2030 and 1.44 million in 2050. Obesity falls by 14 percentage points; against the status quo level of 55.5%, this represents a decrease of 25%. Perhaps more importantly, class 3 obesity would be 3.4% in 2050, lower than its 2004 level. A similar reduction would occur for class 2 obesity. This implies that in 2030, total medical costs for the age 51+ population would decrease by $21.6 billion. By 2050, the decline would be $24.8 billion, which would represent roughly 1% of medical costs at that time. More than half this decline would come from Medicare and Medicaid. Both revenues and Social Security benefits would increase slightly due to higher life expectancy. The federal government enjoys net savings of $11.4 billion in 2030, as the medical cost savings offset increases in the annuity burden. By 2050, increases in life expectancy largely erase these gains. Overall, obesity interventions have limited net fiscal effects, as medical cost savings are offset by a higher annuity burden. The net social value is nonetheless substantial both in 2030 ($63.3 billions) and 2050 ($245.7 billions). This is because we include the benefits from life extension in this calculation. The social value grows with time because population is growing faster. This growth is due to life extension.
Table 4.
Current Eligibility Population Estimates 2004–2050
| Relative Change to Status Quo |
Absolute Change to Status Quo |
|||
|---|---|---|---|---|
| Year | Year | |||
| 2030 | 2050 | 2030 | 2050 | |
| Population size (Million) | 0.4% | 1.0% | 0.47 | 1.51 |
| Population 65+ (Million) | 0.6% | 1.8% | 0.42 | 1.44 |
| Prevalence of selected conditions | ||||
| Obese 3 (BMI >=40) (%) | −73.6% | −76.1% | −0.08 | −0.12 |
| Obese 2 (35 <= BMI < 40)(%) | −43.0% | −45.2% | −0.06 | −0.07 |
| Obese 1 (30 <= BMI < 35)(%) | 10.1% | 20.8% | 0.03 | 0.05 |
| Overweight (25<=BMI<30) (%) | 31.0% | 45.0% | 0.10 | 0.12 |
| Diabetes | −5.4% | −7.5% | −0.02 | −0.02 |
| Heart disease | −1.6% | −2.2% | 0.00 | −0.01 |
| Hypertension | −0.6% | −0.7% | 0.00 | 0.00 |
| Public revenues from age 51+ ($bn) | 0.00 | 0.00 | ||
| Federal personal income taxes | 0.4% | 0.5% | 1.11 | 2.01 |
| Social security payroll taxes | 0.3% | 0.5% | 0.35 | 0.59 |
| Medicare payroll taxes | 0.3% | 0.5% | 0.08 | 0.14 |
| Total Revenue Effect | 0.4% | 0.5% | 1.54 | 2.73 |
| Public expenses from age 51+ ($bn) | ||||
| Old Age and Survivors Insurance (OASI) | 0.4% | 1.3% | 5.37 | 22.54 |
| Disability Insurance benefits (DI) | −1.3% | −1.7% | −0.69 | −1.36 |
| Supplementary Security Income (SSI) | 0.6% | 1.8% | 0.23 | 1.07 |
| Medicare costs | −1.2% | −1.1% | −11.35 | −16.27 |
| Medicaid costs | −1.0% | −0.4% | −3.37 | −2.31 |
| Medicare + Medicaid | −1.1% | −0.9% | −14.72 | −18.58 |
| Total Expenditure Effect | −0.4% | 0.1% | −9.82 | 3.67 |
| Net revenue effect ($bn) | 0.5% | 0.0% | 11.37 | −0.94 |
| Total medical cost for age 51+ ($bn) | −0.9% | −0.7% | −21.56 | −24.80 |
| Net social value ($bn) | 63.3 | 245.7 | ||
Notes: Table reports the effect relative to status quo for the current eligibility scenario in 2030 and 2050. Both relative (%) and absolute ($) changes reported. The net revenue effect is defined as the change in total revenue minus the change in total public expenditures (excluding private medical expenditures). The net social value is defined in the text and takes account of the value of life extension.
Next, we look at a scenario where we extend the population eligible for surgical and pharmaceutical intervention. Results are reported in Table 5. The additional cases treated are now likely to benefit less. The aggregate effects on population are larger, mostly because more people are treated. The extended scenario does not do much to further reduce the obesity rate, although there is a further reduction in class 2 obesity, which falls by 10% instead of 7%. Total medical cost savings are $22.4 billion in 2030 and $32.2 billion in 2050; this is roughly 50% larger than in the current eligibility scenario. The net fiscal effect remains positive ($18.9 billion in 2030 and $8.6 billion in 2050) and is substantially larger than in the current eligibility scenario, although still very modest in the context of total federal spending. The net social value calculations show that the net social value in this scenario is similar to the ones found for the current eligibility scenario. It is slightly lower in 2030 ($53.5 vs. $63.3 billion) but larger in 2050 ($283.7 vs. $245.7 billion).
Table 5.
