Abstract
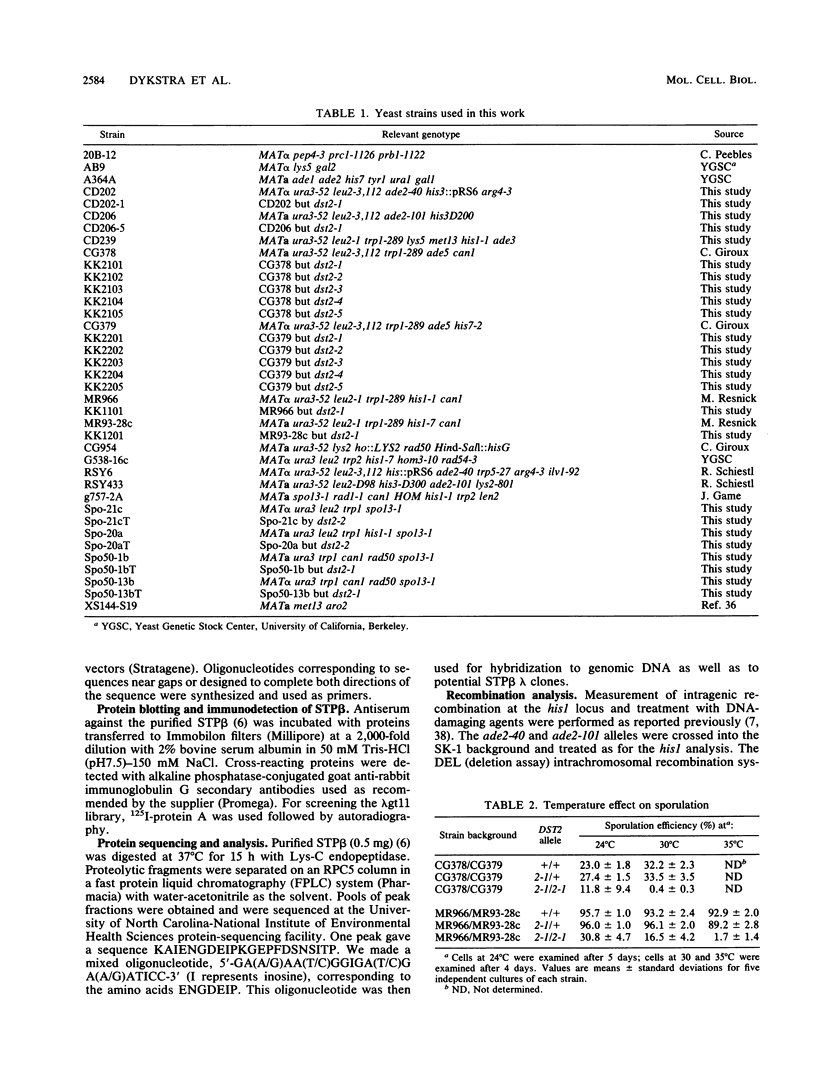
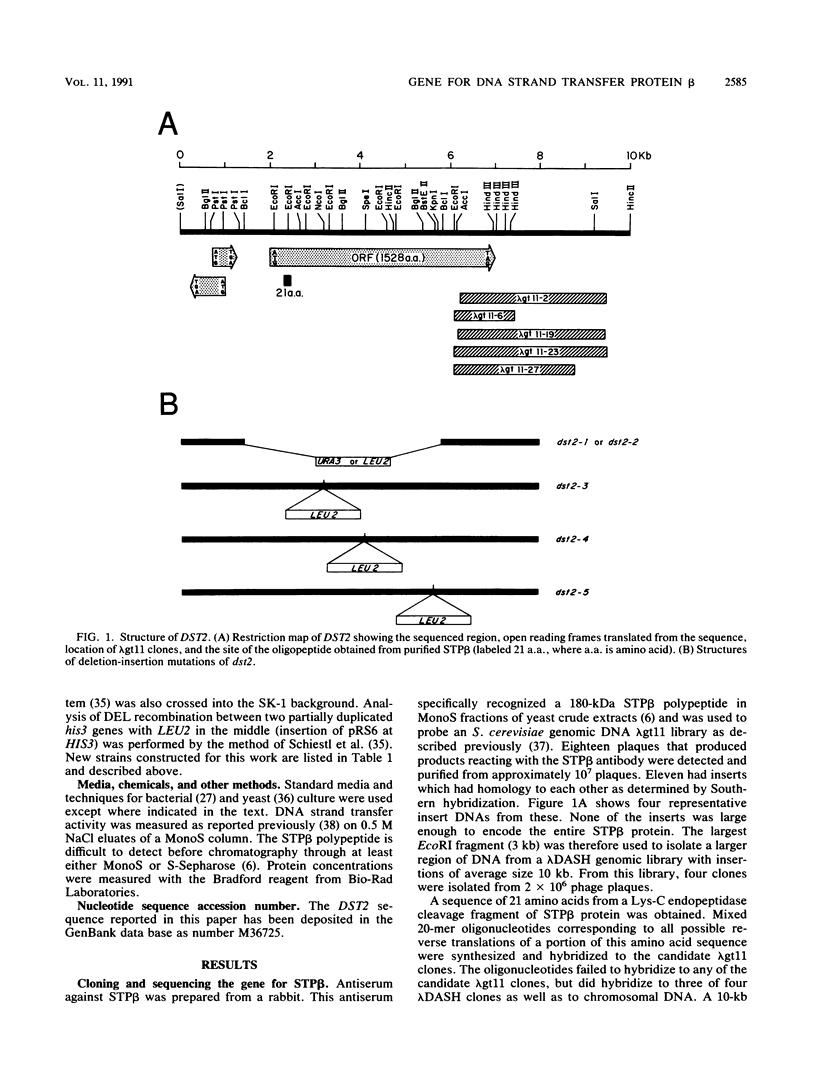
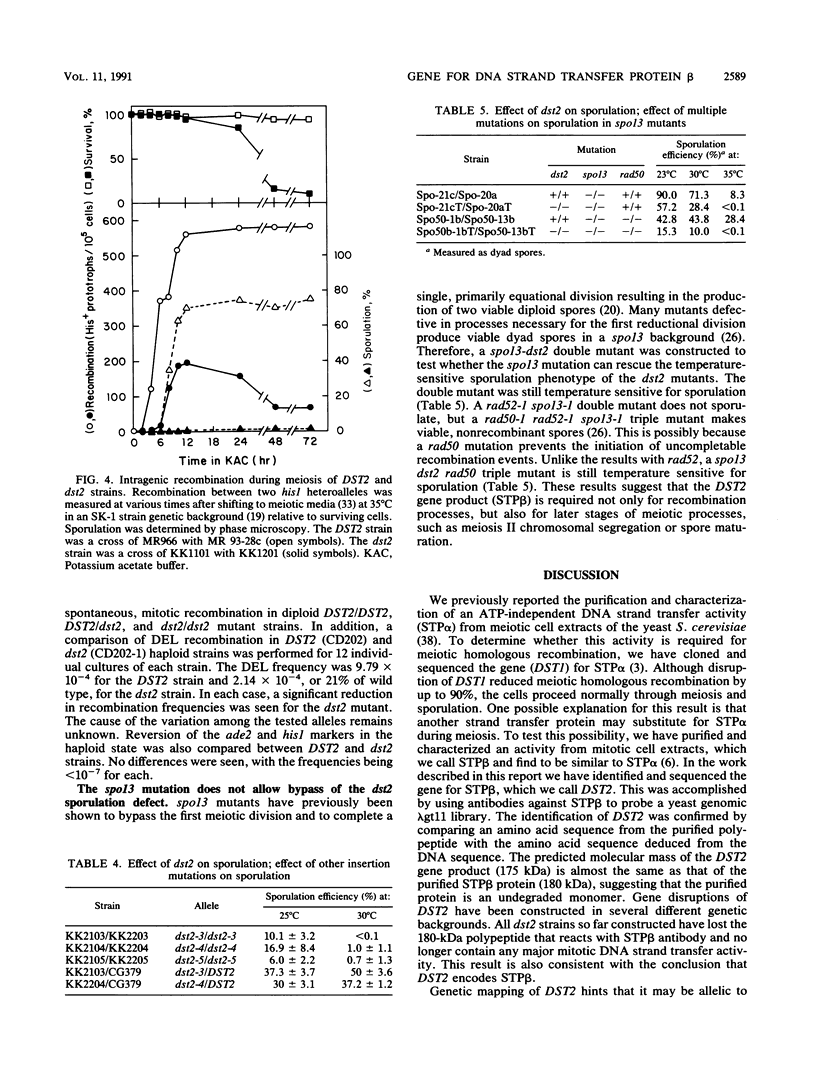
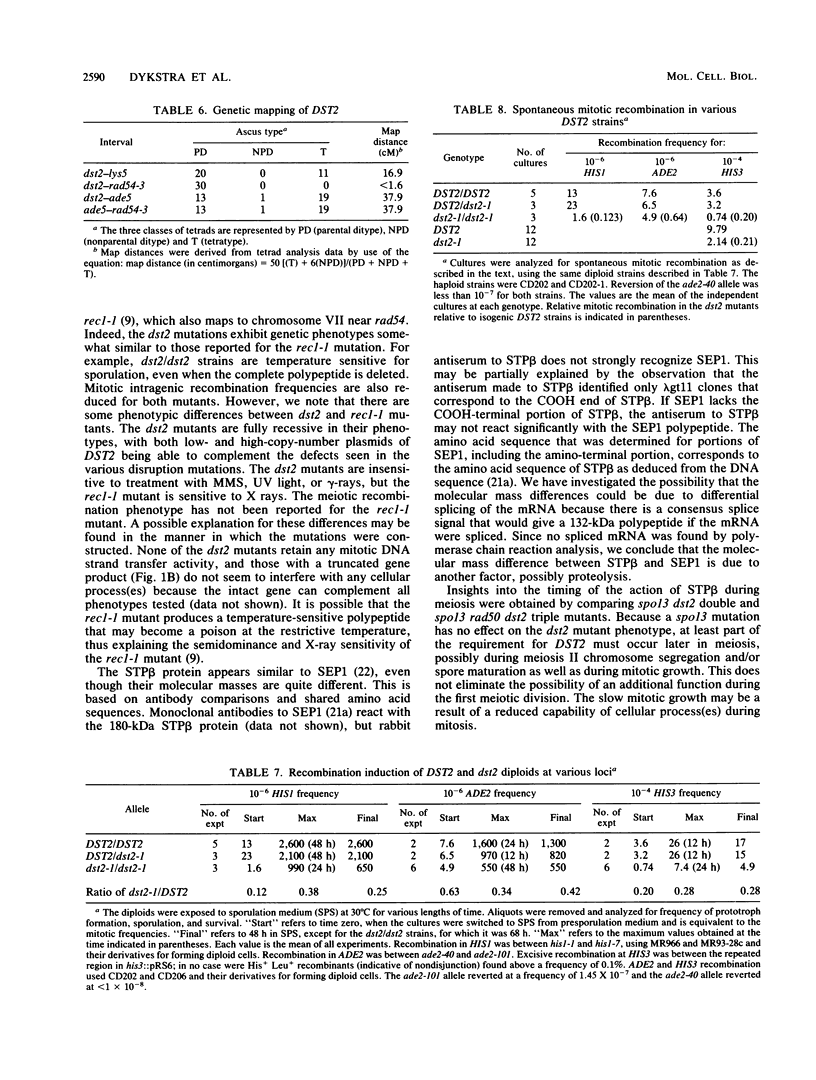
The gene encoding the 180-kDa DNA strand transfer protein beta from the yeast Saccharomyces cerevisiae was identified and sequenced. This gene, DST2 (DNA strand transferase 2), was located on chromosome VII. dst2 gene disruption mutants exhibited temperature-sensitive sporulation and a 50% longer generation time during vegetative growth than did the wild type. Spontaneous mitotic recombination in the mutants was reduced severalfold for both intrachromosomal recombination and intragenic gene conversion. The mutants also had reduced levels of the intragenic recombination that is induced during meiosis. Meiotic recombinants were, however, somewhat unstable in the mutants, with a decrease in recombinants and survival upon prolonged incubation in sporulation media. spo13 or spo13 rad50 mutations did not relieve the sporulation defect of dst2 mutations. A dst1 dst2 double mutant has the same phenotype as a dst2 single mutant. All phenotypes associated with the dst2 mutations could be complemented by a plasmid containing DST2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani E., Subbiah S., Kleckner N. The yeast RAD50 gene encodes a predicted 153-kD protein containing a purine nucleotide-binding domain and two large heptad-repeat regions. Genetics. 1989 May;122(1):47–57. doi: 10.1093/genetics/122.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini A., Parker R., McMahon J., Guthrie C., Rossi J. Activation of a cryptic TACTAAC box in the Saccharomyces cerevisiae actin intron. Mol Cell Biol. 1986 May;6(5):1571–1578. doi: 10.1128/mcb.6.