Abstract
Recognition of sweet, bitter and umami tastes requires the non-vesicular release from taste bud cells of adenosine 5′-triphosphate (ATP), which acts as a neurotransmitter to activate afferent neural gustatory pathways1. However, how ATP is released to fulfill this function is not fully understood. Here we show that calcium homeostasis modulator 1 (CALHM1), a voltage-gated ion channel2,3, is indispensable for taste stimuli-evoked ATP release from sweet-, bitter- and umami-sensing taste bud cells. Calhm1 knockout mice have severely impaired perceptions of sweet, bitter and umami compounds, whereas sour and salty taste recognition remains mostly normal. Calhm1 deficiency affects taste perception without interfering with taste cell development or integrity. CALHM1 is expressed specifically in sweet/bitter/umami-sensing type II taste bud cells. Its heterologous expression induces a novel ATP permeability that releases ATP from cells in response to manipulations that activate the CALHM1 ion channel. Knockout of Calhm1 strongly reduces voltage-gated currents in type II cells and taste-evoked ATP release from taste buds without affecting the excitability of taste cells to taste stimuli. Thus, CALHM1 is a voltage-gated ATP release channel required for sweet, bitter and umami taste perception.
Tastes are sensed by dedicated receptor cells in taste buds (TB), which are composed of three anatomically distinct types of cells: types I, II and III cells. Only the sour-sensing type III cells have neuron-like features, including expression of neurotransmitter biosynthesis enzymes and synaptic vesicles at classical synapses with sensory nerve fibers1. Type II sweet-, bitter- and umami-sensing cells share a common signal transduction pathway. However, they lack classical synaptic structures, yet transmit taste information to gustatory neurons by releasing ATP as a neurotransmitter4,5. Our work, described below, implicates CALHM1 as a critical component responsible for this ATP release.
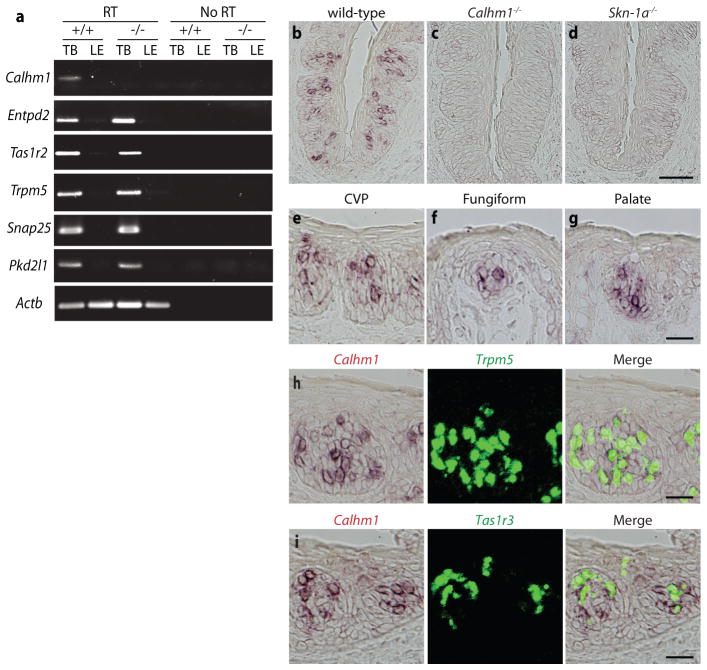
Calhm1 (calcium homeostasis modulator 1)6 (also known as FAM26C) encodes the pore-forming subunit of a voltage-gated non-selective plasma membrane ion channel involved in neuronal excitability2 and potentially the pathogenesis of Alzheimer’s disease6–8. Calhm1 expression was identified in primate TB9, suggesting that CALHM1 may have physiological functions outside the brain. We confirmed that Calhm1 was expressed in mouse TB but not in surrounding epithelium (Figs. 1a, 1b and 1e–g). To examine CALHM1 function, we generated a constitutive Calhm1−/− mouse and verified loss of Calhm1 expression in TB (Figs. 1a and 1c). Calhm1−/− mice were viable and fertile, with no overt morphological abnormalities in their TB, nor altered expression of taste-related marker genes (Figs. 1a and S1). Loss of Calhm1 signal in TB of Skn-1a (also known as Pou2f3) knockout mice in which type II cells are developmentally absent10 (Figs. 1d and S2), and the co-expression of Calhm1 and Trpm5 (Fig. 1h), demonstrated that Calhm1 expression is confined to type II cells. Expression profiling by RT-PCR of pools of isolated type II and type III cells and individual taste cells also supported the confined expression of Calhm1 to type II cells (Fig. S3). Calhm1 expression was observed not only in Tas1r3-expressing sweet and umami taste cells, but also in other type II cells, indicating that Calhm1 is expressed in sweet, bitter and umami taste cells (Fig. 1i).
Figure 1. CALHM1 is selectively expressed in type II taste bud cells.
(a) RT-PCR of mRNA of Calhm1, Actb (β-actin) and taste cell marker genes from laser micro-dissected circumvallate papillae (CVP) taste buds (TB) and lingual epithelium (LE) in wild-type (+/+) and Calhm1 knock-out (−/−) mouse tongues. RT, reverse transcriptase. (b–d) in situ hybridization of Calhm1 in CVP TB of WT (b), Calhm1−/− (c) and Skn-1a−/− (d) mice. Scale bar, 50 μm. Calhm1 is expressed in subsets of CVP (e), fungiform (f), and palate (g) TB cells. (h) Double-label in situ hybridization directly illustrates cellular co-expression of Calhm1 and Trpm5 in CVP TB. Most cells expressing Trpm5 also express Calhm1, with Calhm1 expression absent in Trpm5 negative cells. (i) CVP TB illustrating that Tas1r3 is expressed in a subset of Calhm1 positive cells. Scale bars for (e–i), 20 μm.