Extended Eligibility Population Estimates 2004–2050
| Relative Change to Status Quo |
Absolute Change to Status Quo |
|||
|---|---|---|---|---|
| Year | Year | |||
| 2030 | 2050 | 2030 | 2050 | |
| Population size (Million) | 0.4% | 1.2% | 0.51 | 1.77 |
| Population 65+ (Million) | 0.7% | 2.1% | 0.47 | 1.71 |
| Prevalence of selected conditions | ||||
| Obese 3 (BMI >=40) (%) | −78.1% | −79.6% | −0.08 | −0.12 |
| Obese 2 (35 <= BMI < 40)(%) | −67.7% | −68.5% | −0.09 | −0.10 |
| Obese 1 (30 <= BMI < 35)(%) | −26.3% | −19.2% | −0.07 | −0.05 |
| Overweight (25<=BMI<30) (%) | 33.4% | 47.8% | 0.10 | 0.13 |
| Diabetes | −9.8% | −12.8% | −0.03 | −0.04 |
| Heart disease | −2.1% | −3.0% | −0.01 | −0.01 |
| Hypertension | −1.7% | −2.0% | −0.01 | −0.01 |
| Public revenues from age 51+ ($bn) | ||||
| Federal personal income taxes | 0.4% | 0.6% | 1.31 | 2.27 |
| Social security payroll taxes | 0.4% | 0.5% | 0.40 | 0.65 |
| Medicare payroll taxes | 0.4% | 0.5% | 0.09 | 0.15 |
| Total Revenue Effect | 0.4% | 0.6% | 1.80 | 3.07 |
| Public expenses from age 51+ ($bn) | ||||
| Old Age and Survivors Insurance benefits (OASI) | 0.5% | 1.6% | 5.90 | 27.05 |
| Disability Insurance benefits (DI) | −1.3% | −1.7% | −0.70 | −1.36 |
| Supplementary Security Income (SSI) | 0.3% | 1.6% | 0.11 | 0.96 |
| Medicare costs% | −1.8% | −1.9% | −17.65 | −28.51 |
| Medicaid costs% | −1.4% | −0.6% | −4.72 | −3.67 |
| Medicare + Medicaid | −1.7% | −1.5% | −22.37 | −32.17 |
| Total Exependiture Effect | −0.7% | −0.1% | −17.06 | −5.53 |
| Net Revenue Effect | 0.9% | 0.3% | 18.86 | 8.60 |
| Total medical cost for age 51+ ($bn) | −1.4% | −1.2% | −34.07 | −45.02 |
| Net social value ($bn) | 53.5 | 283.7 | ||
Notes: Table reports the effect relative to status quo for the extended eligibility scenario. Both relative (%) and absolute ($) changes reported. The net fiscal effect is defined as the change in total revenue minus the change in total public expenditures (excluding private medical expenditures)
We computed the net present value in 2004 of the stream of social net benefits from 2004 to 2050. We discounted this stream using a 3% annual real rate of discount. We also counted the number of individuals who initiate treatment each year. This allows us to compute the average per capita net social value today of the population who would be treated during this period. This identifies the net social value each treated patient will expect to receive on average, from the year 2010 perspective. Under the current eligibility scenario, the net social effect is $827.5 billion, which represents $3,342.8 per capita. Under the extended eligibility scenario, we obtain a net social effect of $266 billion, which represents $733.8 per capita. The lower discounted value for the extended eligibility scenario is due to the increase in upfront treatment costs, which yield much lower benefits down the road. Because of projected trends in obesity and the size of entering cohorts, this number is much lower than in the cohort based analysis.
We varied a number of assumptions in these long-term scenarios. None of these assumptions, even those that are rather extreme, appears to affect the basic conclusion from the population analysis. Fiscal benefits are rather small in the aggregate whereas there is a substantial cut in the obesity rate in the population. The net social value calculations do not vary much across scenarios.
E. Discussion
For cohorts suffering from obesity, bariatric surgery promises substantial gains in life expectancy. The cohort of 50 year-olds eligible for surgery according to current guidelines can expect 1.5 years of additional life and 2.9 years of additional healthy life. Treatment leads to modestly higher social costs, primarily due to non-health care expenditures by the federal government, but these are more than justified by the increase in life expectancy. Formally, bariatric surgery is highly cost-effective from a social point of view, costing society less than $9,000 per life-year saved. This would continue to be true if the use of surgery were expanded past current eligibility guidelines, even though the absolute gains in health would be smaller for the marginally treated patients.
Pharmacotherapy does not appear to be cost-effective from a social point of view. Its absolute effect on health and life expectancy is much smaller and on the order of a few months. Moreover, estimates of net benefits are imprecise and do not rule out negative net social values. Taken together, the largest gains in life expectancy and the highest social value are likely to come from bariatric surgery, rather than currently available pharmacotherapies.
The above results describe the social value of these therapies to obese cohorts. From a population-wide perspective, there are also benefits in terms of reduced obesity rates, and lower public spending. From a societal perspective, per period medical costs fall almost immediately with the reduction in obesity, and increases in financial liability – from increased life expectancy -- take time to emerge. From a fiscal perspective, society sees upfront cost-savings from improved health. These savings erode over time with rising annuity burdens. The fiscal effects are thus relatively small. However, the net social effect is large, for the most part due to the increase in life expectancy of those treated.
The net value of obesity-treatment balances morbidity-reduction, longevity gain and changes in spending. Improvements in longevity generate private value, but also impose greater financial liabilities on public annuity and medical insurance schemes. The arithmetic tends to favor obesity treatment, because: the value of longevity improvement is high; the costs of greater longevity are delayed; and the benefits of morbidity reduction accrue in the very short-term.
Our analysis quantifies the substantial social value associated with the successful treatment of obesity using technologies available today. A significant unknown is the direction of future innovation, which could yield even more effective treatments for obesity, but potentially at higher cost. Modeling the likely course of future innovations to treat obesity is a natural next step.
Figure F.2. Summary Statistics of Outcomes in the Transition Model.
Table G.2.
Cost-Effectiveness: Pharmacotherapy
| Current Eligibility | Extended Eligibility | |
|---|---|---|
| Additional Medical Costs | −343 | −462 |
| Additional earnings | 300 | 91 |
| Additional Deadweight Loss | 44 | −94 |
| Treatment Cost | 5 000 | 5 000 |
| Total Economic Cost | 4 401 | 4 353 |
| Life Expectancy Gain | 0.09 | 0.04 |
| Value of Life Expectancy Gain | 17 235 | 7 062 |
| Net Value | 12 8234 | 2 710 |
| 95% CI | (−19 628 to 60 921) | (−20 250 to 34 691) |
| Incremental Cost-Effectiveness Ratio | 51 066 | 123 260 |
Notes: averages from 1000 simulations of the projection for the cohort age 50 in 2010. Real discount rate is 3%. All dollar figures are in terms of 2010 dollars. See text for definition of the scenarios. The additional medical costs minus additional earnings plus deadweight loss (see text for definition) are added to the treatment cost to define the total economic cost. The value of the life expectancy gain is computed using a value of $200,000 per statistical life year. The net value is defined as the difference between the value of the life expectancy gain minus the total economic cost. A 95% confidence interval is reported for this value by taking the 2.5 and 97.5 percentile of the net values over 1000 simulations. The incremental cost effectiveness ratio is the total economic cost divided by the gain in life years from the intervention.
