Abstract
Objective
To test the hypothesis that cochlear implantation surgery before 12 months of age yields better spoken language results than surgery between 12–18 months of age.
Study Design
Language testing administered to children at 4.5 years of age (± 2 months).
Setting
Schools, speech-language therapy offices, and cochlear implant (CI) centers in the US and Canada.
Participants
69 children who received a cochlear implant between ages 6–18 months of age. All children were learning to communicate via listening and spoken language in English-speaking families.
Main Outcome Measure
Standard scores on receptive vocabulary, expressive and receptive language (includes grammar).
Results
Children with CI surgery at 6–11 months (N=27) achieved higher scores on all measures as compared to those with surgery at 12–18 months (N=42). Regression analysis revealed a linear relationship between age of implantation and language outcomes throughout the 6–18 month surgery-age range.
Conclusion
For children in intervention programs emphasizing listening and spoken language, cochlear implantation before 12 months of age appears to provide a significant advantage for spoken language achievement observed at 4.5 years of age.
INTRODUCTION
Since the introduction of cochlear implant (CI) surgery for children there have been many changes in clinical practice, several of which are attributable to shifts in candidacy criteria (1–4). One of the most notable changes has been the lowering of the approved surgery age by the US Food and Drug Administration: to 24 months of age in 1990, then to 18 months in 1998, and then to 12 months in 2000. The impetus for changing the guidelines has been a steady stream of reports from researchers and clinicians showing better benefit in speech perception, speech production and language learning for children who receive a CI at younger ages.
The vast majority of children who are born with severe-profound hearing loss have parents with normal hearing sensitivity. For most parents, the goal of CI surgery is to facilitate the development of spoken language in their child. Adequately testing the impact of earlier implantation on this desired outcome goes beyond measuring the recognition of speech or imitation of speech sounds. It should focus on the degree to which a child perceives, internalizes, and utilizes the spoken vocabulary and sentence structure (grammar) of the surrounding language community. Studies that specifically examine outcomes in receptive and expressive spoken language reveal that children who receive a CI between the ages of 12–24 months achieve higher levels of understanding and use of language and/or experience faster growth rates in language than those who receive a CI just one year later (5–9). There is a fairly strong consensus in the literature on this point and the next question becomes whether moving the surgery age below 12 months is warranted.
Why provide the CI earlier?
There are several compelling reasons why a move to CI surgery under the age of 12 months might be advantageous. First, recently published studies show that normally-hearing infants in the first year are life are capable of a wide range of auditory perceptual discriminations and abilities not previously recognized. These include a preference for human speech over rhesus vocalizations by 3 months of age (10), the development of word segmentation abilities between 7.5–10.5 months of age (11), the ability to associate words with salient persons, common objects and body-parts at 6 months (12–14), and recognition of change in the identity of a speaker at 7 months (15). Clearly these very early abilities involve some of the foundational skills of human speech communication.
Second, all of the emerging abilities of infants to process spoken linguistic content depend upon adequate hearing and attention to the sounds of the infant’s native language community, likely beginning at birth. To the extent that significant amounts of input and experience are necessary for language learning to occur, infants will benefit from hearing as early as possible, in order to take advantage of this important developmental period of growth. Building upon many studies indicating that there is a critical, or sensitive, window of development within which spoken language should be available in order to achieve optimal language acquisition, Gordon and colleagues (16) argue forcefully that there should be an urgency to early diagnosis and early cochlear implantation of children with severe-profound hearing loss in order to maximize auditory development and subsequent language learning.
Finally, the cost savings of very early implantation may be significant over the long term. Colletti et al. (17) project the costs to individual families and to society of educating a deaf child receiving a CI before the age of 12 months as significantly less than for the child receiving a CI between 12–23 months of age. The savings to individual families for younger implantation was more dramatic than the educational or medical system savings and derived primarily from a reduction in costs associated with missed work and costs of travel, hearing aids, and therapy prior to CI surgery.
Risks for infant surgery
Any surgical procedure that is suggested for young infants must be evaluated for additional surgical and anesthetic risks and complications. A recent review by Cosetti & Roland (18) concluded that these risks were not appreciably greater for young infants during or immediately after the cochlear implantation procedure as compared with those for older children. Empirical papers from multiple cochlear implant centers in the United States and Europe similarly reveal few problems (19–22) though best outcomes may be observed in centers which perform many pediatric implant surgeries and have access to pediatric anesthesiological services (23).
