Abstract
Hypercalcemia is a common medical problem with an estimated prevalence of 15% among hospitalized patients. Multiple myeloma (MM) and primary hyperparathyroidism (PHPT) are among the most common causes of hypercalcemia but coexistence of both pathologic processes in a patient is an extremely rare phenomenon. In this paper we have discussed a patient presenting with this rare phenomenon. We have also provided a comprehensive review of the scientific literature published on codiagnosis of MM and PHPT.
1. Introduction
Hypercalcemia is a common clinical problem with an estimated prevalence of 15% among hospitalized patients [31]. The etiology of hypercalcemia is complex with many factors playing a pathogenic role. From a clinical standpoint, it may present with changes in mental status, generalized weakness, polyuria, and constipation. Multiple myeloma (MM) and primary hyperparathyroidism (PHPT) are among the most common causes of hypercalcemia but coexistence of the two pathologic processes in one patient is an extremely rare phenomenon. In this paper, we have discussed a patient presenting with this rare phenomenon and have reviewed the relevant scientific literature.
2. Case Presentation
A 92-year-old Caucasian female with a past medical history of Alzheimer's dementia, seizure disorder, osteoporosis, and osteoarthritis was admitted to the hospital for an evaluation of a new onset confusion and constipation. Review of symptoms during admission was significant for anorexia, weight loss, constipation for the last three weeks, and history of a fall one month prior to the presentation. Family history was significant for MM in a sister. At the time of presentation, the patient was using donepezil, memantine, vitamin D with calcium, calcium carbonate (calcium containing antacid), and levetiracetam. Vitals at the time of admission were blood pressure 140/58, pulse 68, respiratory rate 18, oxygen saturation 98% on room air, and temperature 97.4. On physical examination, the patient was alert and oriented in place and person but not in time. Other significant findings were diastolic murmur in right second intercostal space, petechiae over lower extremities, and back tenderness, which the patient attributed to a recent fall. Lumbar spine X-ray was done three weeks prior to the presentation that showed degenerative changes with no evidence of fracture. Basic blood workup including complete blood count and comprehensive metabolic panel was done, which revealed anemia, leucopenia, and hypercalcemia. Home medications were held for concerns of hypercalcemia and confusion.
Endocrinology and neurology services were consulted. MRI of the brain was done, which showed lytic lesions as shown in Figure 1. MM was suspected; serum protein electrophoresis (SPEP), urine protein electrophoresis (UPAP) and bone marrow biopsy were done which confirmed the diagnosis of MM (IgG kappa) (International Staging System stage II). Bone marrow biopsy showed mildly hypercellular bone marrow with plasmacytosis (30%) as shown in Figure 2. Skeletal survey showed diffuse lytic lesions throughout long bones, pelvis, and skull (Figure 3). Surprisingly, intact PTH came back high suggesting primary hyperparathyroidism (PHPT). The data on laboratory tests are presented in Table 1.
Figure 1.
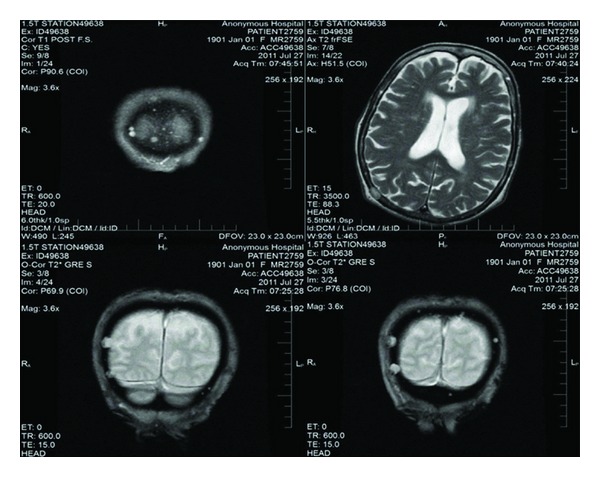
MRI demonstrating lytic lesions.
Figure 2.