Acknowledgments
This research was funded by Employee Benefits Security Administration US Department of Labor through Contract J-9-P-2-0033, the National Institutes of Aging through grant R01AG030824, the Roybal Center for Health Policy Simulation and the MacArthur Foundation Research Network on an Aging Society. Michaud acknowledges support from the Programme d’aide financière à la recherche et création (PAFARC) at UQAM. The authors are solely responsible for the content.
F. Appendix: Overview of the Future Elderly Model
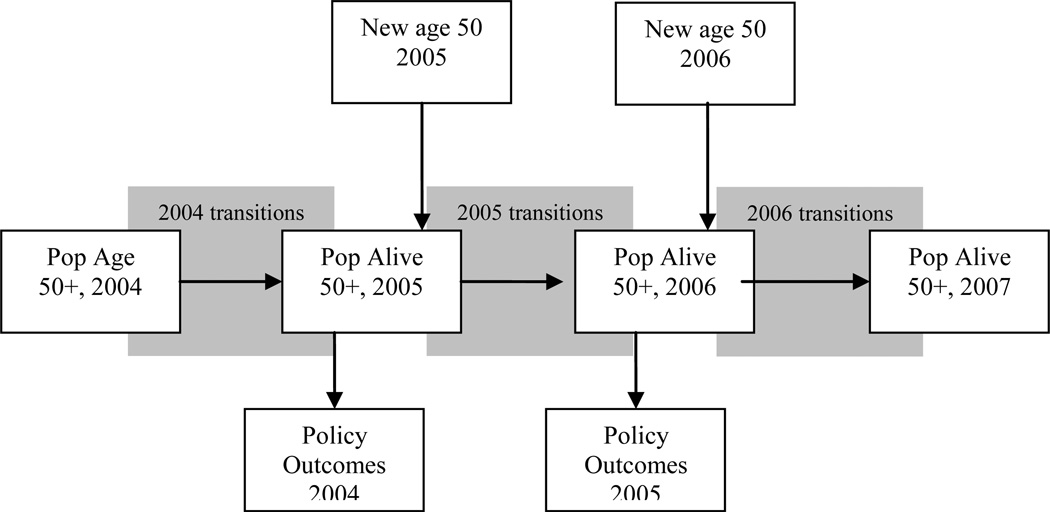
Figure F.1 gives an overview of the mechanics of the model. In this appendix, we focus on the key aspects of the model for this paper. Namely, we focus our attention on the key assumptions in the transition model and the health care cost model. A complete technical appendix containing details on the modeling is available online at https://sites.google.com/site/pcmichaud/Home/programs
Figure F.1. Overview of the Future Elderly Model.
We start in 2004 with an initial population aged 51+ taken from the HRS. We then predict outcomes using our estimated transition probabilities. Those who survive make it to the end of that year, at which point we calculate policy outcomes for the year. We then move to the following time period (two years later), when a new cohort of 51 and 52 year-olds enters. This entrance forms the new age 51+ population, which then proceeds through the transition model as before. This process is repeated until we reach the final year of the simulation.
F.1 Health Transition Model
We consider a large set of outcomes for which we model transitions. We list these outcomes in the next table along, along with summary statistics.
Since we have a stock sample from the age 51+ population, each respondent goes through an individual-specific series of intervals. Hence, we have an unbalanced panel over the age range starting from 51 years old. Denote by ji0 the first age at which respondent i is observed and jiTi the last age when he is observed. Hence we observe outcomes at ages ji = ji0, ⋯, jiTi.
We first start with discrete outcomes which are absorbing states (e.g. disease diagnostic, mortality, benefit claiming). Record as hi, ji, m = 1 if the individual outcome m has occurred as of age ji. We assume the individual-specific component of the hazard can be decomposed in a time invariant and variant part. The time invariant part is composed of the effect of observed characteristics xi and permanent unobserved characteristics specific to outcome m, ηi, m. The time-varying part is the effect of previously diagnosed outcomes hi, ji−1, −m, (outcomes other than the outcome m) on the hazard for m.6 We assume an index of the form zm, ji = xiβm + hi, ji−1, −mγm + ηi, m. Hence, the latent component of the hazard is modeled as
| (1) |
We approximate am, ji with an age spline. After several specification checks, a node at age 75 appears to provide the best fit. This simplification is made for computational reasons since the joint estimation with unrestricted age fixed effects for each condition would imply a large number of parameters.
The outcome, conditional on being at risk, is defined as
| (2) |
As mentioned we consider 8 outcomes which are absorbing states. The occurrence of mortality censors observation of other outcomes in a current year. Mortality is recorded from exit interviews.
First, we have binary outcomes which are not an absorbing state. We specify latent indices as in (1) for these outcomes as well but where the lag dependent outcome also appears as a right-hand side variable. This allows for state-dependence.
Second, we have ordered outcomes. These outcomes are also modeled as in (1) recognizing the observation rule is a function of unknown thresholds ζm. Similarly to binary outcomes, we allow for state-dependence by including the lagged outcome on the right-hand side.
The third type of outcomes we consider are censored outcomes, earnings and financial wealth. Earnings are only observed when individuals work. For wealth, there is a non-negligible number of observations with zero and negative wealth. For these, we consider two part models where the latent variable is specified as in (1) but model probabilities only when censoring does not occur. In total, we have M outcomes. We also include a set of other controls including gender, race and education level. The estimation strategy is explained in the online technical appendix.
