Abstract
Background
The significance of heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) is unknown. Using a multinational collection of isolates from methicillin-resistant S. aureus (MRSA) infective endocarditis (IE), we characterized IE patients with and without hVISA, and genotyped the infecting strains.
Methods
MRSA bloodstream isolates from 65 patients with definite IE from 8 countries underwent PCR for 31 virulence genes, pulsed-field gel electrophoresis, and multilocus sequence typing. hVISA was defined using population analysis profiling (PAP).
Results
Nineteen (29.2%) of 65 MRSA IE isolates exhibited hVISA by PAP. Isolates from Oceania and Europe were more likely to exhibit hVISA than isolates from the United States (77.8% vs. 35.0% vs. 13.9%; P < .001). The prevalence of hVISA was higher among isolates with a vancomycin minimum inhibitory concentration of 2 mg/L (P = .026). hVISA-infected patients were more likely to have persistent bacteremia (68.4% vs. 37.0%; P = .029) and heart failure (47.4% vs. 19.6%; P = .033). Mortality of hVISA- and non-hVISA-infected patients did not differ (42.1% vs. 34.8%, P = .586). hVISA and non-hVISA isolates were genotypically similar.
Conclusions
In these analyses, hVISA occurred in over one-quarter of MRSA IE isolates, was associated with certain IE complications, and varied in frequency by geographic region.
Keywords: hVISA, Methicillin-resistant Staphylococcus aureus, endocarditis, genotype
INTRODUCTION
Infections caused by heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) are poorly understood [1]. Defined as the presence of subpopulations (typically at a rate of 1 organism per 105–106) of MRSA with intermediate vancomycin resistance [2], the hVISA phenotype has now been reported in clinical MRSA isolates from many parts of the world [3–7]. However, fundamental questions involving hVISA - including its prevalence, global distribution, and clinical significance - persist.
Nowhere is the issue of antibiotic efficacy more important than in infective endocarditis (IE) caused by MRSA. Vancomycin - often out of necessity rather than choice - remains the first-line treatment for this condition. However, vancomycin’s efficacy is hindered by its slowly bactericidal mechanism of action and poor penetration into valvular vegetations [8, 9]. Furthermore, several studies have reported an association between the hVISA phenotype and deep-seated endovascular infections such as IE [3, 7, 10, 11]. Because of these observations, we reasoned that patients with MRSA IE would be at particularly high risk for the presence of the hVISA phenotype, and that bloodstream isolates from such patients would be ideal to address many of the unresolved issues regarding the prevalence, global distribution, and clinical significance of hVISA. However, the relative infrequency of MRSA IE in any single center made a study focusing upon this infection impractical until now.
The current study had two major objectives: 1) to define the prevalence and clinical significance of the hVISA phenotype in MRSA IE; and 2) to evaluate the genotypic characteristics of MRSA IE bloodstream isolates exhibiting the hVISA phenotype. To address these issues, we used MRSA bloodstream isolates obtained from a large, contemporary, international cohort of prospectively-identified and clinically well-characterized patients with definite IE.
PATIENTS AND METHODS
Patients and settings
Data from the International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) were used for this study. The ICE-PCS Microbiological Repository contains more than 1,500 bloodstream isolates of bacteria obtained from prospectively-identified patients with definite IE from more than 16 countries between November 13, 1999 and January 20, 2006 [12, 13]. For the current study, bloodstream isolates from all patients with definite MRSA IE were eligible for inclusion.