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. B., Dykstra C. C., Sugino A. Isolation, DNA sequence, and regulation of a Saccharomyces cerevisiae gene that encodes DNA strand transfer protein alpha. Mol Cell Biol. 1991 May;11(5):2576–2582. doi: 10.1128/mcb.11.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. M., Schild D., Lovett S. T., Mortimer R. K. Regulation of RAD54- and RAD52-lacZ gene fusions in Saccharomyces cerevisiae in response to DNA damage. Mol Cell Biol. 1987 Mar;7(3):1078–1084. doi: 10.1128/mcb.7.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Dykstra C. C., Hamatake R. K., Sugino A. DNA strand transfer protein beta from yeast mitotic cells differs from strand transfer protein alpha from meiotic cells. J Biol Chem. 1990 Jul 5;265(19):10968–10973. [PubMed] [Google Scholar]
- Engebrecht J., Roeder G. S. Yeast mer1 mutants display reduced levels of meiotic recombination. Genetics. 1989 Feb;121(2):237–247. doi: 10.1093/genetics/121.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Brown J. T. Conditional hyporecombination mutants of three REC genes of Saccharomyces cerevisiae. Curr Genet. 1990 Jan;17(1):7–12. doi: 10.1007/BF00313242. [DOI] [PubMed] [Google Scholar]
- Fishel R. A., Detmer K., Rich A. Identification of homologous pairing and strand-exchange activity from a human tumor cell line based on Z-DNA affinity chromatography. Proc Natl Acad Sci U S A. 1988 Jan;85(1):36–40. doi: 10.1073/pnas.85.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Halbrook J., McEntee K. Purification and characterization of a DNA-pairing and strand transfer activity from mitotic Saccharomyces cerevisiae. J Biol Chem. 1989 Dec 15;264(35):21403–21412. [PubMed] [Google Scholar]
- Hamatake R. K., Dykstra C. C., Sugino A. Presynapsis and synapsis of DNA promoted by the STP alpha and single-stranded DNA-binding proteins from Saccharomyces cerevisiae. J Biol Chem. 1989 Aug 5;264(22):13336–13342. [PubMed] [Google Scholar]