F.2 Health Care Costs
In the FEM, a cost module links a person’s current state—demographics, economic status, current health, risk factors, and functional status—to 4 types of individual medical spending. The FEM models: total medical spending (medical spending from all payment sources), Medicare spending7, Medicaid spending (medical spending paid by Medicaid), and out of pocket spending (medical spending by the respondent). These estimates are based on pooled weighted least squares regressions of each type of spending on risk factors, self-reported conditions, and functional status, with spending inflated to constant dollars using the medical component of the consumer price index. We use the 2002–2004 Medical Expenditure Panel Survey (n = 14,098) for these regressions for persons not Medicare eligible, and the 2002–2004 Medicare Current Beneficiary Survey (n = 33, 231) for spending for those that are eligible for Medicare. Those eligible for Medicare include persons eligible due to age (65+) or due to disability status.
In the baseline scenario, this spending estimate can be interpreted as the resources consumed by the individual given the manner in which medicine is practiced in the United States at the beginning of the 21st century. Since Medicare spending has numerous components (Parts A and B are considered here), models are needed to predict enrollment. In 2004, 98.4% of all Medicare enrollees, and 99%+ of aged enrollees, were in Medicare Part A, and thus we assume that all persons eligible for Medicare take Part A. We use the 1999–2004 MCBS to model take up of Medicare Part B for both new enrollees into Medicare, as well as current enrollees without Part B. Estimates are based on weighted probit regression on various risk factors, demographic, and economic conditions. The HRS starting population for the FEM does not contain information on Medicare enrollment. Therefore another model of Part B enrollment for all persons eligible for Medicare is estimated via a probit, and used in the first year of simulation to assign initial Part B enrollment status. The MCBS data over represents the portion enrolled in Part B, having a 97% enrollment rate in 2004 instead of the 93.5% rate given by Medicare Trustee’s Report.
Since both the MEPS and MCBS are known to under-predict medical spending, we applied adjustment factors to the predicted three types of individual medical spending so that in year 2004, the predicted per-capita spending in FEM equal the corresponding spending in National Health Expenditure Accounts (NHEA), for age group 55–64 and 65 and over, respectively. For example, the predicted per-capita total medical spending for aged 65 and over in FEM 2004 is $13,920, while the corresponding number in NHEA is $14,797. The adjustment factor is calculated as $14,797 divided by $13,920, which is 1.06. Therefore the total medical spending for each aged 65 and over in FEM will be multiplied by 1.06.
The Medicare Current Beneficiaries Survey (MCBS) 2006 contains data on Medicare Part D. The data gives the capitated Part D payment and enrollment. When compared to the summary data presented in the CMS 2007 Trustee Report, the per capita cost is comparable between the MCBS and the CMS. However, the enrollment is underestimated in the MCBS, 53% compared to 64.6% according to CMS. To account for both the initial under reporting of Part D enrollment in the MCBS, as well as the CMS prediction that Part D enrollment will rise to 75% by 2012, the constant in the probit model is increased by 0.22 in 2006, to 0.56 in 2012 and beyond. The per capita Part D cost in the MCBS matches well with the cost reported from CMS. An OLS regression using demographic, current health, and functional status is estimated for Part D costs.
F.3 Revenues and Other Expenditures
We consider Federal, State and City taxes paid at the household level. We also calculate Social Security taxes and Medicare taxes. HRS respondents are linked to their spouse in the HRS simulation. We take program rules from the OECD’s Taxing Wages Publication for 2004. Households have basic and personal deductions based on marital status and age (>65). Couples are assumed to file jointly. Social Security benefits are partially taxed. The amount taxable increases with other income from 50% to 85%. Low income elderly have access to a special tax credit and the earned income tax credit is applied for individuals younger than 65. We calculate state and city taxes for someone living in Detroit, Michigan. The OECD chose this location because it is generally representative of average state and city taxes paid in the U.S. Since Social Security administrative data cannot be used jointly with Geocoded information in the HRS, we apply these hypothetical taxes to all respondents.
Workers with 40 quarters of coverage and of age 62 are eligible to receive their retirement benefit. The benefit is calculated based on the Average Indexed Monthly Earnings (AIME) and the age at which benefits are first received. If an individual claims at his normal retirement age (NRA) (65 for those born prior to 1943, 66 for those between 1943 and 1957, and 67 thereafter), he receives his Primary Insurance Amount (PIA) as a monthly benefit. The PIA is a piece-wise linear function of the AIME. If a worker claims prior to his NRA, his benefit is lower than his PIA. If he retires after the NRA, his benefit is higher. While receiving benefits, earnings are taxed above a certain earning disregard level prior to the NRA. An individual is eligible to half of his spouse’s PIA, properly adjusted for the claiming age, if that is higher than his/her own retirement benefit. A surviving spouse is eligible to the deceased spouse’s PIA. Since we assume prices are constant in our simulations, we do not adjust benefits for the COLA (Cost of Living Adjustment) which usually follows inflation. We however adjust the PIA bendpoints for increases in real wages.
Workers with enough quarters of coverage and under the normal retirement age are eligible for their PIA (no reduction factor) if they are judged disabled (which we take as the predicted outcome of DI receipt) and earnings are under a cap called the Substantial Gainful Activity (SGA) limit. This limit was $9720 in 2004. We ignore the 9 month trial period over a 5 year window in which the SGA is ignored.
Self reported receipt of supplemental security income (SSI) in the HRS provides estimates of the proportion of people receiving SSI under what administrative data would suggest. To correct for this bias, we link the HRS with administrative data from the social security administration identifying those receiving SSI. In the linked administrative data, 3.96% of the population receives supplementary security income, while only 2.79% of the sample reports social security income. We therefore estimate a probit of receiving SSI as a function of self-reporting social security income, as well as demographic, health, and wealth. The benefit amount is taken from the average monthly benefits found in the 2004 Social Security Annual Statistical Supplement. We assign monthly benefit of $450 for person aged 51 to 64, and $350 for persons aged 65 and older.