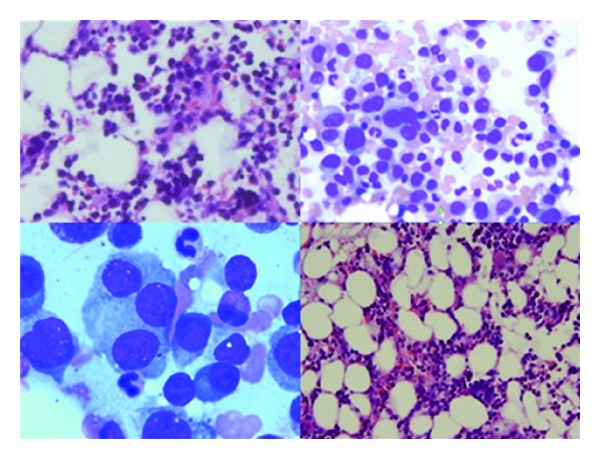
Mildly hypercellular bone marrow with plasmacytosis (30%), consistent with multiple myeloma.
Figure 3.
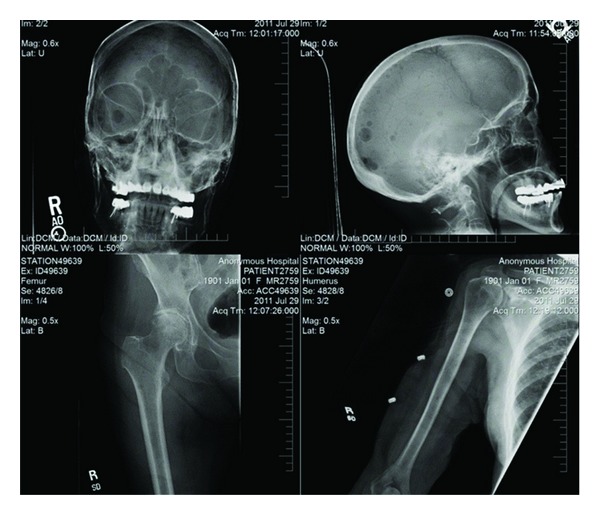
Skeletal survey showing lytic lesions in long bones and skulls.
Table 1.
| Result name | Results | Reference range |
|---|---|---|
| WBC | 2.9 K/mm cu | 4.2–11.0 |
| Platelet | 156 K/mm cu | 140–400 |
| Hemoglobin Hb | 9.0 g/dL | 12.0–15.0 |
| Hematocrit | 27.1% | 36.0–47.0 |
| Reticulocyte | 0.7% | 0.5–2.8 |
| Blood urea nitrogen | 22 mg/dL | 5–20 |
| Creatinine | 1.11 mg/dL | 0.0–1.00 |
| Sodium | 143 mmol/L | 135–145 |
| Potassium | 4.0 mmol/L | 3.4–5.1 |
| Chloride | 104 mmol/L | 98–109 |
| Bicarbonate | 33 mmol/L | 23–31 |
| Calcium | 13.3 mg/dL | 8.4–10.5 |
| Total protein | 7.0 g/dL | 6.4–8.3 |
| Albumin | 4.0 g/dL | 3.4–5.2 |
| Aspartate amino transferase | 20 IU/L | 0–32 |
| Alanine amino transferase | 10 IU/L | 0–40 |
| Alkaline phosphatase | 67 IU/L | 35–104 |
| Bilirubin total | 0.2 mg/dL | 0–10.0 |
| Haptoglobin | 157 mg/dL | 36–195 |
| Vitamin B12 | 532 pg/mL | 211–946 |
| TSH | 1.160 uIU/mL | 0.400–5.400 |
| Vitamin D25 OH | 47.0 ng/mL | 30.0–100.0 |
| 25 Hydroxy D3 | 26 pg/mL | |
| 25 hydroxy D2 | <8 | |
| Vitamin D 1,25(OH)2 | 26 | 18–72 |
| Folate | >20.0 ng/mL | 3.1–17.5 |
| Ferritin | 64 ng/mL | 13–150 |
| Phosphorous | 2.8 mg/dL | 2.0–4.0 |
| Lactate dehydrogenase | 137 IU/L | 135–214 |
| Total iron | 30 ug/dL | 30–160 |
| Unsaturated IBC | 234.0 ug/dL | 110.0–370.0 |
| Total IBC | 264.0 | 228.0–428.0 |
| Percentage of iron saturation | 11% | 20–55 |
| PTH intact on day of presentation | 70.5 pg/mL | 15.0–65.0 |
| PTH 7 months later | 540.0 pg/mL | |
| PTH-related protein | 18 pg/mL | 14–27 |
| Beta-2 microglobulin | 3.3 mg/L (5.8 mg/L four months later) | 0.8–2.2 |
| Serum viscosity | 1.5 relative to H2O | 1.5–1.9 |
| PT/INR | 10.6/1.0 sec | 9.211.8/0.9–1.1 |
| APTT | 29 sec | 24–33 |
| Immunoglobulins | ||
| IGA | 29 mg/dL | 50–400 |
| IGG | 692 mg/dL (1200, 5 months later) | 600–1500 |
| IGM | 6 mg/dL | 50–300 |
| Free kappa light chains | 1510 | 3.3–19.4 mg/L |
| Free lambda light chains | 2.4 | 5.7–26.3 |
| Free Kappa/lambda | 629.17 | 0.26–1.65 |
| Urine protein electrophoresis | ||
| Urine volume 24 hours | 1150 mL/24 hour | |
| Urine-protein electrophoresis (UPE) | 253 mg/24 hour | 0–165 |
| Albumin UPE | 30.6% | |
| Alpha1 | 16.1% | |
| Alpha2 | 14.1% | |
| Beta | 17.1% | |
| Gamma | 22.1% | |
| Immunofixation | Free kappa light chains | |