- Holden D. W., Spanos A., Banks G. R. Nucleotide sequence of the REC1 gene of Ustilago maydis. Nucleic Acids Res. 1989 Dec 25;17(24):10489–10489. doi: 10.1093/nar/17.24.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Meyn M. S., Camerini-Otero R. D. Partial purification and characterization of a recombinase from human cells. Cell. 1986 Mar 28;44(6):885–894. doi: 10.1016/0092-8674(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Gene conversion between duplicated genetic elements in yeast. Nature. 1981 Jul 23;292(5821):306–311. doi: 10.1038/292306a0. [DOI] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Ljungdahl P. O., Fink G. R. kem mutations affect nuclear fusion in Saccharomyces cerevisiae. Genetics. 1990 Dec;126(4):799–812. doi: 10.1093/genetics/126.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapholz S., Esposito R. E. Recombination and chromosome segregation during the single division meiosis in SPO12-1 and SPO13-1 diploids. Genetics. 1980 Nov;96(3):589–611. doi: 10.1093/genetics/96.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Homologous pairing of DNA molecules promoted by a protein from Ustilago. Cell. 1982 Jun;29(2):367–374. doi: 10.1016/0092-8674(82)90153-2. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Evans D. H., Morrison P. T. Purification and characterization of an activity from Saccharomyces cerevisiae that catalyzes homologous pairing and strand exchange. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5560–5564. doi: 10.1073/pnas.84.16.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Larimer F. W., Stevens A. Disruption of the gene XRN1, coding for a 5'----3' exoribonuclease, restricts yeast cell growth. Gene. 1990 Oct 30;95(1):85–90. doi: 10.1016/0378-1119(90)90417-p. [DOI] [PubMed] [Google Scholar]