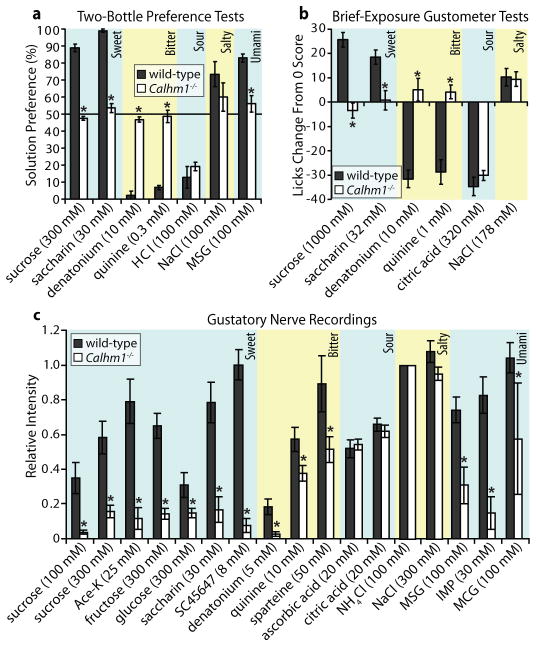
We used two behavioral tests and nerve recordings to evaluate the impact of the loss of CALHM1 expression on taste perception. Two-bottle preference tests revealed that Calhm1−/− mice displayed a nearly complete loss of preference for sweet and umami compounds and avoidance of bitter compounds (Figs. 2a, S4a, S4c and S4d, Tables S1 and S2). In contrast, there were little-or-no differences between wild-type (WT) and Calhm1−/− mice in their responses to salty and sour compounds (Figs. 2a and S4a, Tables S1 and S2). Brief-access taste tests reproduced these phenotypes (Figs. 2b and S4b, Table S3), demonstrating that the taste phenotype in Calhm1−/− mice is a sensory defect. Recordings of the whole chorda tympani nerve revealed strongly reduced responses to sweet, bitter and umami compounds in Calhm1−/− mice, whereas responses to NaCl and the acid compounds were not different from WT (Figs. 2c and S5), indicating that the sensory defect in Calhm1−/− mice was due to the knockout of CALHM1 function in the peripheral taste cell system. Together, these results indicate that CALHM1 plays a crucial role in taste-sensing type II cells1,11 (See Supplementary Information for further discussion).
Figure 2. CALHM1 is essential for sweet, bitter and umami taste perception.
(a) Mean preference % (taste compound vs. water) from 48 hr two-bottle preference tests and (b) brief-access lick scores to indicated compounds in Calhm1−/− mice and WT littermates. Error bars, s.e. (8–12 mice per group, 4–6 month-old); *P < 0.01 (post hoc Bonferroni’s test for (a) and Student’s t-test for (b)). (c) Summary of responses from whole-chorda tympani nerve recordings stimulated with indicated compounds and normalized to response to NH4Cl from WT (n = 7) and Calhm1−/− (n = 8) mice. MSG, monosodium glutamate; IMP, inosine 5′-monophosphate; MCG, monocalcium di-L-glutamate. Error bars, s.e.; *P < 0.05 (Student’s t-test).
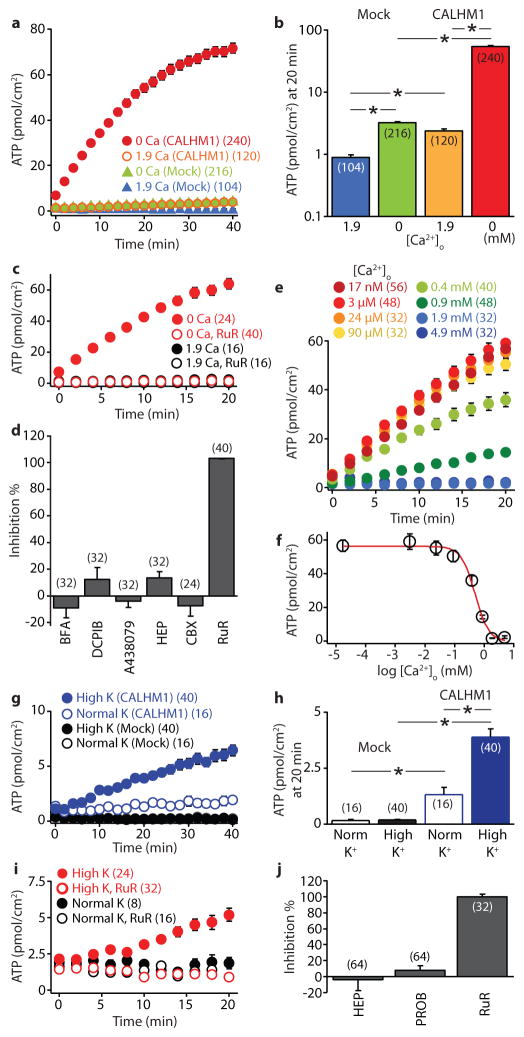
Type II cells signal to the nervous system by non-vesicular ATP release to nearby afferent gustatory nerves5,12–14. Although connexin hemichannels and pannexin 1 have been proposed, the molecular identity of the ATP release mechanism remains uncertain1,12,13,15. The CALHM1 pore diameter is ~14Å3, similar to that estimated for connexins. We therefore examined the possibility that CALHM1 mediates ATP release. The CALHM1 ion channel can be activated by membrane depolarization or reduction of extracellular Ca2+ concentration ([Ca2+]o)2. We first exploited the latter mechanism to activate CALHM1 in heterologous expression systems to determine if CALHM1 can form an ATP release channel. Lowering [Ca2+]o from 1.9 mM to essentially zero (17 nM) activated ATP release from human CALHM1 (hCALHM1)-expressing HeLa cells, whereas little ATP efflux was induced in mock-transfected cells (Figs. 3a and 3b). Similar low [Ca2+]o-induced ATP release was observed in hCALHM1-expressing COS-1 cells and Xenopus oocytes (Fig. S6). Neither CALHM1 expression nor low [Ca2+]o exposure caused cell damage (Fig. S7). Involvement of other possible mechanisms16 was ruled out because ATP release was unaffected by Brefeldin A (vesicular release), DCPIB (volume-sensitive Cl− channels), A438079 (P2X7 receptors), heptanol (connexin hemichannels) or carbenoxolone (pannexins and connexins at 30 μM) (Fig. 3d). In contrast, ruthenium red (RuR), which inhibits CALHM1 ion currents2, abolished low [Ca2+]o-evoked ATP release from hCALHM1-expressing cells (Figs. 3c and 3d). Thus, CALHM1 expression induces a novel ATP permeability.