Definition
Definite IE was defined according to modified Duke criteria [14]. Chronic immunosuppressive therapy was defined as the administration of recognized immunosuppressive agents (including oral corticosteroids or other agents such as those used in solid organ transplantation or rheumatologic disorders) for more than 30 days at the time of IE diagnosis. A cardiac device was defined as a permanent pacemaker, cardioverter-defibrillator, or prosthetic cardiac valve. Intravascular access devices were defined as an arterial-venous fistula or an indwelling vascular catheter. A chronic indwelling central catheter was defined as a tunneled or cuffed catheter, or a subcutaneous port catheter. A stroke was defined as an acute neurological deficit of vascular etiology lasting more than 24 hours [15]. Vascular/immunologic evidence of endocarditis was defined as the presence of physical examination findings of endocarditis present on initial evaluation, including Osler’s nodes, Janeway lesions, Roth spots, conjunctival hemorrhage, splinter hemorrhages, or peripheral vascular embolic events. Congestive heart failure was defined according to the New York Heart Association classification system [16]. Persistent bacteremia was defined as >3 days of bacteremia despite receipt of an antibiotic to which the isolate was susceptible in vitro. Health care associated IE was defined as either nosocomial infection or non-nosocomial health care associated infection. Nosocomial infection was defined as IE developing in a patient hospitalized for more than 48 hours prior to the onset of signs/symptoms consistent with IE. Non-nosocomial health care associated infection was defined as IE diagnosed within 48 hours of admission in an outpatient with extensive health care contact as reflected by any of the following criteria: (1) received intravenous therapy, wound care, or specialized nursing care at home within the 30 days prior to the onset of S aureus IE; (2) attended a hospital or hemodialysis clinic or received intravenous chemotherapy within the 30 days before the onset of S. aureus IE; (3) was hospitalized in an acute care hospital for 2 or more days in the 90 days before the onset of S aureus IE; or (4) resided in a nursing home or long-term care facility [17]. The remaining clinical, echocardiographic, and outcome variables were defined as reported elsewhere [12].
Laboratory methods and susceptibility definition
All S. aureus isolates were identified with use of standard methods. The minimum inhibitory concentration (MIC) of vancomycin was determined by E-test (AB Biodisk, Solna, Sweden) according to the manufacturer’s instructions, and by broth microdilution method according to manufacturer protocol (Siemens, Berlin, Germany) and Clinical and Laboratory Standards Institute recommendations [18]. Except where noted, all vancomycin MIC values in the text were defined according to E-test. Reference strain ATCC 29213 was used as a positive hVISA control strain. The MIC of daptomycin was determined using E-test according to the manufacturer’s instructions. All MICs were interpreted by a single blinded investigator (JFF).
Population Analysis Profiling
All MRSA isolates were assessed by population analysis profile-area under the curve (PAP-AUC) using the technique described by Wootton et al. [19]. Organisms were cultured in tryptone soy agar plate from an overnight growth, adjusted to a 108 CFU/ml density, and spiral plated (Eddy jet spiral platers, NY, USA) onto brain-heart infusion agar (Difco, Detroit, MI, USA) plates containing 0, 0.5, 1, 1.5, 2, 3, 4, and 8 mg/L vancomycin. Colonies were counted after 48 hours incubation at 37°C and the viable count was plotted against vancomycin concentration using GraphPad Prism (GraphPad; San Diego, CA, USA). This was then used to calculate an area under the curve. Isolates were defined as hVISA if they had a PAP-AUC ratio of >0.9 and <1.3 when compared with Mu3 reference strain (ATCC 700698).
Multiplex PCR
Genomic DNA was prepared as previously described [20]. Bacterial determinants including adhesins, toxins, agr group I-IV and other genes were screened by multiplex PCR as described before [20, 21]. The isolates were classified as Staphylococcal chromosomal cassette mec (SCCmec) type I, II, III, or IV by multiplex PCR, as previously described [22].
Pulsed Field Gel Electrophoresis (PFGE)
PFGE with SmaI was performed on all isolates, and the gels analyzed using BioNumerics version 4.0 (Applied Maths, Kortrijk, Belgium) as previously described [23].
Multilocus Sequence Typing (MLST)
MLST was performed as described previously [24, 25]. PCR fragments of 7 housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL) were obtained from chromosomal DNA and were directly sequenced. MLST allele names and sequence type (ST) were derived from http://www.mlst.net. Clonal complexes were assigned to groups of isolates sharing 6 of 7 alleles by use of eBURST (http://eburst.mlst.net).