| 24-hour-urine protein | 310.5 mg/24 hour | 0–150 |
| 24-hours-urine creatinine | 0.7 g/24 hour | 0.74–1.57 |
| 24-hour-urine volume | 1150 cc | |
| 24-hour-urine creatinine | 0.5 g/24 hour | 0.74–1.57 |
| 24-hour-urine volume | 900 mL (repeat test) | |
| 24-hour-urine calcium | 239 mg/24 hour | 100–300 |
| Serum protein electrophoresis | ||
| Albumin | 3.3 g/dL | 3.1–5.0 |
| Alpha 1 | 0.3 g/dL | 0.2–0.5 |
| Alpha2 | 0.7 g/dL | 0.5–1.1 |
| Beta | 0.6 g/dL | 0.6–1.1 |
| Gamma | 1.5 g/dL | 0.7–1.7 |
| Albumin/globulin | 1.0 | |
| M spike | 1.09 g/dL | |
| Total protein | 6.5 g/dL | 6.4–8.3 |
| Immunofixation | Monoclonal paraprotein of class IgG kappa | |
| CD56 NK cells | 63% | 3–35 |
| CD 138 marker | 26% | |
| Lambda B-cell marker | 1% | 1–7 |
| Kappa B-cell marker | 73% | 2–14% |
| CD45 LCA | 98% | 92–100 |
| CD38 Marker | 26% | 1–17 |
Pathology. Normal female bone marrow karyotype. No clonal, structural, or numerical chromosome abnormalities identified. FISH analysis indicates normal hybridization signals with MM probe panel. This excludes majority of chromosome rearrangements known to be associated with MM.
Surgical Pathology. Mildly hyper cellular bone marrow with plasmacytosis consistent with MM.
Leukemia/lymphoma panel. Bone marrow aspirate shows 30–40% plasma cells with kappa light chain restrictions. (plasma cell dyscrasia).
Peripheral Smear. Lymphocytes with foamy cytoplasm, no rouleaux formation, adequate polys with occasional platelet clumps.
Hypercalcemia was managed with intravenous hydration, calcitonin, bisphosphonates, and furosemide. The patient was started on melphalan and prednisone, which were later switched to lenalidomide with a high dose of dexamethasone due to a poor treatment response. After one and a half year, the patient is still following in our outpatient oncology center being on a low dose of lenalidomide with a stable M protein.
3. Discussion
Hypercalcemia is common in patients with MM and occurs in 28% of myeloma cases [32]. MM may cause hypercalcemia through multiple mechanisms. First, plasma cells produce various cytokines, including TNF-β and IL-6, that activate osteoclasts and lead to calcium washout from bones to the bloodstream [33]. Second, some studies suggest that MM cells may secrete parathyroid hormone-related peptide similarly to other malignancies, such as squamous cell lung carcinoma [34, 35]. Third, serum calcium may be falsely elevated because of a binding to immunoglobulin [36, 37].