- Lowenhaupt K., Sander M., Hauser C., Rich A. Drosophila melanogaster strand transferase. A protein that forms heteroduplex DNA in the absence of both ATP and single-strand DNA binding protein. J Biol Chem. 1989 Dec 5;264(34):20568–20575. [PubMed] [Google Scholar]
- Moore S. P., Fishel R. Purification and characterization of a protein from human cells which promotes homologous pairing of DNA. J Biol Chem. 1990 Jul 5;265(19):11108–11117. [PubMed] [Google Scholar]
- Mortimer R. K., Schild D., Contopoulou C. R., Kans J. A. Genetic map of Saccharomyces cerevisiae, edition 10. Yeast. 1989 Sep-Oct;5(5):321–403. doi: 10.1002/yea.320050503. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Fungal recombination. Microbiol Rev. 1985 Mar;49(1):33–58. doi: 10.1128/mr.49.1.33-58.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Nitiss J., Edwards C., Malone R. E. Meiosis can induce recombination in rad52 mutants of Saccharomyces cerevisiae. Genetics. 1986 Jul;113(3):531–550. doi: 10.1093/genetics/113.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Sugino A., Nitiss J., Chow T. DNA polymerases, deoxyribonucleases, and recombination during meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2811–2817. doi: 10.1128/mcb.4.12.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Igarashi S., Hastings P. J. Analysis of the mechanism for reversion of a disrupted gene. Genetics. 1988 Jun;119(2):237–247. doi: 10.1093/genetics/119.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Elledge S., Sweetser D., Young R. A., Davis R. W. Lambda gt 11: gene isolation with antibody probes and other applications. Methods Enzymol. 1987;154:107–128. doi: 10.1016/0076-6879(87)54073-3. [DOI] [PubMed] [Google Scholar]
- Sugino A., Nitiss J., Resnick M. A. ATP-independent DNA strand transfer catalyzed by protein(s) from meiotic cells of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3683–3687. doi: 10.1073/pnas.85.11.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]