Previous studies of language outcomes with CI surgery < 1 year of age
Cochlear implantation surgery involving children younger than 12 months of age is still considered an “off-label” practice in the United States and therefore is not practiced by all surgeons and those reports that exist do not involve large numbers of children. Some studies involve comparison of infants with an age of implantation (AOI) younger than 12 months to children with an AOI between 12–24 months and others compare the target group to normally-hearing children of the same age or to normative test data. Complicating matters of comparison may be differing modes of communication used in education and testing (speech only vs speech/sign together), differing ages of onset of deafness, and different pre-implant residual hearing levels. All of these variables, and others, must be considered when seeking to isolate and test the effect of early age of implantation. Ideally, groups of children with different AOIs should be as homogeneous as possible on all other variables that could compete for explanatory power in comparisons of group differences.
Short-term outcome studies involving CI surgeries before the age of 12 months have measured speech perception (24), early infant communication (25) and auditory perception (21–22,26). A 2010 meta-analysis (27) concludes that the published outcome studies as of that date did not support the increasing practice of providing CIs to children below one year of age. Studies that specifically test for outcomes in the realm of language development are still quite rare and involve relatively small numbers of children. Dettman and colleagues (28) report on the expressive and receptive growth rates of 11 children with AOI at ≤ 12 months of age as compared to a group with AOI at 12.5 – 24 months of age. For both language domains, those in the youngest AOI group progressed significantly faster than those in the older group over the first 1–3 years of CI use. Similarly, Colletti et al. (29) found that after 10 years of use, a group of 19 children with AOI from 2–11 months were significantly more likely to score higher on a receptive vocabulary test and significantly more likely to score at the 75th percentile or higher on a test of receptive grammar than those with later surgeries.
Factors Influencing AOI Comparisons
Selecting comparison groups
Grouping children into AOI categories (e.g., 12–23 months versus 24–36 months) for statistical comparison can sometimes obscure the continuous relation between surgery age and language outcome. Further, means derived from AOI groupings may be unduly influenced by the distribution of implant age within each group. For example, when reporting a mean outcome score for children with AOI within 12–23 months of age the mean of that group may be heavily influenced if either end of this continuum is overly represented. For this reason, we believe it to be important to analyze outcome data, when possible, (a) by using very narrow age ranges when grouping and (b) to also separately consider outcomes as continuous variables. Therefore, we will limit the comparison in the present paper to small AOI ranges (6–11 vs 12–18 months of age). For those who are located in areas with newborn hearing screening and an interest in the potential benefits of earlier CI surgery, differences in outcomes between AOI at 6–11 months versus 12–18 months should be the comparison of most interest. Because we test children from programs across the United States and Canada, we have data from more children in these particular AOI ranges than most single-center-based studies.
In addition, we will explore the continuous relation between AOI from 6–18 months and predicted language outcome scores. In an earlier paper, we presented a regression function for language quotient scores at age 4.5 as a function of AOI for children with surgery ages from 12–38 months of age. That function predicted scores closer to age-appropriate levels for each month’s decrease in surgery age from 30 to 12 months (8). In the present study we will include a similar analysis with a younger but slightly overlapping age range. This will examine whether the positive effect of each month’s decrease in implant age on expected outcome continues below 12 months.
Duration of use
Language outcome level has been shown to improve with increased duration of CI experience (30). When chronological age at testing is the same for all children, those implanted at younger ages will necessarily have longer durations of CI use. Therefore, duration of CI use should also be examined for its relation to any outcome variables.
Number of implants
Increasing numbers of young children with CIs have received two devices, either simultaneously or with an inter-surgical interval of weeks, months or years (31). There have been reports in the literature of benefits in sound localization (32) and speech perception in noise (33) but considerably fewer studies report bilateral benefits for outcomes in spoken language. Nonetheless, any comparison of CI outcomes must take this change in practice into account either in participant selection, statistical control, or both.
Mother’s education level
Studies of children without hearing loss have documented a significant language advantage related to mothers’ education level, with mean PPVT scores of 110 for children whose mothers completed college, 101 for those who completed high school, and 90 for those who had less than a high school education (34). This strong influence on language learning may also extend to those learning language with the aid of a CI. It may not be unreasonable to find that some children with CIs who have college-educated mothers may eventually achieve standard scores above the normative mean of 100.
Objectives of the present study
Given the growing consensus that obtaining a CI close to a child’s first birthday is better for spoken language outcomes than obtaining one just a year later, the most interesting new comparison is between children with surgeries in the months shortly before and after the 12-month mark. Given the relatively large individual differences that are seen in most studies of language development (both in hearing and deaf children), significant differences between two relatively small windows of AOI on either side of the 1st birthday would be quite meaningful and hold important implications for clinical practice and perhaps, eventually, federal guidelines for the lower limit for pediatric cochlear implantation. Therefore, objectives for the present study were:
To determine whether significant differences in spoken language exist between children who receive a 1st CI at 6–11 months of age versus 12–18 months of age.