Clubb et al. [38] described first-case linking PHPT and paraproteinemia in 1964. Drezner and Lebovitz were the first who described a case of concomitant MM and PHPT in 1979 [30]. Some researchers speculate that the association between MM and PHTP may not be coincidental [39, 40], although mechanisms explaining codiagnosis are not known. Arnulf et al. showed that the prevalence of monoclonal gammopathy is higher in patients with PHTP as compared to general population [40]. Pest et al. hypothesized that elevated PTH may mediate the induction of MM through the downstream biological effects of IL-6 [1]. This hypothesis was supported by the study performed by Pirih et al., who showed that PTH decreases apoptotic cell death of the hematopoietic stem cells via the IL-6 [41].
PHPT leads to hypercalcemia via direct bone resorption [42] mediated by osteoclasts. Another important mechanism is through an increased calcium absorption in the duodenum and greater reabsorption in the kidneys.
The above-mentioned pathogenic mechanism gives an insight to how PHPT and MM may be linked. Some studies have suggested that calcium may act as a mitogenic factor [43], whereas others suggest that myelomatous proteins may interfere with polypeptide hormone synthesis bind their circulating fractions, and/or block their peripheral effects that may secondarily stimulate parathyroid gland [29]. However, both of these diseases are common among elderly and may share similar risk factors, such as ionizing radiation [44, 45], and a simple coincidence may be the case.
Summary of published cases [1–28] is presented in Table 2. Codiagnosis of PHPT and MM should be suspected in cases of difficult-to-control hypercalcemia. Most of the cases of coexistent MM and PHPT have been observed in females (23 out of 29 reported cases). The youngest patient with codiagnosis was a 45-year-old female and the oldest patient was a 92-year-old female. PHPT is more common in females, whereas the opposite is true for MM. Differences in incidence of the two diseases may explain female preponderance (MM less frequent than PHPT). Initial diagnosis was highly variable, eleven cases had primary diagnosis of hyperparathyroidism, ten had primary diagnosis of MM and seven had both diagnosis made at presentation. The type of immunoglobulin chains of MM observed in all the cases was variable as six patients had light chain MM, remaining patients had a combination of heavy and light chain MM, one patient had nonsecretory type of MM. All the patients had calcium ≥11 mg/dL at the time of presentation. Majority of patients had parathyroid adenoma as a cause of PHPT, few had chief cell hyperplasia, and none had parathyroid cancer. Parathyroidectomy, combination of radiotherapy, and chemotherapy had been used for treatment of this coexistent condition with variable success. Rao et al. [2] suggested that parathyroidectomy in patients with coexistent PHPT and MM serves three folds; first, it removes confusion about etiology of hypercalcemia; second, it alters prognosis of myeloma; third, calcium can be used as a tumor marker in cases if there is a recurrence of tumor. Considering age, our patient was not a candidate for surgery, in such patient population medical alternative to parathyroidectomy is needed. Ten out of 29-patients died within 5 years after codiagnosis, and out of those ten, eight died within one year.
Table 2.