F.4 Trend Assumptions
The baseline model uses the SSA intermediate growth assumptions. We test the sensitivity of our model by using the SSA high and low growth assumptions. As would be anticipated, this has little effect on our predictions of medical expenditures in 2050, but has effects on future OASI/DI expenditures, as well as on tax revenues. Under the SSA low growth assumptions we find a reduction in OASI expenditures of $234.6 billion and also a reduction in tax revenues of $66 billion. The SSA high growth assumptions provide the opposite result that tax revenues go up by about $78 billion, and that OASI expenditures increase by $280 billion.
The baseline model also uses the SSA assumptions on mortality improvements, but the Census bureau uses alternative assumptions which result in greater mortality improvements over time. We thus test the sensitivity of our model by using the Census mortality assumptions. As would be expected, this increases the aged population in 2050 and thus both the population medical expenditures (by $303 billion) and OASI payments ($139 billion).
The other long term economic trend assumed in the model is that of real medical cost growth. To assess the effect of this assumption, we alternatively assume a higher medical cost growth (1.5% in 2033, .9% in 2053 linearly interpolated as compared with 1% in 2033 and 0.4% in 2053 in the baseline). This assumption has only moderate effects on social security expenditures, but increase medical expenditures by $586 Billion in 2050 for the aged population.
G. Appendix: Pharmacotherapy
G.1 Interventions
Only two drugs have been approved for long-term treatment of obesity so far: orlistat and sibutramine.8 In a review of clinical trials, Rucker et al. (2007) concluded that almost all studies reported weight loss up to four years after starting treatment. The average BMI in these clinical trials was 35, and some trials focused on populations with particular pre-existing conditions (e.g., diabetes). The average effect on weight relative to placebo ranged from 3% (orlistat) to 4.5% (sibutramine). In the control group, average weight loss ranged from 1% to 2% of pre-intervention weight. Hence, the average effect ranges between 4% and 6.5% of baseline weight. Hence, these effects are no where near those of surgery.
Effects appeared larger in populations with higher weight and more pre-existing conditions (such as diabetes). Adverse effects also vary across the drugs. Some patients taking orlistat had adverse effects in terms of gastrointestinal problems (7–20%). This led to attrition in some trials. On the other hand, patients taking sibutramine had on average higher blood pressure (1.7 mm Hg systolic and 2.4 mm Hg diastolic) and pulse rate (4.5 beats per minute). Based on private health insurance pharmacy claims data in the Ingenix Touchstone database9, the daily cost of orlistat was on average $6.22 in year 2007.10 Hence, the cost for one year of treatment is $2,270.
G.2 Scenarios
We use different eligibility criteria for pharmacotherapy, because there is no official guideline for prescribing these drugs. However, their effectiveness has been demonstrated for individuals with an average BMI of 35 or greater. Hence, we use the following two eligibility criteria:
Current Eligibility: eligibility is defined as having a BMI between 35 and 40, or a BMI of 30 to 35 with qualifying comorbidities.
Extended Eligibility: eligibility is extended to those with BMI between 30 and 35 and with no qualifying comorbidities, and to those with a BMI between 25 and 30 with qualifying comorbidities (any of the following: heart disease, hypertension, diabetes and stroke).
We consider a short-term reduction in weight of 5%, based on the literature discussed above. Given the evidence on side-effects and duration of treatment, we end the treatment after 2 years, and allow weight to return gradually to the trajectory predicted by the model (on average 5 years after treatment initiation). Hence, we do not assume permanent weight loss. In a sensitivity analysis, we allow the pill to be twice as effective as assumed (10% effectiveness in the short-run). This has the effect of increasing the age at which on average the weight is regained in the simulations. There are 1.4 million individuals eligible in the current eligibility scenario and 2.4 million under the extended eligibility scenario. Since the daily cost of drugs is $6.22 in 2007, we obtain a treatment cost of approximately $5,000 (in 2010 dollars) for two years of treatment.
Table G.1 shows the results of the simulations.
Table G.1.
Pharmacotherapy Cohort Simulation Results
| Current Eligibility | Expanded Eligibility | |||||
|---|---|---|---|---|---|---|
| Baseline | Intervention | Effect | Baseline | Intervention | Effect | |
| Disability free life expectancy | 21.94 | 22.09 | 0.16 | 22.78 | 22.82 | 0.04 |
| Life expectancy | 29.68 | 29.76 | 0.09 | 29.64 | 29.67 | 0.04 |
| Medical costs ($2010) | ||||||
| Medicare | 133 845 | 133 684 | −161 | 123 610 | 123 400 | −210 |
| Medicaid | 50 117 | 50 095 | −22 | 45 877 | 45 825 | −52 |
| Private Medical Cost | 162 745 | 162 585 | −160 | 151 983 | 151 783 | −200 |
| Total | 346 707 | 346 364 | −343 | 321 470 | 321 007 | −462 |
| Other fiscal outcomes ($2010) | ||||||
| Tax Revenue | 79 426 | 79 534 | 107 | 84 092 | 84 134 | 41 |
| SSI, DI, OASI | 125 162 | 125 517 | 355 | 134 154 | 134 320 | 166 |
| Earnings | 231 060 | 231 360 | 300 | 238 000 | 238 091 | 91 |
Notes: averages from 1000 simulations of the projection for a cohort entering at age 50 in 2010. Real discount rate is 3%. All amounts in $2010 dollars. SSI = Supplemental Security Income, DI = Disability insurance, OASI = old-age social insurance. Private medical costs cover other costs not covered by Medicare and Medicaid (e.g. costs covered by employer). Please refer to text for definition of current and expanded eligibility.
