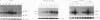Abstract
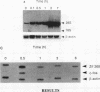
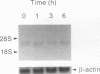
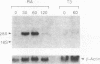
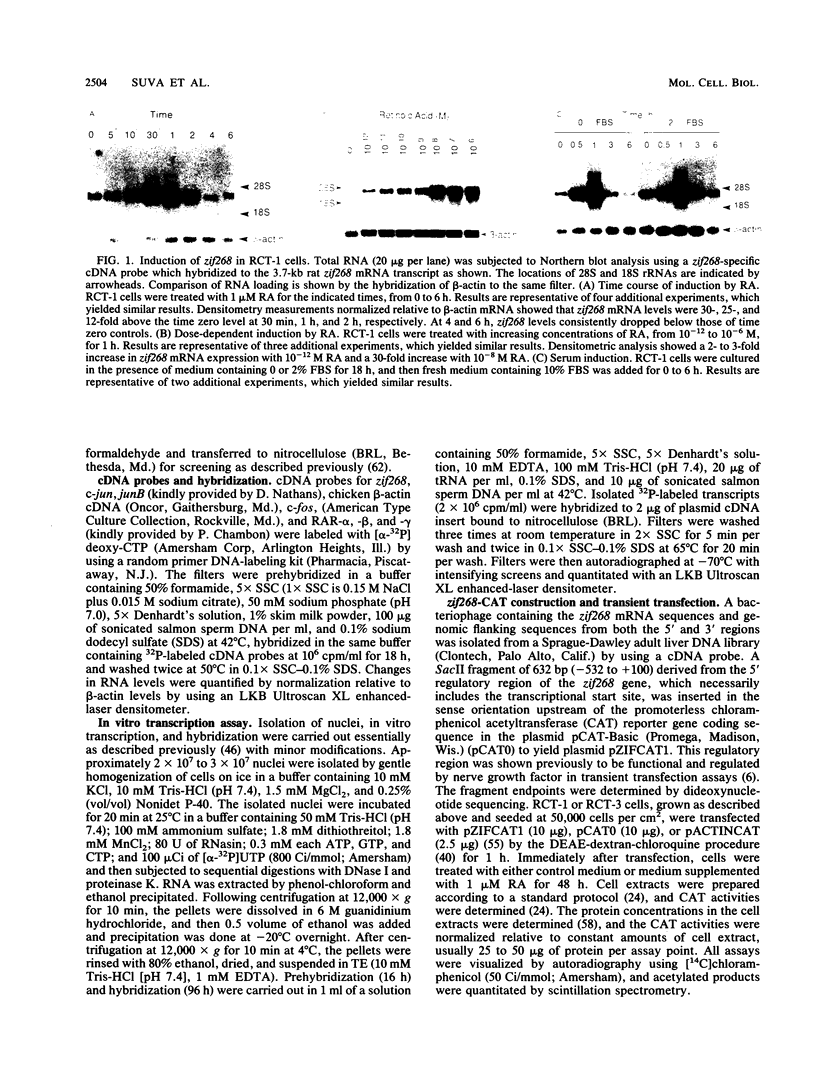
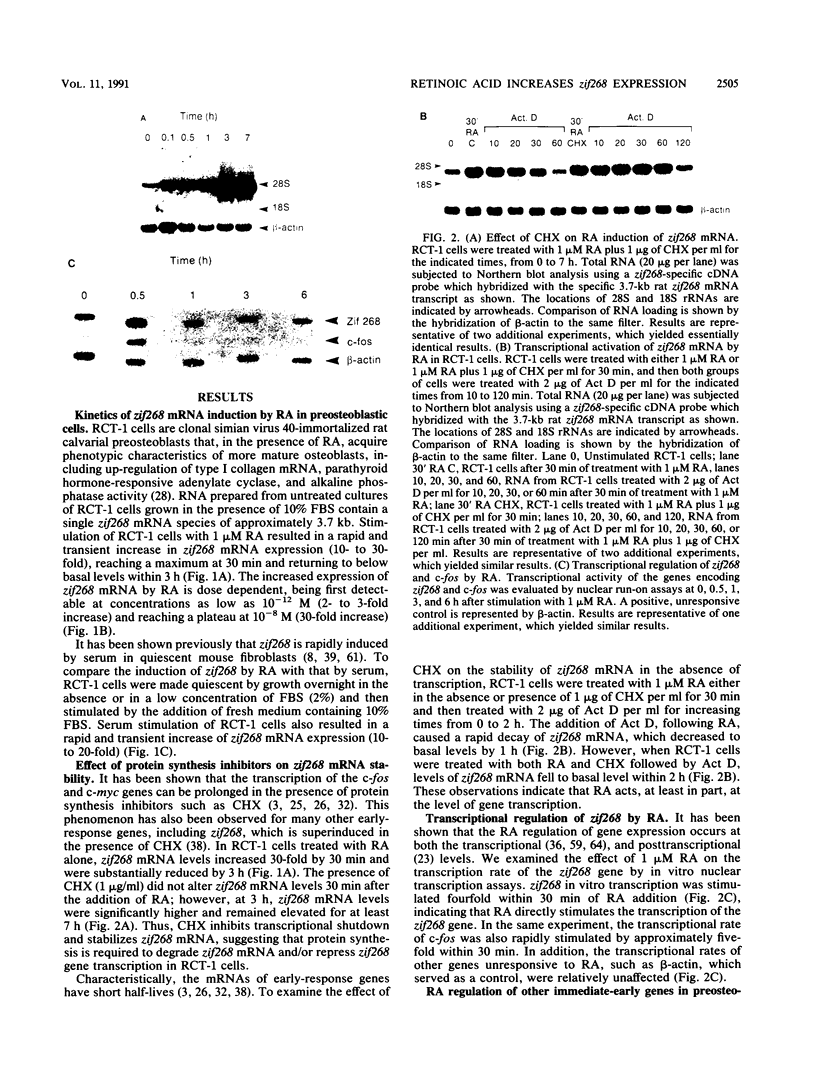
In this study we demonstrate that retinoic acid (RA) increases the expression of transcription factor zif268 mRNA in primary cultures of fetal rat calvarial cells and in simian virus 40-immortalized clonal rat calvarial preosteoblastic cells (RCT-1), which differentiate in response to RA, but not in the more differentiated RCT-3 and ROS 17/2.8 cells. The increased expression of zif268 mRNA is rapid (maximal within 1 h), transient (returns to basal levels by 3 h), detectable at RA doses of 10(-12)M, and independent of protein synthesis. The relative stimulation of zif268 mRNA by RA was much larger than that of other early genes, including c-fos, c-jun, and junB. The rate of transcription of RA-stimulated RCT-1 cells, estimated by nuclear run-on assays, was elevated, suggesting that RA regulation of zif268 gene transcription was at least in part transcriptional. Moreover, RA stimulated the transcriptional activity of a Zif268CAT (chloramphenicol acetyltransferase) plasmid containing 632 bp of zif268 5' regulatory sequences in RCT-1 cells but not in the more differentiated RCT-3 cells. These in vitro data support the in vivo observations which localize zif268 and RA receptor-gamma transcripts to bone and cartilage during development, suggesting that both RA and zif268 may play a role in osteoblast differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Caubet J. F., Bernaudin J. F. Expression of the c-fos proto-oncogene in bone, cartilage and tooth forming tissues during mouse development. Biol Cell. 1988;64(1):101–104. doi: 10.1016/0248-4900(88)90100-1. [DOI] [PubMed] [Google Scholar]
- Changelian P. S., Feng P., King T. C., Milbrandt J. Structure of the NGFI-A gene and detection of upstream sequences responsible for its transcriptional induction by nerve growth factor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):377–381. doi: 10.1073/pnas.86.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christy B. A., Lau L. F., Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with "zinc finger" sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B., Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8737–8741. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B., Nathans D. Functional serum response elements upstream of the growth factor-inducible gene zif268. Mol Cell Biol. 1989 Nov;9(11):4889–4895. doi: 10.1128/mcb.9.11.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Robertson K. A., Mueller L. Retinoic acid-induced granulocytic differentiation of HL-60 myeloid leukemia cells is mediated directly through the retinoic acid receptor (RAR-alpha). Mol Cell Biol. 1990 May;10(5):2154–2163. doi: 10.1128/mcb.10.5.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Ernst M., Heath J. K., Rodan G. A. Estradiol effects on proliferation, messenger ribonucleic acid for collagen and insulin-like growth factor-I, and parathyroid hormone-stimulated adenylate cyclase activity in osteoblastic cells from calvariae and long bones. Endocrinology. 