Statistics
Simple descriptive statistics were used to describe the demographics and other characteristics of the patients and for the genetic and antibiotic sensitivity profile of the bacterial isolates. Statistics are presented as medians with 25th and 75th percentiles for continuous variables, and as frequency counts and percentages for categorical factors. The statistical significance of associations between variables was calculated using the Kruskal-Wallis test for continuous measures and with Fisher’s exact test for cross-classifications of categorical data. For all tests, a p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SAS, version 9.1.2 (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics and outcome
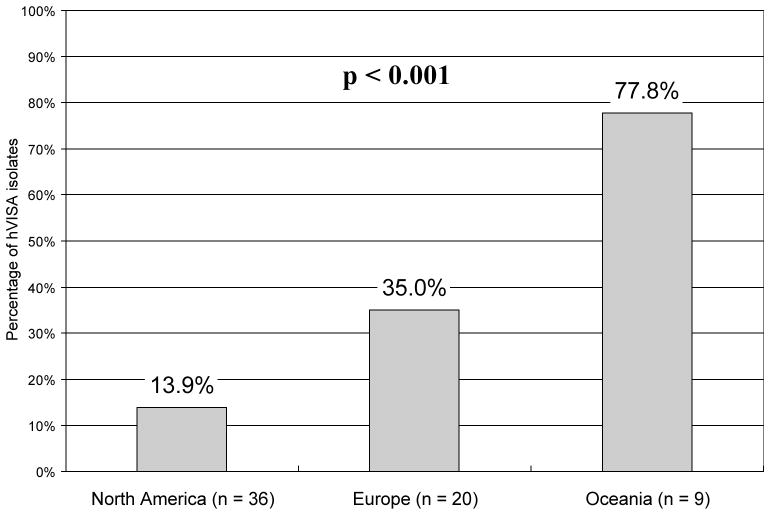
Isolates from a total of 65 prospectively-enrolled patients from 13 centers in 8 countries with definite MRSA IE were available in this study. Of these, 19 (29.2%) exhibited the hVISA phenotype using the PAP-AUC method. Patients with IE due to hVISA were older (median age, 73.1 vs. 60.6 years; P = .037) (table 1), were more likely to be from Oceania or Europe (P < .001) (figure 1), and were more likely to have native valve IE (94.4% vs. 53.3%; P = .005). None of the 65 patients were injection drug users. Patients with hVISA had a higher rate of persistent bacteremia (68.4% vs. 37.0%; P = .029) and congestive heart failure (47.4% vs. 19.6%; P = .033). Other complications and inhospital mortality did not differ significantly between groups.
Table 1.
Clinical characteristics and outcome of 65 patients with infective endocarditis due to heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) and vancomycin-susceptible S. aureus (VSSA)
| Characteristic | VSSA (n=46) | hVISA (n=19) | P |
|---|---|---|---|
| Patient demographics : | |||
| Male sex | 26 (56.5) | 9 (47.4) | 0.589 |
| Age, years, median (25th–75th percentiles) | 60.6 (47.6–74.1) | 73.1 (59.9–79.6) | 0.037 |
| Predisposing conditions : | |||
| Diabetes mellitus | 20 (43.5) | 8/18 (44.4) | 1.00 |
| Hemodialysis dependent | 12 (26.1) | 3 (15.8) | 0.522 |
| History of cancer | 6 (13.0) | 3 (15.8) | 0.714 |
| Chronic immunosuppressive therapy | 8/45 (17.8) | 3 (15.8) | 1.00 |
| Other chronic disease | 34 (73.9) | 10 (52.6) | 0.144 |
| Recent Invasive procedures | 19 (41.3) | 9 (47.4) | 0.795 |
| Presumed place of acquisition : | 0.26 | ||
| Nosocomial | 18 (39.1) | 12 (63.2) | |
| Non-nosocomial health care-associated | 18 (39.1) | 5 (26.3) | |
| Community-acquired | 10 (21.7) | 2 (10.5) | |
| Type of infective endocarditis : | 0.005 | ||
| Native | 24/45 (53.3) | 17/18 (94.4) | |
| Prosthetic | 12/45 (26.7) | 1/18 (5.6) | |
| Other | 9a/45 (20.0) | 0/18 (0) | |
| Clinical findings : | |||
| Fever, > 38°C | 39/42 (92.9) | 16/17 (94.1) | 1.00 |
| Vascular/ immunologic evidence of endocarditisb | 9 (19.6) | 7 (36.8) | 0.20 |
| New cardiac murmur | 8 (17.4) | 3 (15.8) | 1.00 |
| Septic pulmonary infarcts | 2 (4.3) | 2 (10.5) | 0.574 |
| Echocardiographic findings : | |||
| New regurgitation | 18 (39.1) | 10 (52.6) | 0.411 |
| Intracardiac vegetation : | 43 (93.5) | 14 (73.7) | 0.041 |
| Aortic and/or mitral valves | 26 (60.5) | 11 (78.6) | |
| Tricuspid valve | 7 (16.3) | 2 (14.3) | |
| Aortic/mitral plus tricuspid valves | 1 (2.3) | 1 (7.1) | |
| Other | 9a (20.9) | 0 (0) | |
| Treatment : | |||