Figure 3. CALHM1 mediates ATP release.
(a) Time courses of extracellular ATP levels due to release from mock- and hCALHM1-transfected HeLa cells exposed to normal (1.9 mM) or zero (17 nM) [Ca2+]o. Error bars mostly hidden behind symbols. (b) Summary of extracellular ATP levels at 20 min in (a). (c) Low [Ca2+]o-induced ATP release in CALHM1-expressing cells is abolished by ruthenium red (RuR). (d) Effects on low [Ca2+]o-stimulated ATP release from hCALHM1 cells of 0.5 μg/ml brefeldin A (BFA); 10 μM DCPIB; 3 μM A438079; 1 mM 1-heptanol (HEP); 30 μM carbenoxolone (CBX); 20 μM RuR. (e) Time courses of ATP release from hCALHM1 cells induced by various [Ca2+]o. (f) ATP levels at 20 min in (e) plotted against [Ca2+]o and fitted to a Hill equation. (g) Depolarization by high [K+]o (117.5 mM K+) induces ATP release specifically from hCALHM1 cells. (h) ATP levels at 20 min in (g). (i) Depolarization-induced ATP release from CALHM1-expressing cells is abolished by RuR. (j) Pharmacological sensitivities of depolarization-induced ATP release from hCALHM1 cells. 1 mM HEP, 1 mM probenecid (PROB); 20 μM RuR. Number of wells in parentheses throughout. Error bars, s.e.; *P < 0.01 (Student’s t-test).
CALHM1 ion channel gating is regulated by [Ca2+]o with an apparent half-maximal inhibitory [Ca2+] of ~ 220 μM and Hill coefficient of 2.12. Extracellular Ca2+ inhibited ATP release with IC50 = 495 μM and Hill coefficient of 1.9 (Figs. 3e and 3f). CALHM1 ion currents are also activated by membrane depolarization2. In normal [Ca2+]o, hCALHM1-expressing but not mock-transfected cells released ATP in response to high [K+]o- induced depolarization (Figs. 3g and 3h). Depolarization-induced ATP release was inhibited by RuR but not by heptanol or probenecid, connexin and pannexin-117 blockers, respectively (Figs. 3i and 3j). Expression of mouse CALHM1 conferred ATP release with similar properties (Fig. S8). Regulation of ATP release in hCALHM1-expressing cells is therefore similar to that of CALHM1 channel gating, indicating that the CALHM1 channel is a conduit for ATP release.
The three types of TB cells can be classified based on whole-cell electrophysiological fingerprints13,18. We verified these fingerprints by recording whole-cell currents in single isolated TB cells from TRPM5-GFP mice with GFP expressed specifically in type II cells (Fig. S9). With tetraethylammonium in the bath and Cs+ as the major cation in the pipette solution (to block K+ currents)2, the electrophysiological subtypes were identified by the combination of voltage-gated Na+ currents (INa)and non-selective slowly-activating outward currents (Islow) with inward tail currents at −70 mV (Itail) (Figs. 4a–c and S9). To identify CALHM1 ion currents, whole-cell currents were recorded in isolated TB cells from WT and Calhm1−/− mice (Figs. 4a–c). Loss of CALHM1 substantially reduced the amplitudes of Islow and Itail without affecting the amplitude of INa in type II cells (Figs. 4d–f), whereas no differences were observed in types I and III cells (Figs. 4b, 4c and 4g). The slowly-activating outward current in type II cells was inhibited by the non-specific CALHM1 blocker Gd3+ but not by probenecid or heptanol, ruling out contributions of pannexin-1 or connexins (Fig. 4h). These data demonstrate that CALHM1 channel conductance is present in type II cells and contributes the major component of the slowly-activating outward current. Notably, the amount of depolarization-induced ATP release from type II cells is correlated with the magnitude of Islow13.
Figure 4. CALHM1 is required for taste-evoked ATP release from taste cells.
(a–c) Electrophysiological phenotypes of types I, II and III cells identified in WT (red) and Calhm1−/− (blue) taste cells. Cells held at −70 mV and pulsed from −80 to +80 mV in 20 mV increments with 1 sec duration. INa (●), Islow at end of pulses (■), and Itail (▲) measured in (d–f) for type II (n = 9 WT, 10 Calhm1−/−), and (g) type III (n = 9 WT, 6 Calhm1−/−) cells. Type I current recorded from 16 WT, 9 Calhm1−/− cells. (h) Sensitivities of Islow in GFP-positive cells from TRPM5-GFP mice to Gd3+ (100 μM), probenecid (1 mM), 1-heptanol (1 mM) (n = 4). (i) [Ca2+]i in type II cells from WT (left, 9 cells) and Calhm1−/− (right, 12 cells) mice. Type II cells identified by robust [Ca2+]i response to a mix of sweet and bitter substances (gray bar). Basal (j) and taste-evoked responses (k) are comparable in WT and Calhm1−/− cells. (l) Taste-evoked ATP release from gustatory CVP tissue and non-gustatory LE. Bitter mix elicits considerable ATP release from CVP vs. LE in WT mice that is abolished in Calhm1−/− mice and by 1 μM tetrodotoxin (TTX). Error bars, s.e.; *P < 0.05; **P < 0.01. (Student’s t-test). (m) Schematic illustration of signal transduction cascade in type II taste receptor cells with CALHM1 as the ATP release pathway.
Type II cells detect sweet, bitter and umami compounds via G protein-coupled taste receptors4 that stimulate a common signal transduction cascade involving activation of PLCβ2, inositol 1,4,5-trisphosphate-mediated Ca2+ release and Ca2+-dependent activation of TRPM5 channels11 that depolarizes the plasma membrane to generate action potentials and subsequent non-vesicular release of ATP. Importantly, no differences in basal [Ca2+]i and taste-evoked [Ca2+]i responses were observed between WT and Calhm1−/− type II cells (Figs. 4i–k). Furthermore, Calhm1 deficiency was without effects on voltage-gated Na+ currents (Fig. 4f), TRPM5 expression in type II cells (Figs. 1a, S1i and S2) or ATP content of taste buds (Fig. S10). Strikingly, however, taste-evoked release of ATP from circumvallate papillae was abolished in Calhm1−/− mice (Figs. 4l and S11). This strongly suggests that CALHM1 contributes to the major ATP release mechanism in sweet/bitter/umami-sensing TB cells (Figs. 4l and 4m).