Compared to surgery, the effects of pharmacotherapy on total and healthy life expectancy are modest. In the current eligibility scenario, total life expectancy improves by slightly more than 1 month (0.09 year), while healthy life expectancy rises by 2 months (0.16 year). Effects are even more modest in the extended eligibility scenario where both healthy and total life expectancy rise by less than 2 weeks. There are modest savings in medical costs, between $343 (current) and $462 (extended), and modest increases in taxes ($107 and $41). Other fiscal expenditures go up by $355 in the current eligibility scenario and $166 in the extended eligibility scenario. Earnings increase by $300 in the current eligibility scenario and $91 in the extended eligibility scenario. As mentioned before, the 2-year treatment cost is set to $5,000. Taking into account additional medical costs, additional earnings, additional deadweight loss, as well as treatment cost, the total economic cost is $4,401 in the currently eligible scenario, but $4,353 under the extended eligibility scenario.
H. Appendix: Sensitivity Analysis of Cohort Simulations
In Table H.1, we consider 3 sensitivity analyses for bariatric surgery and 1 for pharmacotherapy. We show these for the currently eligible population only, as results are qualitatively similar for the extended eligibility population. First, we consider variation in the effectiveness of bariatric surgery by assuming that it reduces weight by 35%, rather than 25%. This raises the total life expectancy gain from 1.55 to 1.76. Total economic cost is smaller: $9,249 rather than $12,666 under the baseline. The net value increases to $342,751. The impact of the effectiveness parameter is non-linear, as we see in the next scenario, which lowers effectiveness by 10% (surgery reduces weight by 15%, rather than 25%). The life expectancy gains are cut considerably, from 1.55 years to just under one year. Significantly, the net value remains positive (181,421) and the ICER remain below the NICE thresholds ($16,405). Finally, instead of permanent weight loss, we allow the eligible population to regain 50% of their weight loss after 10 years. This has an effect similar to lowering effectiveness by 10%. The net value remains positive and the ICER remains low ($12,318). Overall, these sensitivity analyses demonstrate that effectiveness matters for our results, but the implied cost-effectiveness ratios continue to hover below $20,000 per life-year. Hence, the results for bariatric surgery appear to be robust to variation in the effectiveness of treatment.
Table H.1.
Sensitivity Analysis Cohort Simulations
| Total economic cost ($2010) |
LE Gain | Net Value | Cost- effectiveness ratio |
|
|---|---|---|---|---|
| Bariatric Surgery (no change) | 12 666 | 1.55 | 297 362 | 8 171 |
| 95% CI | (102 266 to 475 271) | |||
| 10% more effective | 9 249 | 1.76 | 342 751 | 5 255 |
| 95% CI | (179 134 to 561 457) | |||
| 10% less effective | 16 308 | 0.99 | 181 421 | 16 405 |
| 95% CI | (59 221 to 370 942) | |||
| 50% relapes after 10 years | 14 997 | 1.06 | 197 043 | 12 318 |
| 95% CI | (61 577 to 357 937) | |||
| Pharmacotherapy (no change) | 4 401 | 0.09 | 12 834 | 51 066 |
| 95% CI | (−19 628 to 60 921) | |||
| 5% more effective | 3 379 | 0.16 | 28 789 | 21 008 |
| 95% CI | (−29 213 to 107 755) | |||
Notes: Table reports the average of 1000 simulations where we vary treatment effectiveness. The real discount rate is 3%. The total economic cost includes treatment cost, additional medical expenditures, deadweight loss minus additional earnings. Bariatric surgery effectiveness is allowed to increase to 35% (line 2), and decrease to 15% (line 3). At line 4, we let patients receiving the surgery regain 50% of their weight after 10 years. We also allow the pharmacotherapy to be 5% more effective (10% effectiveness). Net value is defined as the value of life expectancy gain (using a value of a statistical life year equal to $200,000) minus the total economic cost. A 95% confidence interval based on 1000 replications is reported by taking the 2.5 and 97.5 percentile of the net values over the 1000 simulations
Pharmacotherapy demonstrated very low effectiveness. Hence, we also double the effectiveness of pharmacotherapy by assuming 10%, instead of 5%, weight loss over a two-year period. The total economic cost is $3,379, slightly lower than $4,401 in the baseline. This increases the net value modestly, but we still cannot rule out a negative net value. The ICER is much lower, however, and decreases by half (from $51,066 to $21,008). Hence, with higher efficacy, pharmacotherapy has the potential of delivering a reasonable ICER.
I. Sensitivity Analysis of Long-Term Scenarios
We perform sensitivity analyses on the current eligibility scenario, reported in Table I.1. For each modification of our assumptions, we report the percentage change relative to the baseline scenario assumptions, for both 2030 and 2050. The first two provide the baseline results, for convenience; these are identical to those reported earlier in Table 3. We first check the robustness of our results to a change in the exogenous rate of growth in medical spending. As sensitivity analyses, we assume higher cost growth of 1.5% in 1992–2004, 1.5% in 2033, 0.9% in 2053, and 0.3% in 2083. In the baseline scenarios we had assumed 1% until 2033 and 0.4% until 2053 in the baseline. These results are reported in the 3rd and 4th column of Table 9. Medical savings become larger, which slightly improves the net absolute fiscal effects. But the basic conclusion remains the same: net aggregate fiscal effects are small. The net social value does not vary much either.
Table I.1.