To examine the role of duration of CI use, mother’s education, and bilateral implantation in spoken language outcomes.
To estimate the implant age below which children can be expected to achieve language levels commensurate with normally-hearing peers by age 4.5.
MATERIALS AND METHODS
Participants
This study was conducted with a sample of 69 children with cochlear implants drawn from across the US and Canada. Of those children with CIs, 27 had a first CI surgery age between 6–11 months of age and 42 between 12–18 months of age. All children were presumed deaf since birth, had no significant visual, motor, or cognitive problems and came from families in which English was the primary language spoken in the household. All had received listening and spoken language (LSL) intervention since at least the time of CI surgery.
Average characteristics of each of the AOI groups are summarized in Table 1. In addition to significantly younger AOI, the 6–11 month AOI group had significantly longer duration of CI use, younger age at diagnosis of deafness, a smaller proportion of female participants, and a larger proportion receiving a second CI than the 12–18 month AOI group. The groups were not significantly different in age at test or mother’s education level. At the time of testing, 36 children had unilateral and 33 had bilateral implants. Of those with bilateral implants, 12 received the two CIs in a simultaneous implantation surgery and the remaining had sequential surgeries with intervals ranging from 1 to 12 months. Approximately 80% of children had mothers who had graduated from college. Children were excluded from this study if they had previously experienced an interruption of CI use for more than 30 days. Children were recruited through schools for deaf children, speech-language therapists, and cochlear implant centers. Candidates who met the study criteria were identified by on-site staff and provided with information about the study and a method to volunteer to participate if interested. The study protocol was approved by the Human Research Protections Office of the first author’s institution.
Table 1.
Participant characteristics and t-test comparison of means.
| Age of Implantation Group | |||
|---|---|---|---|
| 6–11 months N = 27 |
12–18 months N = 42 |
Mean comparisons a p – value |
|
| Age at Test (months) | 54.4 (1.5) | 54.8 (1.3) | t(67) = − 1.16, ns |
| Age 1st CI surgery (months) | 9.6 (1.3) | 14.7 (2.5) | t(66)= −11.15, p < .001 |
| Duration of CI Use (months) | 44.9 | 40.1 | t(67)= 7.61, p < .001 |
| Age Diagnosis HL (months) | 1.5 (2.4) | 4.8 (4.5) | t(65) = − 4.01, p < .001 |
| Bilateral CIs | N = 17 (63%) | N = 16 (38%) | χ2(1) = 4.07, p < .05 |
| Mother’s Educ (years) | 16.2 | 16.5 | t(66) = − 0.51, NS |
| Gender | F = 9 (33%) M = 18 |
F = 26 (62%) M = 16 |
χ2(1) = 5.37, p < .05 |
two-tailed t-tests
N = 21
N = 41
Data Collection Procedures
Testing occurred when all children were 4.5 years of age (+ 2 months). Experienced examiners from the study staff travelled to the school, speech-therapy office, or cochlear implant center in the child’s home city for testing. All children were administered the Peabody Picture Vocabulary Test –III (PPVT)(35) and the Preschool Language Scale-IV (PLS)(36). The PPVT is a standardized, age-normed test of receptive vocabulary in which an examiner says “Show me…” followed by a single word. The child is shown four pictures and responds by pointing to the one that most accurately depicts or represents the spoken word. The PLS is a standardized, age-normed test of overall language ability, i.e., covering semantics, morphology, syntax, and vocabulary and involves both the understanding and production of language by the child. Test administration was delivered in spoken English and only spoken language was credited (except for response points as allowed by the testing protocol, such as PPVT). Standard scores were calculated for receptive vocabulary (PPVT), receptive and expressive language (Auditory Comprehension and Expressive Communication Scales of the PLS, respectively). The test norms (based on normally-hearing children) for both the PPVT and PLS have mean standard scores of 100 and standard deviations of 15.
RESULTS
Comparison of AOI groups
Mean scores of children in the two groups are shown in Table 2, along with standard deviations and results of t-tests comparing the mean scores of the groups on each language test. On every test, the mean of the 6–11 months AOI group was significantly higher than that of the 12–18 months AOI group. When compared to the tests’ normative data the percentage of children in the 6–11 months group scoring within the average range or higher (standard score of 85+) was 93% for receptive vocabulary, 85% for receptive language, and 77% for expressive language. The corresponding percentages for the 12–18 months group were 77%, 60%, and 57%, respectively.
Table 2.