Our study sheds light on a novel cellular ATP releasing mechanism by demonstrating that CALHM1 is a voltage-gated ion channel that mediates tetrodotoxin-sensitive ATP release in TB (Fig. 4l) as an essential mechanism of sweet, bitter and umami taste perception. As such, CALHM1 provides a missing link in the signal transduction cascade in type II cells, connecting taste receptor activation and the generation of Na+ action potentials to the activation of afferent gustatory neural pathways1 (Fig. 4m). It has not escaped our attention that other CALHM isoforms6 as well as pannexin 1 and connexins are also present in TB9,12,13 and they might also be involved in ATP release in taste, perhaps acting in parallel with or in a complex with CALHM1. Signaling by extracellular ATP is a widespread phenomenon that regulates many physiological activities19, including neurotransmission20,21, intercellular communication22,23, vascular tone24, and sensory transduction5,25–27. CALHM1 and its isoforms may participate in physiologically important ATP release elsewhere.
Method Summary
All animal experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania, the Feinstein Institute for Medical Research, the Monell Chemical Senses Center, and the University of Minnesota Duluth.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Methods
Calhm1−/− mice
Calhm1 +/− founder mice were generated at genOway (Lyon, France) as described previously2. Briefly, Calhm1 exon 1 deletion was performed by homologous recombination in 129Sv embryonic stem (ES) cells. Positive clones were screened by PCR and confirmed by Southern blot analysis. The resultant ES cells were injected into blastocysts derived from C57BL/6J mice to obtain chimeric mice that possessed germline transmission of the targeted Calhm1 locus. Mice on the mixed 129Sv × C57BL/6J genetic background were used in this study. WT (+/+) and Calhm1 KO (−/−) F1 mice derived from littermate mice were used for two-bottle preference tests. All other experiments were performed with WT and KO littermates. In some mice, the neo cassette was removed and the resulting strain was backcrossed with C57BL/6J mice. Briefly, Calhm1 +/− mice were mated to mice bearing an EIIa-Cre transgene (B6.FVB-Tg (EIIa-cre)C5379Lmgd/J, The Jackson Laboratory) on the C57BL/6J background to remove the neomycin resistance cassette by cre-loxP-mediated excision. The resulting Calhm1 +/− pups were further backcrossed to C57BL/6J (The Jackson Laboratory) for at least five generations before being made homozygous. The removal of the neomycin cassette was confirmed by PCR. Backcrossed mice were used for two-bottle preference tests. Loss of CALHM1 expression in Calhm1−/−taste buds was verified by RT-PCR and in situ hybridization.
Laser capture microdissection, RNA amplification, and reverse transcriptase-PCR (RT-PCR)
Circumvallate taste tissue from Calhm1 +/+ and −/− mice was embedded in cryomolds using O.C.T. freezing medium. Twelve micron-thick tissue sections were cut on a Leica CM1850 cryostat (Leica Microsystems, Wetzlar, Germany), collected on RNase-free membrane slides (Leica), and stained with cresyl violet. Taste buds from the circumvallate papilla (CVP) and surrounding lingual epithelium (LE) were isolated using a Leica laser microdissection system LMD7000 (Leica). Taste buds and surrounding epithelium were pooled from a total of four mice of each genotype. Total RNA from taste bud and LE samples was purified using a RNAqueous-Micro Kit (Ambion). Total RNA was amplified using two sequential rounds with MessageAmp II aRNA kit (Ambion), as per manufacturer’s instructions. One microgram of RNA was transcribed into cDNA using Invitrogen’s SuperScript III First-Strand Synthesis System for RT-PCR with random hexamers, according to the supplied protocol. A 50 μL PCR reaction was run with the following final concentrations: 450 ng of each primer (see Table S4 for PCR primer sequences), 2 mM MgCl2, 0.3 mM dNTPs, 2.5 U Taq polymerase (Promega GoFlex DNA polymerase) and 1 μL of 10 ng/μL DNA. PCR cycling conditions used for Calhm1 were: 95°C for 3 min; 35 cycles of 95°C for 30 sec, 65°C for 30 sec, and 72°C for 45 sec; 72°C for 7 min; 4°C (hold).
In situ hybridization
Oral epithelia containing taste buds were dissected from adult male mice deeply anesthetized with an overdose of sodium pentobarbital (Abbott Laboratories, North Chicago, IL) and embedded in the frozen O.C.T. Compound (Sakura Finetech USA, Torrance, CA). Fresh-frozen sections of 8 μm thickness were prepared using a cryostat (CM3050S, Leica Microsystems, Wetzlar, Germany). The in situ hybridization procedure was described previously28. In brief, digoxigenin- and fluorescein-labeled antisense RNAs prepared using RNA labeling mix (Roche Diagnostics, Indianapolis, IN) and an RNA polymerase (Stratagene) were used for hybridization after fragmentation under alkaline conditions to a length of about 150 bases. Fresh-frozen sections were fixed with 4% PFA, treated with diethylpyrocarbonate, prehybridized with salmon sperm DNA for 2 h at 65°C, and hybridized with antisense riboprobe(s) for 40 h at 65°C except single labeling for Tas2r108 and Trpm5 for which prehybridization and hybridization were carried out at 58°C. After hybridization, the sections were washed in 0.2 × SSC at 58°C or 65°C and blocked with 0.5% blocking reagent (Roche Diagnostics) in Tris–buffered saline. Chromogenic signals were developed for one day except Calhm1 using alkaline phosphatase–conjugated anti-digoxigenin antibody (1:500, Roche Diagnostics) and 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate as chromogenic substrates. Chromogenic Calhm1 signals were developed for 2 days to clarify the cells expressing Calhm1 mRNA. No Calhm1 signal was observed in Skn-1 or Calhm1 knockout taste tissues after signal development for 3 days. Stained images were obtained with a Nikon eclipse 80i microscope (Nikon Instruments Inc., NY) equipped with a DXM1200C digital camera (Nikon). Fluorescent signals were developed using biotin–conjugated anti-fluorescein antibody (1:500, Vector laboratory, Burlingame, CA), avidin-biotin complex (Vector laboratory), tyramide signal amplification biotin system (1:50, PerkinElmer), and Alexa 488–conjugated streptavidin (4 μg/ml, Invitrogen). Fluorecent images were obtained with a Leica SP2 confocal scanning microscope (Leica). For double labeling of Calhm1 with Trpm5 and Tas1r3, fluorescent signals were first developed and obtained, then chromogenic signals were developed and obtained as described above, and the images were superimposed using Photoshop CS3 (Adobe). RNA probes generated were to nucleotides 1–894 of Tas2r108 (accession no. AF227148), nucleotides 310–3491 of Trpm5 (accession no. AF228681), nucleotides 525–2725 of Tas1r3 (accession no. AF337039), and nucleotides 1–1407 and 2148–2369 of Calhm1 cDNA fragment which contains 1047 bp of the entire coding sequence and 1322 bp of 3′-noncoding region.