| n | Author | Age/ Gender | Type of MM | Ca (mg/dL) | Therapy for MM and PHPT | Parathyroid histology | Outcome | Initial diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | Pest et al. [1] | 76 F | IgA-? | 13.2 | Hydration, bisphosphonates, Lasix, melphalan, cyclophosphamide, and steroids | Adenoma | Survived | PHPT |
| 2 | Rao et al. [2] | 54 M | IgG-lambda | 11.2 | Adriamycin, melphalan, prednisone, cyclophosphamide, and parathyroidectomy | Adenoma | Died after 12 years | Both |
| 3 | Jackson and Orland [3] | 45 F | IgG-lambda | 17.1 | Hydration, Lasix, prednisone, and melphalan | Adenoma | — | MM |
| 4 | Chisholm et al. [4] | 80 M | Kappa | 13.1 | Parathyroidectomy, radiotherapy, melphalan, prednisone, vincristine, carmustine, cyclophosphamide, hydration, and Lasix | Adenoma (c-cells) | Died 2 years later | PHPT |
| 5 | Francis et al. [5] | 70F | Lambda | 11.6 | Norethisterone, vincristine, melphalan, and prednisone | Adenoma | Died 3 weeks later | PHPT |
| 6 | Mundis and kyle [6] | 76 F | IgG-kappa | 11.0 | Melphalan, prednisone, and parathyroidectomy | Adenoma (c-cells) | survived | MM |
| 7 | Stone et al. [7] | 47 F | IgA-kappa | 13.7 | Melphalan, prednisone, radiotherapy, parathyroidectomy, hydration, and mithramycin | Adenoma | Died | MM |
| 8 | Hoelzer and Silverberg [8] | 51 F | IgA-lambda | 11.9 | Parathyroidectomy? | Adenoma (c-cells) | — | PHPT |
| 9 | Schneider and Thomas [9] | 74 F | IgG-kappa | 12.0 | Melphalan, prednisone, and parathyroidectomy | Adenoma | Survived | MM |
| 10 | Toussirot et al. [10] | 82 M | Kappa | 15.2 | Melphalan, prednisone, and parathyroidectomy | Hyperplasia | Died | PHPT |
| 11 | Goto et al. [11] | 73 F | Kappa | 13.2 | Parathyroidectomy, melphalan | Adenoma | Died 1 year later | PHPT |
| 12 | Otsuka et al. [12] | 77 F | IgG-lambda | Melphalan, prednisone, bisphosphonates, calcitonin, and parathyroidectomy | c-cells hyperplasia | Survived | — | |
| 13 | Fery-Blanco et al. [13] | 68 F | IgG-kappa | 11.28 | ? chemotherapy and surgery refused | Adenoma | Died | Both |
| 14 | Sarfati et al. [14] | 62 F | IgA-kappa | 16.4 | Mithramycin, lasix, plasmaphoresis, Adriamycin, vincristine, prednisone, and parathyroidectomy | Adenoma | Survived | MM |
| 15 | Rosen et al. [15] | 81 M | IgG-kappa | 13.4 | Hydration, bisphosphonates, melphalan, prednisone, radiotherapy, needle aspiration of parathyroid gland, and refused surgery | Adenoma | Survived | MM |
| 16 | Tomon et al. [16] | 60 F | IGA-kappa | — | — | — | — | MM |
| 17 | Fanari et al. [17] | 59 F | lambda | 12.7 | Hydration, bisphosphonates, cinacalcet, bortezomib and dexamethasone | Possible Adenoma | Died 4 months later | Both |
| 18 | Bogas et al. [18] | 72 F | IgG-kappa | 13.66 | Melphalan, prednisone, and Interferon? | Adenoma | Died 4 years later | Both |
| 19 | Katayama et al. [19] | 50 F | PHPT | |||||
| 20 | Romagnoli et al. [20] | 70 F | — | — | Parathyroidectomy, steroids and chemotherapy | Adenoma | — | PHPT (MEN-1) |
| 21 | Toh and Winocour et al. [21] | 71 M | 12.0 | Melphalan, prednisone, and bisphosphonates | Died 6 weeks later | MM | ||
| 22 | Sopea et al. [22] | 77 F | Kappa (ns) | 12.9 | Bisphosphonates, refused surgery, or chemotherapy | Died 1 year later | Both | |
| 23 | Khandwala and Boctor [23] | 72 F | — | 11.7/ 16.6* | Parathyroidectomy, bisphosphonates, calcitonin, melphalan, and prednisone | Adenoma | — | PHPT |
| 24 | Patel et al. [24] | 73 F | IgG- kappa | 13.5 | Bisphosphonates, steroids, thalidomide, plicamycin, and parathyroidectomy | Adenoma | — | MM |
| 25 | Avcioglu et al. [25] | 52 F | IgG-kappa | 12.6 | Parathyroidectomy and steroids | Adenoma | — | Both |
| 26 | Chowdhury and Scarsbrook et al. [26] | 87 F | — | — | — | — | — | PHPT |
| 27 | Dalgleish and Gatenby [27] | 59 F | IgG-lambda | 11.68 | Hydration, lasix, prednisone, mithramycin, cyclophosphamide, and parathyroidectomy | Adenoma | Survived | MM |
| 28 | Peters et al. [28] | 73 M | IgA-lambda | 16 | Parathyroidectomy, chemotherapy, and radiotherapy | Hyperplasia | Died 1 week later | PHPT |
| 29 | Our case | 92 F | IgG-kappa | 13 .3 | Bisphosphonates, Lasix, hydration, calcitonin, melphalan, prednisone, lenalidomide, and dexamethasone | — | Survived | Both |
| 30 | Johansson and Werner [29] mentioned 3 cases of MM and PHPT (no detail of the cases is given), one other such as has been described by Drezner and Lebovitz [30] without much detail. | |||||||
*Calcium at time of diagnosis of MM.