| Glycopeptide therapyc | 43d (93.5) | 18 (94.7) | 1.00 |
| Cardiac surgery during this hospitalization | 14 (30.4) | 3 (15.8) | 0.353 |
| Complications and Outcome : | |||
| Stroke | 9 (19.6) | 4 (21.1) | 1.00 |
| Congestive heart failure | 9 (19.6) | 9 (47.4) | 0.033 |
| Intracardiac abscess | 4 (8.7) | 3 (15.8) | 0.408 |
| Persistent bacteremia | 17 (37.0) | 13 (68.4) | 0.029 |
| New conduction abnormalities | 2/44 (4.5) | 3 (15.8) | 0.156 |
| In-hospital death | 16 (34.8) | 8 (42.1) | 0.586 |
NOTE. Data are no. (%) of patients, unless otherwise indicated.
Includes infections involving other cardiac devices (7 patients), intracardiac catheter (1 patient), or other myocardial structure (1 patient).
physical examination findings of endocarditis present on initial evaluation, including Osler’s nodes, Janeway lesions, Roth spots, conjunctival hemorrhage, splinter hemorrhages, or peripheral vascular embolic events.
Treatment details missing for 1 VSSA-infected patient and 1 hVISA-infected patient.
One patient received fosfomycin.
Figure 1.
Prevalence by geographic region of heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) phenotype among MRSA bloodstream isolates from patients with infective endocarditis. Percentages of patients with hVISA phenotype are specified above each column.
Patient treatment
Rates of glycopeptide therapy did not differ between groups (43 of 46 [93.5%] VSSA-infected patients vs. 18 of 19 [94.7%] hVISA-infected patients, P = 1.00). Of the 43 VSSA-infected patients treated with glycopeptides, two received teicoplanin. All 18 hVISA-infected patients treated with glycopeptides received vancomycin. Twenty-two (51.2%) of 43 VSSA-infected patients treated with a glycopeptide also received combination antimicrobial therapy: vancomycin plus rifampin (7 patients), vancomycin plus rifampin plus aminoglycoside (6 patients), vancomycin plus aminoglycoside (3 patients), vancomycin plus trimethoprim-sulfamethoxazole (3 patients), vancomycin plus linezolid (1 patient), teicoplanin plus quinupristin-dalfopristin (1 patient), or fosfomycin plus aminoglycoside (1 patient). Eleven (61.1%) of 18 hVISA-infected patients receiving vancomycin also received rifampin (5 patients), aminoglycoside (3 patients), fosfomycin plus imipenem (2 patients), or linezolid (1 patient). No statistically significant differences between VSSA and hVISA–infected patients were noted in rates of surgical therapy (30.4% VSSA vs. 15.8% hVISA; P = .353) or mean time to surgical treatment (20.5 days VSSA vs. 6 days hVISA; Kruskal-Wallis P = .130). The frequency of patients transferred from another facility also did not differ between VSSA- and hVISA-infected patients (20/46 [43.5%] vs. 8/19 [42.1%]; P = 1.00).
Genotypic characteristics of hVISA Isolates
Several genes were highly conserved in both hVISA and VSSA isolates (table 2). For example, spa, ebps, hlg, and efb were present in all of the tested isolates. The hVISA isolates were significantly more likely to contain the genes for fnbA (47.4% vs. 15.2%; P = .011) and seh (21.1% vs. 2.1%; P = .023) than VSSA isolates. The frequency of agr type II polymorphism did not differ between the hVISA and VSSA isolates (P = .586). The distribution of SCCmec types of hVISA isolates was significantly different from that of VSSA isolates (P = .012), with SCCmec type III being more common in hVISA isolates (21.1% vs. 0%; P = .006).