Immunofluorescence staining
For fixed tissue preparation, mice 12-weeks old or older were anesthetized with an overdose of sodium pentobarbital (Abbott Laboratories, North Chicago, IL) and transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde in ice-cold PBS. Tongue epithelia containing CVP were dissected and postfixed with the same fixative at 4°C overnight. Tissue samples were then cryoprotected in 30% sucrose in PBS, embedded in frozen O.C.T. Compound (Sakura Finetech), and sectioned using a cryostat (CM1900, Leica Microsystems, Wetzlar, Germany) at 8 μm. Tissue sections were mounted on tissue-adhesive-coated glass slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C until analyzed. For immunofluorescence staining, slides were rinsed with PBS, incubated in a pre-heated target retrieval solution (S1700, Dako, Carpinteria, CA) at 80°C for 20 min, allowed to cool at room temperature for 20 min still in the target retrieval solution, and washed in PBS for 3 × 10 min. After blocking by one hr-incubation in PBS containing 5% normal donkey serum (PBS-5% NDS) at room temperature, sections were incubated overnight at 4°C with primary antibodies diluted in PBS-5% NDS: 1:3000 for rabbit anti-TRPM5 (Alomone labs, Jerusalem, Israel); 1:500 for rabbit anti-PLCβ2 (Santa Cruz Biotechnology, Santa Cruz, CA); 1:500 for rabbit anti-AADC (Gene Tex, Irvine, CA); 1:500 for goat anti-KCNQ1 (Santa Cruz). The next day, the slides were washed for 3 × 10 min in PBS at room temperature and incubated for 1 hr with Alexa fluor-conjugated antibodies diluted in PBS-5% NDS: 1:500 for Alexa fluor 488 donkey anti-rabbit IgG; 1:500 for Alexa fluor 568 anti-goat IgG (Invitrogen, Grand Island, NY). Finally, sides were washed for 3 × 10 min in PBS at room temperature and mounted in VectaShield with DAPI (H-1500, Vector Laboratories). Stained sections were imaged with a confocal scanning microscope (LSM 710, Carl Zeiss, Thornwood, NY).
Single-taste cell analyses
Circumvallate taste epithelium was enzymatically delaminated, taste buds were harvested from peeled epithelium and dissociated single taste cells were collected, all as detailed previously29. For patch clamp and fura-2 Ca2+ imaging experiments, isolated cells were placed on poly-L-lysine-coated coverslips and allowed to settle for 30 – 60 min.
Total RNA isolated from taste buds and from adjacent non-taste epithelium was used as positive and negative controls, respectively. Individual dissociated taste cells from PLCβ2-GFP (type II) and GAD1-GFP (type III) transgenic mice were selected for collection on the basis of their expression of GFP, as previously described30. Type I taste cells were selected from PLCβ2-GFP × GAD1-GFP double transgenic mice by the absence of GFP expression30. Total RNA isolated from taste cells was either subjected to T7 RNA polymerase-based linear amplification (aRNA, Message BOOSTER kit, Epicentre) or was directly converted to cDNA, both as previously described29. For aRNA-based analyses, 1% of the resulting cDNA was used as template for PCR. For direct RT-PCR, 15% of cDNA from each cell was used to test for diagnostic mRNAs and 30% was used for Calhm1. PCR conditions with HotStarTaq Plus (QIAGEN) were 95°C for 5 min; 45 cycles of 30 sec each at 94°C, annealing temperature, and 72°C. PCR primers, annealing temperatures and product sizes were as in Table S4. Two different primer sets were used for Calhm1 (Table S4). Primers #1, #2 were used for RT-PCR on bulk tissue RNA, as well as for aRNA from individual taste cells (Figs. 1a, S3a, S3b, S3d). Primers #3, #4 span an intron, and were used for the single cell direct RT-PCR to avoid amplifying from genomic DNA (Fig. S3c). The cell type of all single cells and pools of cells was confirmed by RT-PCR for three diagnostic cell-type markers: Entpd2 (type I), Plcb2 (type II), and Snap25 (type III).
Two-bottle preference tests
Calhm1−/− and WT littermate control mice were presented for 48 h with two drinking bottles, one containing water and the other a solution supplemented with ascending concentrations of the specific taste compounds to be tested. All solutions were prepared with tap water and served at room temperature. Mice had ad libitum access to a standard chow diet (Laboratory Rodent Diet 5001, LabDiet, PMI Nutrition International, Brentwood, MO) and drinking solutions. Two independent groups of mice were tested for the following series of taste solutions. The order of testing was: (Group 1) sucrose, saccharin, NaCl, denatonium; (Group 2) MSG, quinine, HCl. Sucrose, saccharin, denatonium benzoate, quinine sulfate, and MSG were from Sigma-Aldrich (St. Louis, MO). HCl and NaCl were from Fisher (Thermo Fisher Scientific Inc., Waltham, MA). The order of stimulus presentation was identical for wild type and KO mice. The mice had 3 days with a single bottle of drinking water between each test series. The two bottles were switched after 24 h to control for a possible effect of the bottle positions. The change in liquid levels was considered to be the mouse’s liquid intake31. Fluid intakes were measured volumetrically to the nearest 0.1 ml. The volumes of water and taste compound solutions consumed were recorded. Solution preference was calculated as the intake of taste solution divided by total liquid intake, and this ratio was expressed as a %. The taste compounds were chosen as exemplars of the sweet, bitter, umami, salty, and sour taste qualities and their concentrations were chosen to span the range between indifference and marked acceptance (for sucrose, saccharin, and MSG) or avoidance (for quinine sulfate, denatonium benzoate, NaCl, and HCl). Results were analyzed by mixed-design analyses of variance with factors of group (WT or Calhm1−/−) and concentration. Differences between the groups in consumption of specific concentrations of taste solution were determined using Bonferroni post-hoc tests. Differences in response of each group to individual concentrations of test compounds relative to water were determined using Student’s t-test. Results presented in Fig. 2a are representative examples taken from the more comprehensive concentration functions displayed in Fig. S4a and Tables S1 and S2.