4. Conclusions
A search for concomitant cause of hypercalcemia should be pursued in cases of difficult-to-control hypercalcemia and in elderly individuals, in whom the incidence of PTHP and MM is common.
References
- 1.Pest EP, McQuaker G, Hunter JA, Moffat D, Stanley AJ. Primary hyperparathyroidsm, amyloid and multiple myeloma: an unusual association. Scottish Medical Journal. 2005;50(1):32–34. doi: 10.1177/003693300505000114. [DOI] [PubMed] [Google Scholar]
- 2.Rao DS, Antonelli R, Kane KR, Kuhn JE, Hetnal C. Primary hyperparathyroidism and monoclonal gammopathy. Henry Ford Hospital Medical Journal. 1991;39(1):41–44. [PubMed] [Google Scholar]
- 3.Jackson RM, Orland MJ. Parathyroid adenoma in a patient with multiple myeloma. Southern Medical Journal. 1979;72(10):1336–1337. doi: 10.1097/00007611-197910000-00034. [DOI] [PubMed] [Google Scholar]
- 4.Chisholm RC, Weaver YJ, Chung EB, Townsend JL. Parathyroid adenoma and light chain myeloma. Journal of the National Medical Association. 1981;73(9):875–880. [PMC free article] [PubMed] [Google Scholar]
- 5.Francis RM, Bynoe AG, Gray C. Hypercalcaemia due to the coexistence of parathyroid adenoma and myelomatosis. Journal of Clinical Pathology. 1982;35(7):732–736. doi: 10.1136/jcp.35.7.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mundis RJ, Kyle RA. Primary hyperparathyroidism and monoclonal gammopathy of undetermined significance. American Journal of Clinical Pathology. 1982;77(5):619–621. doi: 10.1093/ajcp/77.5.619. [DOI] [PubMed] [Google Scholar]
- 7.Stone MJ, Lieberman ZH, Chakmakjian ZH, Matthews JL. Coexistent multiple myeloma and primary hyperparathyroidism. Journal of the American Medical Association. 1982;247(6):823–824. [PubMed] [Google Scholar]
- 8.Hoelzer DR, Silverberg AB. Primary hyperparathyroidism complicated by multiple myeloma. Archives of Internal Medicine. 1984;144(10):2069–2071. [PubMed] [Google Scholar]
- 9.Schneider W, Thomas M. Hypercalcaemia in coexistent parathyroid adenoma and multiple myeloma: problems of differential diagnosis. Deutsche Medizinische Wochenschrift. 1989;114(31-32):1199–1202. doi: 10.1055/s-2008-1066740. [DOI] [PubMed] [Google Scholar]
- 10.Toussirot E, Bille F, Henry JF, Acquaviva PC. Coexisting kappa light chain multiple myeloma and primary hyperparathyroidism. Scandinavian Journal of Rheumatology. 1994;23(1):49–50. doi: 10.3109/03009749409102136. [DOI] [PubMed] [Google Scholar]
- 11.Goto S, Yoshioka M, Nagai K, et al. Primary hyperparathyroidism associated with multiple myeloma. Internal Medicine. 1995;34(10):988–991. doi: 10.2169/internalmedicine.34.988. [DOI] [PubMed] [Google Scholar]
- 12.Otsuka F, Hayakawa N, Ogura T, et al. A case of primary hyperparathyroidism accompanying multiple myeloma. Endocrine Journal. 1997;44(1):105–109. doi: 10.1507/endocrj.44.105. [DOI] [PubMed] [Google Scholar]
- 13.Fery-Blanco C, Prati C, Ornetti P, et al. Hypercalcemia ofdouble origin: association ofmultiple myeloma andectopic parathyroidal adenoma. Revue de Medecine Interne. 2007;28(7):504–506. doi: 10.1016/j.revmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Sarfati E, de Ferron P, Dubost C, Assens P, Veyssier P, Detour B. Multiple myeloma associated with primary hyperparathyroidism caused by an adenoma. Annales de medecine interne. 1985;136(8, article 684) [PubMed] [Google Scholar]