1989 Aug;125(2):825–833. doi: 10.1210/endo-125-2-825. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Giguère V., Shago M., Zirngibl R., Tate P., Rossant J., Varmuza S. Identification of a novel isoform of the retinoic acid receptor gamma expressed in the mouse embryo. Mol Cell Biol. 1990 May;10(5):2335–2340. doi: 10.1128/mcb.10.5.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Devary O. V., Rosenfeld M. G. Multiple cell type-specific proteins differentially regulate target sequence recognition by the alpha retinoic acid receptor. Cell. 1990 Nov 16;63(4):729–738. doi: 10.1016/0092-8674(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Glick A. B., Flanders K. C., Danielpour D., Yuspa S. H., Sporn M. B. Retinoic acid induces transforming growth factor-beta 2 in cultured keratinocytes and mouse epidermis. Cell Regul. 1989 Nov;1(1):87–97. doi: 10.1091/mbc.1.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Petkovich M., Gaub M. P., Kagechika H., Shudo K., Chambon P. The retinoic acid receptors alpha and beta are expressed in the human promyelocytic leukemia cell line HL-60. Mol Endocrinol. 1989 Jul;3(7):1046–1052. doi: 10.1210/mend-3-7-1046. [DOI] [PubMed] [Google Scholar]
- Heath J. K., Rodan S. B., Yoon K., Rodan G. A. Rat calvarial cell lines immortalized with SV-40 large T antigen: constitutive and retinoic acid-inducible expression of osteoblastic features. Endocrinology. 1989 Jun;124(6):3060–3068. doi: 10.1210/endo-124-6-3060. [DOI] [PubMed] [Google Scholar]
- Hu L., Gudas L. J. Cyclic AMP analogs and retinoic acid influence the expression of retinoic acid receptor alpha, beta, and gamma mRNAs in F9 teratocarcinoma cells. Mol Cell Biol. 1990 Jan;10(1):391–396. doi: 10.1128/mcb.10.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Rodan S. B., Rodan G. A. Effects of retinoic acid on alkaline phosphatase messenger ribonucleic acid, catecholamine receptors, and G proteins in ROS 17/2.8 cells. Endocrinology. 1988 Feb;122(2):456–463. doi: 10.1210/endo-122-2-456. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Mendelsohn C., Garnier J. M., Zelent A., Leroy P., Staub A., Chambon P. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2700–2704. doi: 10.1073/pnas.87.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Krieger N. S., Stappenbeck T. S., Stern P. H. Characterization of specific thyroid hormone receptors in bone. J Bone Miner Res. 1988 Aug;3(4):473–478. doi: 10.1002/jbmr.5650030415. [DOI] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammer E. J., Chen D. T., Hoar R. M., Agnish N. D., Benke P. J., Braun J. T., Curry C. J., Fernhoff P. M., Grix A. W., Jr, Lott I. T. Retinoic acid embryopathy. N Engl J Med. 1985 Oct 3;313(14):837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982 Feb 25;295(5851):672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- Martin C. A., Ziegler L. M., Napoli J. L. Retinoic acid, dibutyryl-cAMP, and differentiation affect the expression of retinoic acid receptors in F9 cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4804–4808. doi: 10.1073/pnas.87.12.