Brief-exposure gustometer tests
Taste solution acceptance was assessed with brief-exposure tests using procedures similar to those employed by other groups11,32–35.
Mice
12 Calhm1−/− mice (5 male, 7 female) and 12 WT littermate controls (5 male, 7 females) were maintained in a vivarium at 23°C on a 12:12 h light/dark cycle with lights off at 7 pm. When not being tested, each mouse was housed alone in a plastic “tub” cage (26.5 cm × 17 cm × 12 cm) with a stainless-steel grid lid, and wood shavings scattered on the floor. The mouse ate pelleted AIN-76A diet and drank deionized water according to the regimen described below. Throughout the experiment, each mouse was weighed daily, immediately before it was placed into a gustometer.
Apparatus
Brief-exposure taste acceptance was assessed using three MS160-Mouse gustometers manufactured by DiLog Instruments. Each gustometer consists of a 14.5 × 30 × 15 cm test chamber with a motorized shutter that controls access to a taste solution. Bottles of taste solution are mounted on a rack that is precisely positioned by a stepper motor so that any one of eight different taste solutions can be presented to the mouse. The drinking spout of each bottle is part of a high-frequency alternating current contact circuit so that each lick the mouse makes is detected and recorded. Details of construction and other technical information is available elsewhere34,36. Taste compounds were reagent grade and were purchased from Sigma-Aldrich and dissolved in RT deionized water. Deionized water was used as the water (0 concentration) and for “washout” trials (see below). To avoid any undue influence of subtle differences among the three gustometers we used, we ensured that each mouse was always tested in the same gustometer and that equal numbers of WT and Calhm1−/− mice were tested in each gustometer.
Training
To train the mice to sample taste solutions, they were first water-deprived for 22.5 h and then placed in a gustometer with its shutter open. During this first training session, each mouse had continuous access to water for 25 min from the time it first licked the drinking spout. It was then returned to its home cage and given water for 1 h. On the following two days, this procedure was repeated, except the shutter allowing access to water was closed 5 sec after each time the mouse began to lick, and it was reopened after a 7.5-sec interval. Once again, after 25 min, the mouse was returned to its home cage and given water for 1 h. By the 2nd test using these procedures, all except one WT and one Calhm1−/− mouse had learned to obtain water during the 5-sec access periods. We continued to test the two non-responders and they began to drink during the 3rd or 5th test session (see below).
Testing
The mice received two test sessions with each of the 11 compounds presented in Fig. S4b. These were presented in two cycles, with exemplars of the four basic tastes in the first cycle (sucrose, quinine hydrochloride, HCl, and NaCl) and the other compounds in the second cycle. Only one taste compound was used during a session (see below) and there was only one session a day. The deprivation regimen used to investigate the response to sucrose, saccharin and Polycose differed from that used to investigate the response to the other taste compounds because these are hedonically positive and the other three compounds are hedonically negative34. Prior to a session with a hedonically positive compound, each mouse received free access to food and water for 24 h. It then received 1 g of food and 2 mL of water, and the session began 24 h later. After these sessions, the mouse had a recovery day with free access to food and water for 24 h. Its water was then removed for 22.5 h to prepare it for the next session. After sessions with hedonically negative compounds each mouse received water for 1 h in its home cage and it was then water-deprived in preparation for the next session. All sessions lasted 25 min. When hedonically positive compounds were being investigated, the session began with a single test of the highest concentration available in order to kindle the mouse’s interest in the drinking spout. After this, repeated series of 5 concentrations (including water) were presented, in a quasi-random order (a concentration could appear only once in a series of five tests). For each exposure, the shutter was open for 5 sec during which licks of the drinking spout were counted. This was followed by 7.5 sec with the shutter closed, during which a new taste solution was positioned ready for the next presentation. For sessions with hedonically negative compounds, repeated series of 5 or 6 concentrations (including water) were presented in randomized order. Additional 1-sec “washout” trials with water were interposed between test trials. Thus, a mouse received access to a taste solution for 5 sec followed by 7.5 sec with the shutter closed, then access to water for 1 sec followed by 7.5 sec with the shutter closed, followed by the next taste solution for 5 sec, and so on. We think the brief washout trials with water have the effect of cleansing the mouse’s palate and thus prevent it from quitting because it expects only bad-tasting solutions.
Statistical analysis
Separate analyses were conducted for each taste compound. The mean number of licks by each mouse in response to each concentration was obtained by averaging the results of identical exposures together. These values for individual mice were then used in mixed-design analyses of variance with factors of group (WT or Calhm1−/−) and concentration. Mice that did not respond during any presentation of a particular concentration of a taste compound were not included in statistical analyses of that compound. Post hoc LSD tests were used to assess differences among the groups in consumption of specific concentrations of taste solution and differences in response of each group to individual concentrations of each taste compound (Statistica 10, Stat Soft Inc, Tulsa, OK). All analyses were conducted using a criterion for significance of P < 0.05. Results presented in Fig. 2b are representative examples taken from the more comprehensive concentration functions displayed in Fig. S4b and Table S3.