Table 2.
Genotypic characteristics of heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) and vancomycin-susceptible S. aureus (VSSA) isolates from 65 patients with MRSA infective endocarditis.
| Gene | VSSA (n=46) | hVISA (n=19) | P |
|---|---|---|---|
| Adhesins : | |||
| fnbA | 7 (15.2) | 9 (47.4) | 0.011 |
| fnbB | 30 (65.2) | 13 (68.4) | 1.00 |
| clfA | 43 (93.5) | 17 (89.5) | 0.625 |
| clfB | 45 (97.8) | 18 (94.7) | 0.502 |
| cna | 21 (45.7) | 12 (63.2) | 0.277 |
| spa | 46 (100.0) | 19 (100.0) | 1.00 |
| sdrC | 34 (73.9) | 17 (89.5) | 0.203 |
| sdrD | 37 (80.4) | 17 (89.5) | 0.486 |
| sdrE | 34 (73.9) | 17 (89.5) | 0.203 |
| bbp | 43 (93.5) | 17 (89.5) | 0.625 |
| ebps | 46 (100.0) | 19 (100.0) | 1.00 |
| map/eap | 35 (76.1) | 13 (68.4) | 0.547 |
| Toxins : | |||
| eta | 25 (54.3) | 9 (47.4) | 0.785 |
| etb | 1 (2.2) | 1 (5.3) | 0.502 |
| tst | 30 (65.2) | 9 (47.4) | 0.266 |
| sea | 40 (87.0) | 17 (89.5) | 1.00 |
| seb | 1 (2.2) | 3 (15.8) | 0.072 |
| sec | 2 (4.3) | 1 (5.3) | 1.00 |
| sed | 14 (30.4) | 3 (15.8) | 0.353 |
| see | 33 (71.7) | 16 (84.2) | 0.357 |
| seg | 41 (89.1) | 13 (68.4) | 0.067 |
| seh | 1 (2.2) | 4 (21.1) | 0.023 |
| sei | 44 (95.7) | 18 (94.7) | 1.00 |
| sej | 13 (28.2) | 2 (10.5) | 0.196 |
| pvl | 6 (13.0) | 0 (0.0) | 0.169 |
| hlg | 46 (100.0) | 19 (100.0) | 1.00 |
| Other putative virulence genes : | |||
| Efb | 46 (100.0) | 19 (100.0) | 1.00 |
| icaA | 43 (93.5) | 16 (84.2) | 0.347 |
| chp | 34 (73.9) | 11 (57.9) | 0.244 |
| V8 | 36 (78.3) | 18 (94.7) | 0.154 |
| agr genotype : | 0.061 | ||
| Group I | 6 (13.0) | 7 (36.8) | |
| Group II | 30 (65.2) | 11 (57.9) | |
| Group III | 10 (21.7) | 1 (5.3) | |
| SCCmec type : | 0.012 | ||
| Type I | 6 (13.0) | 3 (15.8) | |
| Type II | 29 (63.0) | 7 (36.8) | |
| Type III | 0 (0.0) | 4 (21.1) | |
| Type IV | 11 (23.9) | 5 (26.3) | |
| PFGE type (USA Nomenclature) : | 0.077 | ||
| USA 100 | 20 (43.5) | 6 (31.6) | |
| USA 200 | 9 (19.6) | 1 (5.3) | |
| USA 300 | 3 (6.5) | 0 | |
| USA 400 | 1 (2.2) | 0 | |
| USA 500 | 1 (2.2) | 0 | |
| USA 600 | 2 (4.3) | 0 | |
| USA 800 | 3 (6.5) | 2 (10.5) | |
| Undefined | 7 (15.2) | 10 (52.6) | |
| Clonal complex : | 0.186 | ||
| CC 5 | 23 (50.0) | 8 (42.1) | |
| CC 8 | 5 (10.9) | 6 (31.6) | |
| CC 30 | 10 (21.7) | 1 (5.3) | |
| ST 228 | 4 (8.7) | 3 (15.8) | |
| Other STsa | 4 (8.7) | 1 (5.3) |
NOTE. Data are no. (%) of isolates, unless otherwise indicated.
include 2 ST45, 1 ST1, and 1 ST111 in VSSA isolates and ST59 in an hVISA isolate.