Means and standard deviations on tests of vocabulary, receptive and expressive language with results of mean comparisons.
| Age of Implantation Group | |||
|---|---|---|---|
| 6–11 months (N = 27) | 12–18 months (N = 42) | p-value t-test* | |
| Receptive Vocabulary PPVT-III | 103.07 SD = 11.62 |
94.17 SD = 14.85 |
.005 |
| Receptive Language PLS-Aud Comp | 103.96 SD = 17.33 |
90.45 SD = 19.101 |
.002 |
| Expressive Language PLS-III | 101.04 SD = 19.92 |
90.12 SD = 20.57 |
.016 |
one-tailed
Correlations with potential predictors
It was of interest to discover whether factors seen to influence language performance in CI studies with children who received CIs at older ages would also be important for the children in this study. We examined gender, duration of 1st CI use, mother’s education level, age at diagnosis of HL and whether a child had received 1 versus 2 CIs by the age of 4.5 years (see Table 3). Since scores on the outcome measures were highly inter-correlated (ranging from .732–.787), we averaged the standard scores on the 3 measures for each child and used a resulting “Averaged Language Standard Score” (ALSS) in the correlations below. This ALSS was significantly correlated with age at 1st CI but not correlated with number of CIs, age at diagnosis, gender or mother’s education level. Age at diagnosis of hearing loss was correlated with age of 1st CI (and duration of use). While the number of CIs was not correlated with language outcome scores, it was negatively correlated with age at 1st CI surgery, suggesting that those children in the present sample who received 2 CIs by the age of 4.5 years received those devices at a younger age. While duration of CI use was correlated with AOI, it was not significantly related to the language outcome scores.
Table 3.
Correlations (p-values for two-tailed tests of significance) and cell N for an averaged spoken language performance measure (ALSS = Average Language Standard Score) and potential predictors.
| Variable | ALSS | Age 1st CI | Duration CI Use | Uni- vs Bilateral | Age Dx HL | Mother’s Educ | Gender* |
|---|---|---|---|---|---|---|---|
| ALSS | - | −.325 (.006) | .170 (.163) | .175 (.151) | −.183 (.135) | −.097 (.433) | −.116 (.344) |
| 69 | 69 | 69 | 68 | 68 | 69 | ||
|
| |||||||
| Age 1st CI | - | −.909 (.000) | −.285 (.018) | .309 (.010) | .107 (.384) | .248 (.040) | |
| 69 | .69 | 68 | 68 | 69 | |||
|
| |||||||
| Duration of Use | - | .152 (.213) | −.275 (.023) | −.176 (.150) | −.135 (.270) | ||
| 69 | 68 | 68 | 69 | ||||
|
| |||||||
| Uni - vs Bilateral | - | −.147 (.232) | .025 (.838) | −.043 (.726) | |||
| 68 | 68 | 69 | |||||
|
| |||||||
| Age Dx HL | - | .056 (.650) | .158 (.198) | ||||
| 68 | 68 | ||||||
|
| |||||||
| Mother’s Educ | - | −.209 (.087) | |||||
| 68 | |||||||
For gender: 1 = male, 2 = female
Predicting language outcome scores by Age of Implantation
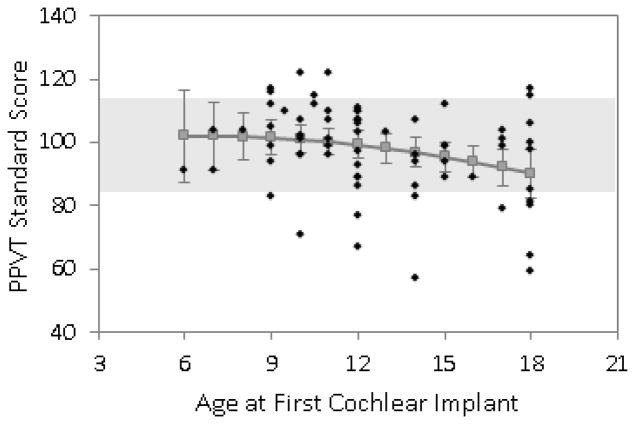
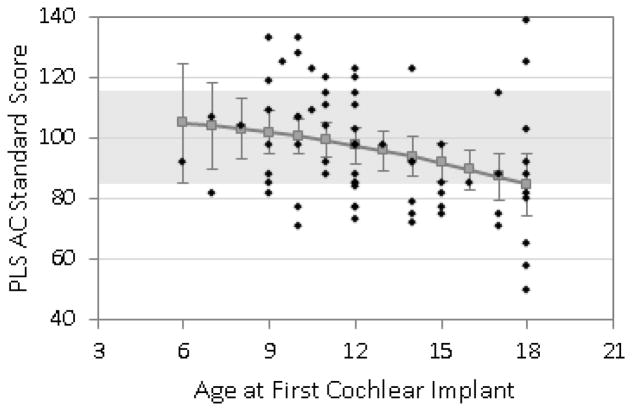
Figures 1, 2, and 3 show the predicted means for receptive vocabulary, receptive language, and expressive language plotted as a function of age of 1st cochlear implantation. Individual data points are plotted on these graphs along with the predicted means and the associated 95% confidence interval for each mean. Regression analyses revealed a significant linear effect of age at surgery on all three types of outcome measures (receptive vocabulary p = .023, receptive language p = .009, expressive language p = .017) but no significant curvilinear effects. This means that there was a steady increase in test scores for each younger age at surgery throughout the entire span of 6–18 months surgical age with no significant change in slope of benefit within this range. Note that on these tests the mean standard score for normally-hearing children is 100 and given a standard deviation of 15 the range for “average” scores is 85–115. This range is depicted in the graphs with light shading. For receptive vocabulary (Fig 1) the expected mean score was within the “average range” for all ages of implantation tested, i.e. through AOI of 18 months. For receptive language (Fig 2) the expected score was in the average range for all AOI below 18 months and for expressive language it was for all AOI below 17 months (Fig 3).