Whole-chorda tympani nerve recordings
The mice were anesthetized with an i.m. injection of a mixture of 1.75 mg/ml ketamine and 1.75 mg/ml xylazine in saline (5 μL/g body wt). Anesthesia was maintained with 0.4–0.6% isoflurane. Body temperature, surgical table temperature, blood oxygen, anesthesia level and heart rate were continuously monitored. The chorda tympani (CT) nerve was dissected free from its junction with the lingual nerve to the tympanic bulla where central part of the CT was cut and the peripheral part was then mobilized in rostral direction so that afferent nerve impulses could be recorded. The nerve impulses were recorded between a silver wire electrode and an indifferent electrode touching the walls of the wound, fed into a custom-made amplifier, monitored over a loudspeaker and an oscilloscope. The nerve impulses were processed by a smoothed absolute value circuit integrator37 and rectified to a DC potential whose amplitude was related to the nerve impulse frequency, here called the summated signal. This signal and a code related to the kind of taste compound used were fed to an oscilloscope and computer. Both the individual nerve impulses and the summated signal were recorded by a recorder (Gould ES 1000). The stimuli were delivered to the tongue with an open flow system controlled by the computer under conditions of constant flow and temperature (33°C)37. Each stimulation lasted for 5 sec with 50 sec rinsing time between stimulations. Care was exercised to make sure that the flow was directed over the fungiform papillae. In each animal, we strived to run the same sequence of stimuli at least three times while we recorded the nerve impulses from the whole CT nerve. The integrated response during stimulation was calculated by subtracting the area of nerve activity preceding stimulation from that during stimulation. Thus, the data reflect the level of activity during stimulation time. The responses to all compounds were expressed relative to the response to 0.1 M NH4Cl in each mouse. The average in each animal and group was calculated, variance determined and significance between the CT responses of Calhm1−/− and WT mice determined. P < 0.05 was considered significant.
Cell culture
HeLa cells (American Type Culture Collection, Rockville, MD) were grown in plastic flasks at 37 °C in a humidified incubator in culture medium containing 90 % (v/v) DMEM, 10 % fetal bovine serum, and 1× Antibiotic-Antimycotic (Invitrogen) with 5 % CO2-in-air.
ATP measurements using cultured cells
Extracellular ATP concentration was measured by the luciferin-luciferase reaction as previously described38. HeLa cells were seeded onto 96-well microplates (Corning Costar, Corning, NY) at a density of 10,000 cells per well one day before transfection. Cells in each well were transfected with 0.2 μg of human CALHM1 cDNA, mouse CALHM1 cDNA, or empty vector (pIRES2.EGFP) using 0.4 μl Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. After 20–30 h, media were removed and cells were incubated for 1 h before 75 μl of the 100 μl bath solution containing 2 mM Ca2+ was replaced with an equal volume of test solution to establish final [Ca2+]o and drug concentrations. The plate was immediately placed in a microplate luminometer (Synergy 2, BioTEK, Winooski, VT) and 10 μl of ATP assay solution (FL-AAM and FL-AAB, Sigma) was dispensed into each well. ATP release was measured every 2 min. ATP concentration was calculated from a standard curve created in each plate. Separate standard curves were made in experiments involving all added drugs at the concentrations used. The bath solution contained (in mM): 150 NaCl, 5 KCl, 10 Hepes, and 10 glucose, pH 7.4 adjusted with NaOH. [Ca2+]o below 90 μM were made by mixing CaCl2 and EGTA, and free [Ca2+]o was calculated by WEBMAXC software (http://maxchelator.stanford.edu). When a drug was tested, cells were pre-incubated for 1 h with the drug before being exposed to low [Ca2+]o solution containing the same drug. The high [K+]o condition (117.5 mM) was established by replacing Na+ with equimolar K+.
ATP measurements using intact taste buds
Taste-evoked ATP release from intact taste buds was recorded with sheets of lingual epithelium as described previously5. The tongue epithelium was cut into pieces, containing the CVP or LE devoid of taste buds, and mounted in a custom Ussing-type chamber that separated the fluid-containing serosal chamber from the apical surface. The apical surface was selectively exposed to a bitter mix (40 mM denatonium benzoate, 1 mM cycloheximide, 10 mM HEPES, pH 7.4) for 45 sec. The serosal fluid (130 μl) was then collected and added to an equal volume of ATP assay solution (FL-AAM and FL-AAB, Sigma) to determine its ATP content by the luciferin-luciferase assay. The solution on the serosal side contained (in mM): 150 NaCl, 5 KCl, 1.5 CaCl2, 1 MgCl2 10 Hepes, and 10 glucose, pH 7.4 adjusted with NaOH.
Single-cell Ca2+ imaging
Isolated taste cells on coverslips were secured in a perfusion chamber and mounted on the stage of an inverted microscope (Nikon Eclipse TE2000). Cells were loaded with Fura-2-AM (Molecular Probes; 2.5 μM) for 45 min at room temperature in the bath solution containing (in mM): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2 10 Hepes, and 10 glucose, pH 7.4 adjusted with NaOH. Cells were continuously perfused with the bath solution and stimulated for 30 sec by bath-perfusion of taste mix (2 mM saccharin, 100 μM SC45647, 1 mM denatonium, 10 μM cycloheximide; dissolved in the bath solution at pH 7.4). Fura-2 was alternately illuminated at 340/380 nm, and the emitted fluorescence intensity at 510 nm was collected with a Perkin Elmer Ultraview imaging system. The background fluorescence was estimated for a region without cells and subtracted during analysis. Changes in [Ca2+]i are presented as changes in fluorescence ratio (F340/F380). Dye calibration was achieved by applying experimentally determined constants to the equation [Ca2+] = Kd β(R –Rmin)/(Rmax – R). Macros used for analysis were custom macros written for IGOR Pro (WaveMetrics). Isolated taste bud cells which responded to a taste stimulus by robust [Ca2+]i increase were identified as type II cells: 24 % of WT cells (9 out of 38 cells from 3 animals), and 39% of Calhm1−/− cells (12 out of 31 cells from 4 animals).