Sensitivity Analysis Population Scenarios
| Baseline | High Medical Cost |
High Economic Growth |
50% Cure for Diabetes |
Census Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2030 | 2050 | 2030 | 2050 | 2030 | 2050 | 2030 | 2050 | 2030 | 2050 | ||
| Population size (Million) | 0.5 | 1.5 | 0.5 | 1.5 | 0.5 | 1.5 | 0.4 | 1.3 | 0.4 | 1.5 | |
| Population 65+ (Million) | 0.4 | 1.4 | 0.4 | 1.4 | 0.4 | 1.4 | 0.4 | 1.3 | 0.4 | 1.4 | |
| Prevalence of selected conditions | |||||||||||
| Obese 3 (BMI >= 40) (%) | −7.7% | −11.7% | −7.7% | −11.7% | −7.7% | −11.7% | −7.7% | −11.7% | −7.6% | −11.6% | |
| Obese 2 (35 <= BMI 40)(%) | −5.6% | −6.9% | −5.6% | −6.9% | −5.6% | −6.9% | −5.5% | −6.9% | −5.5% | −6.9% | |
| Obese 1 (30 <= BMI <35)(%) | 2.5% | 5.2% | 2.5% | 5.2% | 2.5% | 5.2% | 2.5% | 5.2% | 2.5% | 5.0% | |
| Overweight (25<= BMI<30) (%) | 9.5% | 12.1% | 9.5% | 12.1% | 9.5% | 12.1% | 9.5% | 12.1% | 9.5% | 12.1% | |
| Diabetes | −1.5% | −2.5% | −1.5% | −2.5% | −1.5% | −2.5% | −0.9% | −1.4% | −1.5% | −2.5% | |
| Heart disease | −0.5% | −0.7% | −0.5% | −0.7% | −0.5% | −0.7% | −0.5% | −0.7% | −0.5% | −0.7% | |
| Hypertension | −0.4% | −0.5% | −0.4% | −0.5% | −0.4% | −0.5% | −0.4% | −0.5% | −0.4% | −0.5% | |
| Public revenues from age 51+ ($bn) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Federal personal income taxes | 1.1 | 2.0 | 1.1 | 2.0 | 1.2 | 2.3 | 1.0 | 1.8 | 1.1 | 2.0 | |
| Social security payroll taxes | 0.4 | 0.6 | 0.4 | 0.6 | 0.4 | 0.6 | 0.3 | 0.5 | 0.4 | 0.6 | |
| Medicare payroll taxes | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Total Revenue Effect | 1.5 | 2.7 | 1.6 | 2.8 | 1.6 | 3.1 | 1.4 | 2.5 | 1.6 | 2.8 | |
| Public expenses from age 51+ ($bn) | |||||||||||
| Old Age and Survivors Insurance benefits (OASI) | 5.4 | 22.5 | 5.4 | 22.9 | 5.6 | 25.3 | 4.9 | 19.9 | 5.1 | 22.4 | |
| Disability Insurance benefits (DI) | −0.7 | −1.4 | −0.7 | −1.4 | −0.8 | −1.7 | −0.6 | −1.3 | −0.7 | −1.4 | |
| Supplementary Security Income (SSI) | 0.2 | 1.1 | 0.2 | 1.1 | 0.2 | 1.1 | 0.3 | 1.1 | 0.2 | 1.0 | |
| Medicare costs | −11.4 | −16.3 | −12.0 | −18.8 | −11.3 | −16.3 | −8.3 | −9.9 | −12.1 | −20.0 | |
| Medicaid costs | −3.4 | −2.3 | −3.6 | −2.7 | −3.4 | −2.3 | −2.3 | −0.2 | −3.6 | −2.9 | |
| Medicare + Medicaid | −14.7 | −18.6 | −15.6 | −21.5 | −14.7 | −18.6 | −10.6 | −10.1 | −15.7 | −22.9 | |
| Total Expenditure Effect | −9.82 | 3.67 | −10.58 | 1.13 | −9.66 | 6.09 | −6.12 | 9.63 | −11.1 | −0.9 | |
| Net Revenue Effect | 11.37 | −0.94 | 12.14 | 1.64 | 11.26 | −3.04 | 7.56 | −7.12 | 12.6 | 3.6 | |
| Total medical costs for aged 51+ (Billion dollars) | −21.6 | −24.8 | −22.8 | −28.7 | −21.6 | −24.8 | −15.6 | −13.3 | −22.9 | −30.3 | |
| Net social value ($bn) | 63.3 | 245.7 | 46.1 | 196.2 | 63.4 | 244.5 | 58.6 | 243.9 | 65.1 | 251.4 | |
Columns 5 and 6 alter the assumed rate of real earnings growth, which becomes 1.7% annually instead of 1.1%. This flips the sign of the net fiscal effect, due primarily to a higher annuity burden. But the effects remain modest. The third sensitivity analysis lowers the marginal health benefit from the intervention by “curing” diabetes for 50% of the incoming cohort. The net fiscal effect falls as a result ($7.56 billion in 2030 and −$7.12 billion in 2050). The final sensitivity analysis adopts Census assumptions regarding mortality improvements. We calibrate these so as to reach a 65+ population of 87 million in 2050, rather than the 80 million projected by SSA. This tends to yield larger medical cost savings relative to Social Security benefit increases. Hence, the net fiscal effect is larger than in the baseline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Orlistat is the other most commonly prescribed pharmacotherapy.
Both of these are based on an estimated elasticity of 0.4 for taxable income with respect to marginal tax rates, found elsewhere in the literature (Saez, Slemrod and Giertz 2009; Giertz 2004).
Since the cost savings are positive even without including the value of life extension, the results for bariatric surgery are insensitive to the value used.
With some abuse of notation, ji − 1 denotes the previous age at which the respondent was observed.
We estimate annual medical spending paid by Medicare either in total, or for a specific part of Medicare (Parts A, B, and D)
Sibutramine was withdrawn from the U.S. market in October 2010 due to safety issues.
These data were provided to us through an ongoing with Ingenix Inc. a benefits consulting firm.
For 2007, we selected patients who had taken orlistat at some point in the last year (N=722) and compute the average daily cost of dosage by dividing the total cost for the year by the number of days for which the drug was taken. We obtained an average cost of $6.22 per day.
Contributor Information
Pierre-Carl Michaud, Université du Québec à Montréal & RAND.
Dana Goldman, University of Southern California and RAND.
Darius Lakdawalla, University of Southern California and RAND.
Yuhui Zheng, Harvard University.