Figure 1.
Predicted scores for receptive vocabulary (PPVT-III) at 4.5 years of age, based on age at CI surgery.
Figure 2.
Predicted scores for overall receptive language (PLS-III, Auditory Comprehension Scale) at 4.5 years of age, based on age at CI surgery.
Figure 3.
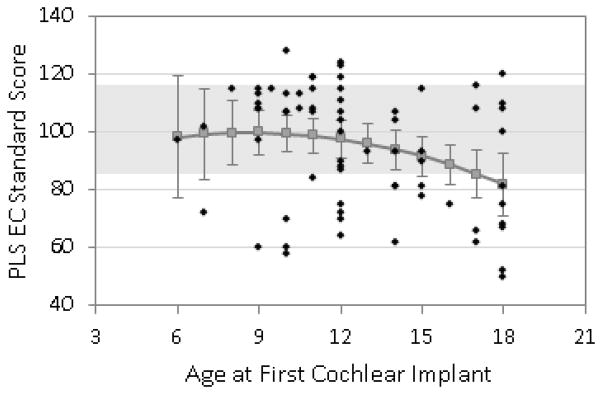
Predicted scores for overall expressive language (PLS-III, Expressive Communication Scale) at 4.5 years of age, based on age at CI surgery.
DISCUSSION
In this paper we reported on receptive vocabulary, receptive language, and expressive language scores of 4.5-year-old children who received a CI at 6–11 months. We compared those scores with those of age-mates who received a CI at 12–18 months of age and also with test norms developed with normally-hearing children with the following objectives:
-
1
To determine whether significant differences in spoken language exist between children who receive a 1st CI at 6–11 months of age versus 12–18 months of age.
Significant advantages for cochlear implantation under 12 months of age were observed for vocabulary, receptive and expressive spoken language.
-
2
To determine the role of duration of CI use, mother’s education, and bilateral implantation in spoken language outcomes.
There was no significant advantage for spoken language provided by longer use of a CI, higher mother education level or receipt of a second CI.
-
3
To estimate the implant age below which children can be expected to achieve language levels commensurate with normally-hearing peers by age 4.5.
CI surgery below 12 months was not necessary for scoring within 1 standard deviation of typically-developing, hearing children by the end of the preschool years. In fact, most (68%) of our entire sample (implant ages up to 18 months) scored within or above the normal range (standard score ≥ 85) on the PLS - receptive and expressive language combined - administered at age 4.5. For the measure of receptive vocabulary (PPVT) the corresponding percentage was 84%. However this result must be considered in light of the demographics of these families. Since families who seek a CI for their child “off-label” (i.e., under 12 months of age) are more likely to be highly educated and have higher incomes than the average family whose child receives a CI at older ages, it was necessary to match those broad demographics in the 12–18 sample as well in order to adequately assess the effects of surgery age. These demographic factors alone are known to be correlated with higher language outcomes in normally-hearing children. Therefore, a caution is warranted about interpreting the generally high language scores exhibited by this sample of children with CIs.
For this sample of relatively advantaged children (i.e., high maternal education level, strong educational programs focusing on listening and spoken language), achieving within one standard deviation of hearing age mates by kindergarten is not entirely unexpected (37). In fact, many of these children could be considered delayed when compared to typically-developing children with similar maternal education level. Almost 80% of the mothers of children in this study had completed college, while only about 20% of those in the normative samples were in that educational category (35–36). The average standard score range on the PPVT for children with college-educated mothers is estimated between 96–124 (34). According to those standards, only 65% of the sample exhibited age-appropriate language outcomes across these three language tests (82% in the 6–11 months group and 55% in the 12–18 month AOI group). When AOI is examined as a continuous variable (see Figures 1–3), the expected mean scores of only those with AOI at or below 12 months of age reach this level of proficiency. While many of these children scored at above-average language levels as compared with test norms, they are likely commensurate with similarly advantaged hearing peers. The important message of these results is not the absolute level of achievement (which will likely not be generalizable to all children) but rather the significant differences in achievement observed by age of implantation surgery.