Single cell electrophysiology
Whole-cell recordings were made in single taste bud cells isolated from Calhm1−/− and WT littermates and TRPM5-GFP mice with a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Union City, CA). TRPM5-GFP mice were a kind gift from Dr. R. F. Margolskee39 and used for selective recordings from type II cells on the basis of their expression of GFP. The pipette solution contained in mM: 155 CsF, 2 MgCl2, 1 CaCl2, 11 EGTA, 10 HEPES, pH 7.3 adjusted with methanesulfonic acid, ~ 308 mOsm. The bath solution contained in mM: 140 Na+, 5.4 K+, 10 TEA+, 20 sucrose, 1.5 Ca2+, 1 Mg2+, 6 Cl− and 10 HEPES, pH 7.4 adjusted with methanesulfonic acid, ~330 mOsm. Patch pipettes were fabricated from thick-walled borosilicate glass capillaries (PG10150-4, World Precision Instruments) and had a resistance of 5–9 MΩ in the recording condition. Electrode capacitance was compensated electronically, and 70 % of series resistance (10–25 MΩ) was compensated with a lag of 20 μs. Currents were low-pass filtered at 5 kHz with 8-pole Bessel characteristics and sampled at 10 kHz. For testing pharmacological properties of Islow in type II cells (Fig. 4h), whole cell currents were recorded in GFP-positive taste cells isolated from TRPM5-GFP mice and Islow was evoked every 5 sec by a voltage pulse to +100 mV with 1 sec duration from a holding potential of −70 mV. Current amplitudes were taken at the end of the pulse and normalized to the average of values before drug addition (initial 50 sec) for each cell.
Supplementary Material
Acknowledgments
This work was supported by a KeySpan award to P.M., several NIH grants (GM56328, MH059937, NS072775 to J.K.F.; DC10393 to M.G.T.; EY13624 to M.M.C.; R03DC011143 to I.M.; Core Grant P30 EY001583 to the University of Pennsylvania; Core Grant P30DC011735 to the Monell Chemical Senses Center), and the University of Minnesota’s Undergraduate Research Opportunities Program to S.L. and M.A. A.T. and M.O. are JSPS Fellows. We thank Dr. Robert F. Margolskee (Monell Chemical Senses Center, PA, USA) for the TRPM5-GFP mice and Dr. Yuzo Ninomiya (Kyushu University, Fukuoka, Japan) for helpful comments.
Footnotes
Author Contributions
A.T., V.V., A.L., Z.M., M.O., I.M., H.Z., L.A, S.L., M.A., G.H., G.D. and N.C. designed and performed experiments. P.M. and V.V. generated the Calhm1 knockout mice. J.K. and P.D. designed experiments. M.G.T., M.M.C., P.M.and J.K.F. designed experiments and helped with data interpretation. A.T., J.K.F., and P.M. wrote the manuscript.
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Z, et al. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc Natl Acad Sci U S A. 2012;109:E1963–1971. doi: 10.1073/pnas.1204023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siebert AP, et al. Structural and Functional Similarities of Calcium Homeostasis Modulator 1 (CALHM1) Ion Channel With Connexins, Pannexins and Innexins. J Biol Chem. 2013 doi: 10.1074/jbc.M112.409789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 5.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 6.Dreses-Werringloer U, et al. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell. 2008;133:1149–1161. doi: 10.1016/j.cell.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koppel J, et al. CALHM1 P86L polymorphism modulates CSF Abeta levels in cognitively healthy individuals at risk for Alzheimer’s disease. Mol Med. 2011;17:974–979. doi: 10.2119/molmed.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert JC, et al. The CALHM1 P86L polymorphism is a genetic modifier of age at onset in Alzheimer’s disease: a meta-analysis study. J Alzheimers Dis. 2010;22:247–255. doi: 10.3233/JAD-2010-100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyer BD, et al. Expression of genes encoding multi-transmembrane proteins in specific primate taste cell populations. PLoS One. 2009;4:e7682. doi: 10.1371/journal.pone.0007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011;14:685–687. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 12.Huang YJ, et al. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romanov RA, et al. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenbeuch A, Zorec R, Kinnamon SC. Capacitance measurements of regulated exocytosis in mouse taste cells. J Neurosci. 2010;30:14695–14701. doi: 10.1523/JNEUROSCI.1570-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romanov RA, et al. Dispensable ATP permeability of Pannexin 1 channels in a heterologous system and in mammalian taste cells. J Cell Sci. 2012 doi: 10.1242/jcs.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1:311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romanov RA, Kolesnikov SS. Electrophysiologically identified subpopulations of taste bud cells. Neurosci Lett. 2006;395:249–254. doi: 10.1016/j.neulet.2005.10.085. [DOI] [PubMed] [Google Scholar]
- 19.Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol. 2011;61:333–372. doi: 10.1016/B978-0-12-385526-8.00011-4. [DOI] [PubMed] [Google Scholar]
- 20.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 21.Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 22.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnstock G. Dual control of local blood flow by purines. Ann N Y Acad Sci. 1990;603:31–44. doi: 10.1111/j.1749-6632.1990.tb37659.x. discussion 44–35. [DOI] [PubMed] [Google Scholar]
- 25.Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- 26.Cockayne DA, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 27.Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–1409. doi: 10.1016/j.gastro.2003.07.008. [DOI] [PubMed] [Google Scholar]
References for Methods
- 28.Ohmoto M, Matsumoto I, Misaka T, Abe K. Taste receptor cells express voltage-dependent potassium channels in a cell age-specific manner. Chem Senses. 2006;31:739–746. doi: 10.1093/chemse/bjl016. [DOI] [PubMed] [Google Scholar]
- 29.Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol. 2009;517:1–14. doi: 10.1002/cne.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinclair MS, et al. Oxytocin signaling in mouse taste buds. PLoS One. 2010;5:e11980. doi: 10.1371/journal.pone.0011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tordoff MG, Bachmanov AA. Mouse taste preference tests: why only two bottles? Chem Senses. 2003;28:315–324. doi: 10.1093/chemse/28.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damak S, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 33.Eddy MC, et al. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses. 2009;34:789–797. doi: 10.1093/chemse/bjp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 35.Hallock RM, Tatangelo M, Barrows J, Finger TE. Residual chemosensory capabilities in double P2X2/P2X3 purinergic receptor null mice: intraoral or postingestive detection? Chem Senses. 2009;34:799–808. doi: 10.1093/chemse/bjp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spector AC, Andrews-Labenski J, Letterio FC. A new gustometer for psychophysical taste testing in the rat. Physiol Behav. 1990;47:795–803. doi: 10.1016/0031-9384(90)90099-p. [DOI] [PubMed] [Google Scholar]
- 37.Hellekant G, Roberts TW. In: Experimental Cell Biology of Taste and Olfaction: Current Techniques and Protocols. Spielman AI, Brand JG, editors. CRC Press; 1995. pp. 277–290. [Google Scholar]
- 38.Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol. 2010;299:C1308–1317. doi: 10.1152/ajpcell.00333.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.