- 15.Rosen C, Segal H, Hartz CE, Mroz F, Carlton E. Primary hyperparathyroidism in an elderly patient with multiple myeloma. Journal of the American Geriatrics Society. 1992;40(7):703–705. doi: 10.1111/j.1532-5415.1992.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 16.Tomon M, Fukase M, Nakata M. A case of multiple myeloma associated with primary hyperparathyroidism. Hormone to Rinsho. 1989;37, article 128 [Google Scholar]
- 17.Fanari Z, Kadikoy H, Haque W, Pacha O, Abdellatif A. Medical management of primary hyperparathyroidism with concommitant multiple myeloma. Internal Medicine. 2010;49(6):581–584. doi: 10.2169/internalmedicine.49.2705. [DOI] [PubMed] [Google Scholar]
- 18.Bogas M, Costa L, Araújo D. Coexistence of primary Hyperparathyroidism and multiple myeloma; association and rare manifestation. Acta Reumatologica Portuguesa. 2008;33(1):98–105. [PubMed] [Google Scholar]
- 19.Katayama Y, Matsuda H, Katoh Y, et al. Multiple myeloma in a patient with primary hyperparathyroidism. Hinyokika Kiyo. 1989;35(8):1369–1372. [PubMed] [Google Scholar]
- 20.Romagnoli E, Minisola S, Carnevale V, Spagna G, D’Erasmo E, Mazzuoli G. Coexistent multiple myeloma and MEN type 1. Postgraduate Medical Journal. 1990;66(780):879–880. doi: 10.1136/pgmj.66.780.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toh V, Winocour P. Multiple myeloma with hyperparathyroidism. Hospital Medicine. 2000;61(10, article 744) [PubMed] [Google Scholar]
- 22.Sopeña B, Rodríguez GJ, De La Fuente J, Martínez-Vázquez C. Two causes of hypercalcemia: learning by the holmesian method. Mayo Clinic Proceedings. 2004;79(5, article 708) doi: 10.4065/79.5.708. [DOI] [PubMed] [Google Scholar]
- 23.Khandwala HM, Boctor MA. Multiple myeloma presenting with recurrent hypercalcemia in a patient with a history of primary hyperparathyroidism: report of case and review of literature. Endocrine Practice. 2004;10(4):345–347. doi: 10.4158/EP.10.4.345. [DOI] [PubMed] [Google Scholar]
- 24.Patel N, Talwar A, Donahue L, John V, Margouleff D. Hyperparathyroidism accompanying multiple myeloma. Clinical Nuclear Medicine. 2005;30(8):540–542. doi: 10.1097/01.rlu.0000170012.06271.76. [DOI] [PubMed] [Google Scholar]
- 25.Avcioglu B, Bayraktaroglu T, Kubat Uzum A, et al. A case with hypercalcemia caused by hyperparathyroidism and multiple myeloma. Endocrine Abstracts. 2007;14, article 458 [Google Scholar]
- 26.Chowdhury FU, Scarsbrook AF. Tc-99m sestamibi uptake mimicking parathyroid adenoma in a patient with primary hyperparathyroidism and occult multiple myeloma. Clinical Nuclear Medicine. 2008;33(3):198–200. doi: 10.1097/RLU.0b013e318162de14. [DOI] [PubMed] [Google Scholar]
- 27.Dalgleish AG, Gatenby PA. Refractory hypercalcaemia: parathyroid adenoma or multiple myeloma? Medical Journal of Australia. 1984;140(2):99–100. [PubMed] [Google Scholar]
- 28.Peters KM, Rosenberger J, Gaczkowski A, Lorenz R. Concomitant occurrence of hyperparathyroidism and multiple myeloma. Internist. 1989;30(2):117–119. [PubMed] [Google Scholar]
- 29.Johansson H, Werner I. Dysproteinemia, malignancy, and hyperparathyroidism. Annals of Internal Medicine. 1975;83(1):121–122. doi: 10.7326/0003-4819-83-1-121_2. [DOI] [PubMed] [Google Scholar]
- 30.Drezner MK, Lebovitz HE. Primary hyperparathyroidism in paraneoplastic hypercalcaemia. The Lancet. 1978;1(8072):1004–1006. doi: 10.1016/s0140-6736(78)90734-1. [DOI] [PubMed] [Google Scholar]
- 31.French S, Subauste J, Geraci S. Calcium abnormalities in hospitalized patients. Southern Medical Journal. 2012;105:231–237. doi: 10.1097/SMJ.0b013e31824e1737. [DOI] [PubMed] [Google Scholar]