It may be worth noting that achievements relative to hearing peers may be easier to achieve in the preschool years when “academic demands” do not exist and progress through age-appropriate developmental milestones may be less dependent upon complex language than they will be later in the school years. Therefore, attainment of scores within and sometimes even above the average range by these children with early CIs may not necessarily be maintained relative to hearing peers as children progress through the school years. These children will face significant and unspecified challenges in the years ahead and may well benefit from an early head start. While we have also recently published data showing that many children with CIs who are achieving at below-average levels at age 4.5 do actually make significant gains relative to normally-hearing peers throughout the early school years (30) it is our firm belief that the earlier in his or her life that a child can achieve language skills commensurate with hearing peers the better those later outcomes are likely to be.
Finally, there are special audiological issues to consider with regard to possible cochlear implant surgery in infants. Current FDA guidelines for candidacy in children 12–23 months of age indicate a hearing loss equal to or exceeding 90 dB HL in both ears. When considering implantation of children of even younger ages, audiologists will want to feel confident about quantification of the child’s residual hearing. Reliable and consistent audiological results are highly desirable and preferably by both electrophysiological methods (auditory brainstem audiometry) and behavioral methods. The point at which audiological threshold data with behavioral measures (i.e., visual reinforcement audiometry) are considered accurate is typically not younger than 6 months of age (38). Further, audiologists should consider providing every child with acoustic stimulation with well-fit hearing aids for some period of time, at least a trial through the period of CI candidacy evaluation. This will allow for (a) a period of observation of auditory development (or lack thereof) with the child’s residual hearing and (b) any possible benefit that might accrue from having even a small amount of pre-CI aided hearing before the surgery, which has sometimes been shown to be helpful in subsequent, post-CI, auditory development (7,39).
The results of this investigation suggest that for children in intervention programs with an emphasis on listening and spoken language, receipt of a cochlear implant below 12 months of age may provide significant additional benefit beyond that achieved by implantation between 12–18 months of age.
Table 4.
Means (SDs) and t-test comparisons between children grouped by unilateral and bilateral (simultaneous and sequential by age 4.5 years) implantation.
| Unilateral (N = 36) | Bilateral (N = 33) | p-value t-test* | |
|---|---|---|---|
|
Receptive Vocabulary: PPVT-III |
96.44 (16.25) | 98.97 (11.86) | n.s. |
|
Receptive Language: PLS-III Auditory Comprehension Scale |
91.64 (18.51) | 100.21 (19.77) | n.s. |
|
Expressive Language: PLS-III Expressive Communication Scale |
91.39 (22.21) | 97.67 (19.11) | n.s. |
1-tailed test
Acknowledgments
We are very grateful to the National Institutes of Health-NIDCD (#R01 DC004168) for funding for this project and to Julia Biedenstein, Sarah Fessenden, and Christine Brenner for testing and data preparation. We also thank the parents and children who participated in the study and the following programs that facilitated testing: Auditory Learning Center (Raleigh NC), Auditory Verbal Center of Atlanta, CCHAT (Sacramento CA), Callier Center (Dallas TX), Center for Communication, Hearing and Deafness (Milwaukee WI), Center for Hearing and Communication (New York NY), Center for Hearing and Speech (Houston TX), Central Institute for the Deaf (St. Louis MO), Child’s Voice School (Wood Dale IL), Cobb County DHH Preschool Program (Marietta GA), Desert Voices (Phoenix AZ), Easter Seals (Salisbury MD), Elwyn EIO (Philadelphia PA), Gaithersburg (MD) Middle School, Hear to Talk (Los Angeles CA), Lois Heymann, SLP (Chestnut Ridge NY), Ionia County Intermediate School District (Ionia MI), Jean Weingarten Peninsula Oral School (Redwood City CA), Laurie Gaylord, Cert AVT (Hobe Sound FL), Lincoln School (Westfield NJ), Listen and Talk (Seattle WA), The Listen Foundation (Greenwood CO), Manhattan Eye, Ear and Throat Hospital (Manhattan NY), Memphis Oral School, Moog Center for Deaf Education (St. Louis MO), Montreal Oral School (Westmount Quebec), Presbyterian Ear Institute (Albuquerque NM), Speech Bananas (Long Beach CA), Shawnee Park Oral Deaf Center (Grand Rapids MI), St. Joseph Institute for the Deaf (St. Louis MO), Summit Speech School (New Providence NJ), Tucker-Maxon School (Portland OR) as well as mainstream schools in eight states.