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cánoves P., Vik D. P., Tack B. F. Mapping of a retinoic acid-responsive element in the promoter region of the complement factor H gene. J Biol Chem. 1990 Nov 25;265(33):20065–20068. [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Cohen D. R., Curran T., Bos T. J., Vogt P. K., Bohmann D., Tjian R., Franza B. R., Jr Fos-associated protein p39 is the product of the jun proto-oncogene. Science. 1988 May 20;240(4855):1010–1016. doi: 10.1126/science.3130660. [DOI] [PubMed] [Google Scholar]
- Rosa F. W., Wilk A. L., Kelsey F. O. Teratogen update: vitamin A congeners. Teratology. 1986 Jun;33(3):355–364. doi: 10.1002/tera.1420330315. [DOI] [PubMed] [Google Scholar]
- Ruberte E., Dolle P., Krust A., Zelent A., Morriss-Kay G., Chambon P. Specific spatial and temporal distribution of retinoic acid receptor gamma transcripts during mouse embryogenesis. Development. 1990 Feb;108(2):213–222. doi: 10.1242/dev.108.2.213. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Setoyama C., de Crombrugghe B. Regulation of a collagen gene promoter by the product of viral mos oncogene. Nature. 1985 Mar 21;314(6008):286–289. doi: 10.1038/314286a0. [DOI] [PubMed] [Google Scholar]
- Seyfert V. L., McMahon S. B., Glenn W. D., Yellen A. J., Sukhatme V. P., Cao X. M., Monroe J. G. Methylation of an immediate-early inducible gene as a mechanism for B cell tolerance induction. Science. 1990 Nov 9;250(4982):797–800. doi: 10.1126/science.2237429. [DOI] [PubMed] [Google Scholar]
- Seyfert V. L., Sukhatme V. P., Monroe J. G. Differential expression of a zinc finger-encoding gene in response to positive versus negative signaling through receptor immunoglobulin in murine B lymphocytes. Mol Cell Biol. 1989 May;9(5):2083–2088. doi: 10.1128/mcb.9.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Murakami K. K., Evans R. M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Kartha S., Toback F. G., Taub R., Hoover R. G., Tsai-Morris C. H. A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res. 1987 Sep-Oct;1(4):343–355. [PubMed] [Google Scholar]
- Suva L. J., Winslow G. A., Wettenhall R. E., Hammonds R. G., Moseley J. M., Diefenbach-Jagger H., Rodda C. P., Kemp B. E., Rodriguez H., Chen E. Y. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987 Aug 21;237(4817):893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Graham W. R. The fos oncogene. Adv Cancer Res. 1987;49:29–52. doi: 10.1016/s0065-230x(08)60793-9. [DOI] [PubMed] [Google Scholar]
- Wagner M., Thaller C., Jessell T., Eichele G. Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature. 1990 Jun 28;345(6278):819–822. doi: 10.1038/345819a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Marchio A., Tiollais P., Dejean A. A novel steroid thyroid hormone receptor-related gene inappropriately expressed in human hepatocellular carcinoma. Nature. 1987 Dec 17;330(6149):667–670. doi: 10.1038/330667a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]