Abstract
Bariatric surgery to treat obesity can also be effective against type 2 diabetes, but it is unclear how such surgical procedures improve glucose metabolism. A new study in rats suggests that nutrient sensing in the jejunum contributes to the antidiabetic effects of duodenal-jejeunal bypass (pages 950–955).
Several bariatric, or ‘weight loss’, surgical procedures are proving effective for treating not only obesity but also type 2 diabetes (T2D). As obesity is a major risk factor for T2D, it seems logical that treating the former would benefit the latter. Surprisingly, however, these surgeries seem to improve T2D through mechanisms that are at least partly independent of weight loss per se.
Perhaps the best-studied bariatric procedure is Roux-en-Y gastric bypass (RYGB), which both reduces the size of the stomach and bypasses the proximal small intestine (the duodenum and the proximal jejunum; Fig. 1). In two recently published randomized trials1, 2, RYGB was shown to fully normalize blood sugar concentrations in up to 75% of treated patients with T2D, a remarkable outcome that was not strongly related to the degree of weight loss in these individuals. Thus, rerouting of or increasing nutrient delivery to the small bowel (or both) has seemingly important potential as a diabetes treatment modality, but how this occurs remains a mystery. A related question is whether weight-loss surgery is appropriate only in the context of obesity—the condition for which it was originally developed—or whether lean people with insulin-deficient type 1 diabetes (T1D) might also benefit.
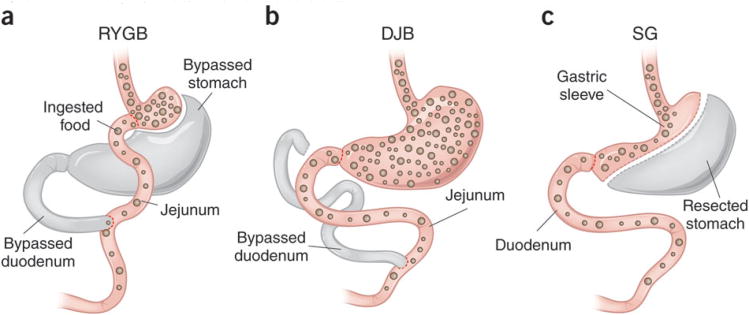
Figure 1. Three gastric surgical procedures with beneficial effects on diabetes.
(a) RYGB bypasses a large portion of the stomach as well as the duodenum and proximal jejunum. (b) DJB leaves most of the stomach intact while the duodenum and proximal jejunum are bypassed. Breen et al.3 now provide evidence that increased delivery of nutrients to the jejunum resulting from DJB may be important in mediating the positive effects of this form of surgery in models of T1D. (c) Sleeve gastrectomy (SG) removes the majority of the stomach while keeping the proximal and distal ends intact, such that intestinal nutrient flow is not altered. Ingested food is depicted with brown spheres.
In this issue of Nature Medicine, Breen et al.3 extend their earlier work4 that suggested that the duodenum senses nutrients when they arrive by showing that a similar arrangement also pertains to the jejunum. Signals generated in response to nutrients in the proximal small bowel are passed on to the brain, which in turn is proposed to activate a neurocircuit innervating the liver that reduces its glucose production. This mechanism has been suggested to ensure that delivery of glucose into the bloodstream from an internal source (the liver) decreases when ingested nutrients arrive at the absorptive surface of the gut. Breen et al.3 now report in normal-weight, nondiabetic rats that, analogous to what they'd previously reported for the duodenum4, infusion of either fats or glucose directly into the jejunum activates a gut-brain-liver neurocircuit that enhances liver insulin sensitivity, thereby reducing hepatic glucose production.
Does this jejunal nutrient-sensing mechanism contribute to the antidiabetic effect of gastric bypass surgery? To investigate this question, Breen et al.3 investigated the effects of duodenal-jejunal bypass (DJB, a stomach-sparing form of RYBG; Fig. 1) in two rat models in which diabetes is induced by the substantial destruction of pancreatic beta cells. This was achieved either by administering the beta cell–targeting toxin streptozotocin or through a genetically driven autoimmune process (in BB (BioBreeding) rats). Thus, both models are nonobese and characterized by a major loss of insulin-secreting beta cells, a situation resembling T1D rather than T2D. In both models, DJB rapidly (2 d after surgery) lowered blood glucose concentrations via a mechanism that was not explained by reduced food intake, required intact jejunal nutrient sensing, was not accompanied by increased insulin secretion and was attributable to reduced hepatic glucose production. By 14 d after the surgery, blood glucose remained at or near normal concentrations, whereas the expected marked elevation of blood glucose persisted in sham-operated diabetic rats. These findings indicate that bariatric surgery can resolve diabetes in these nonobese T1D rodent models, in addition to the earlier evidence that it can improve glucose control in a rat model of nonobese streptozotocin-induced diabetes5 and in rat models of T2D6, 7. More importantly, they suggest that the increased delivery of nutrients to the jejunum resulting from DJB can induce substantial lowering of glucose concentrations without increasing insulin secretion.