Footnotes
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest to report. This work was supported by NIH/NIDCD #R01DC004168 (Nicholas).
Contributor Information
Johanna G. Nicholas, Washington University in Saint Louis.
Ann E. Geers, The University of Texas at Dallas.
References
- 1.Sampaio ALL, Araujo MFS, Oliveira CACP. New criteria of indication and selection of patients to cochlear implant. Int J Otolaryngol. 2011;2011:Article ID 573968, 1–13. doi: 10.1155/2011/573968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heman-Ackah SE, Roland JT, Haynes DS, Waltzman SB. Pediatric cochlear implantation: Candidacy evaluation, medical and surgical considerations, and expanding criteria. Otolaryngol Clinic North Am. 2012;45:41–67. doi: 10.1016/j.otc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Leigh J, Dettman S, Dowell R, Sarant J. Evidence-based approach for making cochlear implant recommendations for infants with residual hearing. Ear Hear. 2011;32:313–322. doi: 10.1097/AUD.0b013e3182008b1c. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien LCG, Kenna M, Neault M, et al. Not a “sound” decision: Is cochlear implantation always the best choice? Int J Pediatr Otorhinolaryngol. 2010;74:1144–1148. doi: 10.1016/j.ijporl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Holt RF, Svirksy MA. An exploratory look at pediatric cochlear implantation: Is earliest always best? Ear Hear. 2008;29:492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto RT, Hay-McCutcheon MJ, Kirk KI, Houston DM, Bergeson-Dana T. Language skills of profoundly deaf children who received cochlear implants under 12 months of age: A preliminary study. Acta Oto-Laryngol. 2008;128:373–377. doi: 10.1080/00016480701785012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27:286–298. doi: 10.1097/01.aud.0000215973.76912.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe-profound hearing loss. J Speech Lang Hear Res. 2007;50:1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholas JG, Geers AE. Expected test scores for preschoolers with a cochlear implant who use spoken language. Am J Speech-Lang Path. 2008;17:121–138. doi: 10.1044/1058-0360(2008/013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vouloumanos A, Hauser MD, Werker JF, Martin A. The tuning of human neonates’ preference for speech. Child Development. 2010;81:517–527. doi: 10.1111/j.1467-8624.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 11.Jusczyk PW. Some critical developments in acquiring native language sound organization during the first year. Ann Otol Rhinol Laryngol. 2002;111:11–15. doi: 10.1177/00034894021110s503. [DOI] [PubMed] [Google Scholar]
- 12.Bergelson E, Swingley D. At 6–9 months, human infants know the meanings of many common nouns. Proc Nat Acad Sci. 2012;109:3253–3258. doi: 10.1073/pnas.1113380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tincoff R, Jusczyk PW. Some beginnings of word comprehension in 6-month-olds. Psychol Sci. 1999;10:172–175. [Google Scholar]
- 14.Tincoff R, Jusczyk PW. Six-month-olds comprehend words that refer to parts of the body. Infancy. 2012;17:432–444. doi: 10.1111/j.1532-7078.2011.00084.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson EK, Westrek E, Nazzi T, Cutler A. Infant ability to tell voices apart rests on language experience. Dev Sci. 2011;14:1002–1011. doi: 10.1111/j.1467-7687.2011.01052.x. [DOI] [PubMed] [Google Scholar]
- 16.Gordon KA, Wong DDE, Valero J, Jewell SF, Yoo P, Papsin BC. Use it or lose it? Lessons learned from the developing brains of children who are deaf and use cochlear implants to hear. Brain Topogr. 2011;24:204–219. doi: 10.1007/s10548-011-0181-2. [DOI] [PubMed] [Google Scholar]
- 17.Colletti L, Mandala M, Shannon RV, Colletti V. Estimated net savings to society from cochlear implantation in infants: A preliminary analysis. Laryngoscope. 2011;121:2455–2460. doi: 10.1002/lary.22131. [DOI] [PubMed] [Google Scholar]
- 18.Cosetti M, Roland JT. Cochlear implantation in the very young child: Issues unique to the under-1 population. Trends Amplif. 2010;14:46–57. doi: 10.1177/1084713810370039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colletti L, Mandala M, Colletti V. Cochlear implants in children younger than 6 months. Otolaryngol - Head Neck Surg. 2012;147:139–146. doi: 10.1177/0194599812441572. [DOI] [PubMed] [Google Scholar]
- 20.Das Purkayastha PK, Jewell S, James AL, Gordon KA, Papsin BC. Soft tissue complications after pediatric cochlear implantation in children younger than 12 months. Otol Neurotol. 2011;32:780–783. doi: 10.1097/MAO.0b013e318214ea88. [DOI] [PubMed] [Google Scholar]