Adam H. Gailey, RAND Corporation
References
- Adams P, Hurd M, McFadden D, Merrill A, Ribeiro T. Healthy, wealthy, and wise? Tests for direct causal paths between health and socioeconomic status. Journal of Econometrics. 2003;112(1):3–56. [Google Scholar]
- Appleby J, Devlin N, Parkin D. NICE’s cost effectiveness threshold. British Medical Journal. 2007;335:358–359. doi: 10.1136/bmj.39308.560069.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendregt J, Bonneux L, van der Maas P. The Health Care Costs of Smoking. New England Journal of Medicine. 1997;337(15):1052–1057. doi: 10.1056/NEJM199710093371506. [DOI] [PubMed] [Google Scholar]
- Chaloupka F, Warner K. The Economics of smoking, in Handbook of Health Economics. part 2. vol. 1. Cuyler and Newhouse; 2000. pp. 1539–1627. [Google Scholar]
- Chouinard HH, Davis D, Lafrance J, Perloff J. Fat Taxes : Big Money for small change. Forum for Health Economics & Policy. 2007;10(2) art. 2. [Google Scholar]
- Craig B, Tseng D. Cost-Effectivness of Gastric Bypass for Severe Obesity. The American Journal of Medicine. 2002;113:491–497. doi: 10.1016/s0002-9343(02)01266-4. [DOI] [PubMed] [Google Scholar]
- Crémieux PY, Buchwald h, Shikora SA, Ghosh A, Yang, Buessing M. A study on the economic effects of bariatric surgery. American Journal of Managed Care. 2008;14(9):589–596. [PubMed] [Google Scholar]
- Cutler D, Glaeser E, Shapiro J. Why have Americans become more obese. Journal of Economic Perspectives. 2003;17(3):93–118. [Google Scholar]
- Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. Journal of American Medical Association. 2008;299(3):316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- Feldstein M. Effects of taxes on economic behavior. National Tax Journal. 2008 Mar;61:131–139. [Google Scholar]
- Finkelstein E, Brown D. A Cost-Benefit Simulation Model of Coverage for Bariatric Surgery Among Full-Time Employees. American Journal of Managed Care. 2005;11(10):641–651. [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. International Journal of Obesity and Related Metabolic Disorders. 1998;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Garber AM, Phelps CE. Economic foundations of cost-effectiveness analysis. Journal of Health Economics. 1997;16:1–31. doi: 10.1016/s0167-6296(96)00506-1. [DOI] [PubMed] [Google Scholar]
- Giertz S. Recent Literature on Taxable-Income Elasticities; Congressional Budget Office Technical Paper 2004–16; 2004. [Google Scholar]
- Giertz S. The Elasticity of Taxable Income: Influences on Economic Efficiency and Tax Revenues, and Implications for Tax Policy. In: Viard Alan., editor. Tax Policy Lessons from the 2000s. Washington, DC: AEI Press; 2009. pp. 101–136. [Google Scholar]
- Goldman D, Michaud P-C, Lakdawalla D, Zheng Y, Gailey A, Vaynman I. The Fiscal Consequences of Trends in Population Health. National Tax Journal. 2010;63(2):307–330. [Google Scholar]
- Goldman D, Zheng Y, Girosi F, Michaud P-C, Olshansky J, Cutler D, Rowe J. The Benefits of Risk Factor Prevention in Americans Aged 51 Years and Older. American Journal of Public Health. 2009;99(11):1–11. doi: 10.2105/AJPH.2009.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, et al. Health status and medical treatment of the future elderly: Final Report. Santa Monica, CA: RAND Corporation; 2004. [Google Scholar]
- James WP, et al. Effect of Sibutramine on Weight Maintenance after Weight Loss: A Randomised Trial. Lancet. 2000;356(9248):2119–2125. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- Lakdawalla D, Philipson T. The Growth of Obesity and Technological Change. Economics and Human Biology. 2009;7(3):283–293. doi: 10.1016/j.ehb.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud P-C, van Soest A : Health and Wealth of Elderly Couples: Causality Tests using Dynamic Panel Data Models. Journal of Health Economics. 2008;27(5):1312–1325. doi: 10.1016/j.jhealeco.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara S, et al. The Clinical Effectiveness and Cost-Effectiveness of Sibutramine in the Management of Obesity: A Technology Assessment. Health Technology Assessment. 2002;6(6):1–97. doi: 10.3310/hta6060. [DOI] [PubMed] [Google Scholar]
- Picot J, et al. The Clinical Effectivness and Cost-Effectivness of Bariatric (weight loss) Surgery for Obesity : A Systematic Review and Economic Evaluation. Health Technology Assessment. 2009;13(41):1–212. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- Ruhm C. Current and Future Prevalence of Obesity and Severe Obesity in the United States. Forum for Health Economics and Policy. 2007;10(2) art 6. [Google Scholar]
- Rucker D, et al. Long Term Pharmacotherapy for Obesity and Overweight : Updated Meta-Analysis. British Medical Journal. 2007:1–10. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez E, Slemrod JB, Giertz S. NBER Working Papers 15012. National Bureau of Economic Research, Inc.; 2009. The Elasticity of Taxable Income with Respect to Marginal Tax Rates: A Critical Review. [Google Scholar]
- Shekelle PG, et al. Evidence Report/Technology Assessment No. 103. AHRQ Publication No. 04-E028-2. Rockville, MD: Agency for Healthcare Research and Quality; 2004. Pharmacological and Surgical Treatment of Obesity. [Google Scholar]
- Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New England Journal of Medicine. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- Smith J. The Impact of Socioeconomic Status on Health over the Life-Course. Journal of Human Resources. 2007;42(4):739–764. [Google Scholar]
- Thomson D, et al. Lifetime Health and Economic Consequences of Obesity. Archives of Internal Medicine. 1999;159:2177–2183. doi: 10.1001/archinte.159.18.2177. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Surgeon General’s call to action to prevent and decrease overweight and obesity. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [PubMed] [Google Scholar]
- Viscusi K, Aldy J. The Value of a Statistical Life : A Critical Review of Market Estimates Throughout the World. Journal of Risk and Uncertainty. 2003;27(1):5–76. [Google Scholar]