- 32.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proceedings. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 33.Mundy GR, Yoneda T, Guise TA. Hypercalcemia in hematologic malignancies and in solid tumors associated with extensive localized bone destruction. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 4th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 1999. pp. 208–211. [Google Scholar]
- 34.Tsujimura H, Nagamura F, Iseki T, Kanazawa S, Saisho H. Significance of parathyroid hormone-related protein as a factor stimulating bone resorption and causing Hypercalcemia in myeloma. American Journal of Hematology. 1998;59:168–170. doi: 10.1002/(sici)1096-8652(199810)59:2<168::aid-ajh11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Kitazawa R, Kitazawa S, Kajimoto K, et al. Expression of parathyroid hormone-related protein (PTHrP) in multiple myeloma. Pathology International. 2002;52(1):63–68. doi: 10.1046/j.1440-1827.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 36.John R, Oleesky D, Issa B, et al. Pseudohypercalcaemia in two patients with IgM paraproteinaemia. Annals of Clinical Biochemistry. 1997;34(6):694–696. doi: 10.1177/000456329703400618. [DOI] [PubMed] [Google Scholar]
- 37.Van Dijk JM, Sonnenblick M, Weissberg N, Rosin A. Pseudohypercalcemia and hyperviscosity with neurological manifestations in multiple myeloma. Israel Journal of Medical Sciences. 1986;22(2):143–144. [PubMed] [Google Scholar]
- 38.Clubb JS, Posen S, Neale FC. Disappearance of a serum paraprotein after Parathyroidectomy. Archives of internal medicine. 1964;114:616–620. doi: 10.1001/archinte.1964.03860110086006. [DOI] [PubMed] [Google Scholar]
- 39.Bellou A, Blain H, Guerci A, Jeandel C. Gammapathie monoclonale et hyperparathyroïdie primitive. À propos de deux observations et revue de la littérature. Revue de Medecine Interne. 1996;17(4):325–328. doi: 10.1016/0248-8663(96)81437-8. [DOI] [PubMed] [Google Scholar]
- 40.Arnulf B, Bengoufa D, Sarfati E, et al. Prevalence of monoclonal gammopathy in patients with primary hyperparathyroidism: a prospective study. Archives of Internal Medicine. 2002;162(4):464–467. doi: 10.1001/archinte.162.4.464. [DOI] [PubMed] [Google Scholar]
- 41.Pirih FQ, Michalski MN, Cho SW, et al. Parathyroid hormone mediates hematopoietic cell expansion through interleukin-6. PLoS One. 2010;5, article 13657 doi: 10.1371/journal.pone.0013657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenfield EM, Shaw SM, Gornik SA, Banks MA. Adenyl cyclase and interleukin 6 are downstream effectors of parathyroid hormone resulting in stimulation of bone resorption. Journal of Clinical Investigation. 1995;96(3):1238–1244. doi: 10.1172/JCI118157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luckasen JR, White JG, Kersey JH. Mitogenic properties of a calcium ionophore, A23187. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(12):5088–5090. doi: 10.1073/pnas.71.12.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis EB. Leukemia, multiple myeloma, and aplastic anemia in american radiologists. Science. 1963;142(3598):1492–1494. doi: 10.1126/science.142.3598.1492. [DOI] [PubMed] [Google Scholar]
- 45.Schneider AB, Gierlowski TC, Shore-Freedman E, Stovall M, Ron E, Lubin J. Dose-response relationships for radiation-induced hyperparathyroidism. Journal of Clinical Endocrinology and Metabolism. 1995;80(1):254–257. doi: 10.1210/jcem.80.1.7829622. [DOI] [PubMed] [Google Scholar]