The notion that intestinal nutrient sensing underlies the rapid diabetes resolution induced by bariatric surgery is not without controversy. For example, many studies have implicated increased insulin secretion in the lowering of glucose concentrations observed after gastric bypass surgery in rat models of T2D6. Yet plasma insulin concentrations were not altered by DJB in the study of Breen et al.3. Dramatic increases in the concentrations of circulating incretin peptides (primarily glucagon-like peptide-1) have also been invoked to explain the beneficial effects of gastric bypass7. Although Breen et al.3 observed increased circulating glucagon-like peptide-1 concentrations in response to DJB, this effect should not reduce hepatic glucose production in the absence of increased insulin (or decreased glucagon secretion, which also was not observed). The conclusion of Breen et al.3 that suppression of hepatic glucose production, resulting solely from activation of a gut-brain-liver reflex arc, is sufficient to ameliorate diabetes is deserving of additional study.
Also controversial is the idea that rerouting of intestinal nutrient delivery is required for bariatric surgery to confer metabolic benefit. Data from both rats8 and humans2 suggest that sleeve gastrectomy, which restricts the size of the stomach but does not redirect intestinal nutrient flow (Fig. 1), is nearly as effective as RYGB for resolving diabetes. Combined with evidence that DJB does not improve insulin resistance or glucose tolerance in an obese rat model9, rerouting intestinal nutrient delivery may not be essential for the effect of bariatric surgery to lower glucose concentrations—simply increasing the rate of gastric emptying may suffice. These considerations challenge the primacy of jejunal nutrient sensing as a mediator of diabetes remission induced by bariatric procedures.
Among many important unanswered questions is whether bariatric surgery can be recommended for nonobese humans with T1D. Without more data on the long-term outcomes of such procedures in obese patients with T2D, this question seems premature. Another consideration is that nonobese humans with T1D are likely to have a far greater deficit of beta cells and thus are more insulin deficient than the rat models studied by Breen et al.3. Additionally, the role of weight loss (which probably had an impact on outcomes reported by these authors, even though they took steps to minimize this effect) in diabetes resolution is complex and awaits further analysis. So, when might we consider undertaking clinical studies that address the safety, tolerability, efficacy and durability of such surgical procedures in nonobese patients with T1D? Perhaps it is not too soon to consider studies that address these questions, but the existence of a glucose-lowering neurocircuit connecting gut, brain and liver raises the possibility that we might one day develop drugs that activate this pathway without the need for surgery. This possibility offers a compelling rationale for ongoing efforts to identify the mechanisms that underlie the impressive effects of bariatric surgery on glucose homeostasis.
References
- 1.Mingrone G, et al. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, et al. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breen DM, et al. Nat Med. 2012;18:950–955. doi: 10.1038/nm.2745. [DOI] [PubMed] [Google Scholar]
- 4.Cheung GW, Kokorovic A, Lam TK. Cell Mol Life Sci. 2009;66:3023–3027. doi: 10.1007/s00018-009-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strader AD, Clausen TR, Goodin SZ, Wendt D. Obes Surg. 2009;19:96–104. doi: 10.1007/s11695-008-9754-x. [DOI] [PubMed] [Google Scholar]
- 6.de Luis D, et al. Am J Surg. 2012 Feb 16; doi: 10.1016/j.amjsurg.2011.07.020. published online. [DOI] [Google Scholar]
- 7.Kindel TL, Yoder SM, Seeley RJ, D'Alessio DA, Tso P. J Gastrointest Surg. 2009;13:1762–1772. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 8.Stefater MA, et al. Gastroenterology. 2010;138:2426–2436. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kindel TL, et al. Obesity (Silver Spring) 2011;19:380–387. doi: 10.1038/oby.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]