- 21.Valencia DM, Rimell FL, Friedman BJ, Oblander MR, Helmbrecht J. Cochlear implantation in infants less than 12 months of age. Int J Pediatr Otorhinolaryngol. 2008;72:767–773. doi: 10.1016/j.ijporl.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Waltzman SB, Roland JT. Cochlear implantation in children younger than 12 months. Pediatrics. 2005;116:e487–e493. doi: 10.1542/peds.2005-0282. [DOI] [PubMed] [Google Scholar]
- 23.Johr M, Ho A, Wagner CS, Linder T. Ear surgery in infants under one year of age: Its risks and implications for cochlear implant surgery. Otol Neurotol. 2008;29:310–313. doi: 10.1097/MAO.0b013e3181661866. [DOI] [PubMed] [Google Scholar]
- 24.Lesinski-Schiedat A, Illg A, Heermann R, Bertram B, Lenarz T. Paediatric cochlear implantation in the first and in the second year of life: A comparative study. Cochear Implants Int. 2004;5:146–159. doi: 10.1179/cim.2004.5.4.146. [DOI] [PubMed] [Google Scholar]
- 25.Tait M, de Raeve L, Nikolopoulos TP. Deaf children with cochlear implants before the age of 1 year: Comparison of preverbal communication with normally hearing children. Int J Pediatr Otorhinolaryngol. 2007;71:1605–1611. doi: 10.1016/j.ijporl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Roland JT, Cosetti M, Wang KH, Immerman S, Waltzman SB. Cochlear implantation in the very young child: Long-term safety and efficacy. Laryngoscope. 2009;119:2205–2210. doi: 10.1002/lary.20489. [DOI] [PubMed] [Google Scholar]
- 27.Vlastarakos PV, Proikas K, Papacharalampous G, Exadaktylou I, Mochloulis G, Nikolopoulos TP. Cochear implantation under the first year of age - The outcomes. A critical review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2010;74:119–126. doi: 10.1016/j.ijporl.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Dettman SJ, Pinder D, Briggs RJS, Dowell RC, Leigh JR. Communication development in children who receive the cochlear implant younger than 12 months: Risks versus benefits. Ear Hear. 2007;28:11S–18S. doi: 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]
- 29.Colletti L, Mandala M, Zoccante L, Shannon RV, Colletti V. Infants versus older children fitted with cochlear implants: Performance over 10 years. Int J Pediatr Otorhinolaryngol. 2011;75:504–09. doi: 10.1016/j.ijporl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Geers AE, Nicholas JG. Enduring advantages of early cochlear implantation for spoken language development. J Speech Lang Hear Res. 2012 doi: 10.1044/1092-4388(2012/11-0347). (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters BR, Wyss J, Manrique M. Worldwide trends in bilateral cochlear implantation. Laryngoscope. 2010;120:S17–S44. doi: 10.1002/lary.20859. [DOI] [PubMed] [Google Scholar]
- 32.Dunn CC, Tyler RS, Oakley S, Gantz BJ, Noble W. Comparison of speech recognition and localization performance in bilateral and unilateral cochlear implant users matched on duration of deafness and age at implantation. Ear Hear. 2008;29:352–359. doi: 10.1097/AUD.0b013e318167b870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunn CC, Noble W, Tyler RS, Kordus M, Gantz BJ, Ji H. Bilateral and unilateral cochlear implant users compared on speech perception in noise. Ear Hear. 2010;31:296–298. doi: 10.1097/AUD.0b013e3181c12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dollaghan CA, Campbell TF, Paradise JL, et al. Maternal education and measures of early speech and language. J Speech Lang Hear Res. 1999;42:1432–1443. doi: 10.1044/jslhr.4206.1432. [DOI] [PubMed] [Google Scholar]
- 35.Dunn LM, Dunn LM. The Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 36.Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale. 3. San Antonio, TX: The Psychological Corp; 1992. [Google Scholar]
- 37.Geers AE, Moog JS, Biedenstein J, Brenner C, Hayes H. Spoken language scores of children using cochlear implants compared to hearing age-mates at school entry. J Deaf Studies Deaf Educ. 2009;14:371–385. doi: 10.1093/deafed/enn046. [DOI] [PubMed] [Google Scholar]
- 38.American Speech-Language-Hearing Association. Guidelines for the Audiologic Assessment of Children from Birth to 5 Years of Age (Guidelines) Available at http://www.asha.org/members/deskref-journals/deskref/default. [PubMed]
- 39.Cowan RSC, DelDot J, Barker EJ, et al. Speech perception results for children with implants with different levels of preoperative residual hearing. Am J Otol. 1997;18:S125–S126. [PubMed] [Google Scholar]