Using MLST, all but 4 of the 65 isolates were included within one of four clonal complexes (CCs) or sequence types (STs): CC5 (31, 47.7%), CC8 (11, 16.9%), CC30 (11, 16.9%), and ST228 (7, 10.8%). The distribution of these groups did not differ significantly between the hVISA and non-hVISA groups. Among the 48 MRSA isolates with a typeable PFGE profile by USA typing schema, 26 isolates (40.0%) demonstrated a USA 100 pattern and 10 isolates (15.4%) had a USA 200 pattern (figure 2). Three isolates demonstrated the USA300 genotype; none exhibited the hVISA phenotype. The distribution of PFGE profiles among the hVISA isolates and VSSA isolates did not differ significantly.
Figure 2.
Dendrogram of pulsed-field gel electrophoresis profiles of 65 MRSA bloodstream isolates from patients with infective endocarditis. The clonal complex/sequence type, geographic region of origin, and heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) status of each isolate is also represented. CC = clonal complex; ST = sequence type as defined by multilocus sequence typing; Region = Geographic region of source patient; Group = vancomycin-susceptible S. aureus (VSSA) and hVISA.
hVISA, Vancomycin MIC, and Clinical Outcome
The hVISA phenotype was significantly associated with higher vancomycin MIC values determined by E-test (P = .026), with all but one of the hVISA isolates having a vancomycin MIC of ≥1.0 mg/L. In addition, the hVISA phenotype was present in 5 of the 6 isolates (80%) of isolates with a vancomycin MIC of 2 mg/L (table 3). To confirm this association between vancomycin MIC and hVISA phenotype, we repeated the analyses using MIC values as defined by the broth microdilution method. These repeat analyses again indicated a significant association between increasing vancomycin MIC and the presence of hVISA phenotype (Fisher’s Exact test, P = .0063) (table 3). All 65 MRSA isolates were susceptible to daptomycin, with a daptomycin MIC ≤ 1.0 mg/L by E-test (data not shown).
Table 3.
Percentage of heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) isolates by population analysis profile according to vancomycin minimum inhibitory concentration (MIC), as defined by E-test and Broth Microdilution. Overall p-value of association for presence of hVISA and increasing vancomycin MIC is 0.026 using E-test method and 0.0063 using Broth Microdilution method.
| E-test
| |||
|---|---|---|---|
| MIC of vancomycin, mg/L | hVISA/MRSA (%)a | VSSA (N = 46) | hVISA (N = 19) |
| 0.5 | 0/3 (0) | 3 (6.5) | 0 |
| 0.75 | 1/11 (9.1) | 10 (21.7) | 1 (5.3) |
| 1.0 | 4/14 (28.6) | 10 (21.7) | 4 (21.1) |
| 1.5 | 9/31 (29.0) | 22 (47.8) | 9 (47.4) |
| 2.0 | 5/6 (83.3) | 1 (2.2) | 5 (26.3) |
|
| |||
|
Broth Microdilution
| |||
| MIC of vancomycin, mg/L | hVISA/MRSA (%)a | VSSA (N = 46) | hVISA (N = 19) |
|
| |||
| <1.0 | 7/40 (17.5) | 33 (71.7) | 7 (36.8) |
| 1.0 | 3/11 (27.3) | 8 (17.4) | 3 (15.8) |
| 2.0 | 8/13 (61.5) | 5 (10.9) | 8 (42.1) |
| 4.0 | 1/1 (100) | 0 | 1 (5.3) |
NOTE. Data are proportion (%) of isolates, unless otherwise indicated.
Ratio and percentages of hVISA phenotype among MRSA isolates.
We also evaluated potential associations between clinical outcome and higher vancomycin MIC values. As shown in table 4, no significant differences in outcome were identified among patients with IE due to MRSA isolates with varying levels of vancomycin MICs.
Table 4.
Characteristics and outcome of 65 patients with MRSA infective endocarditis according to vancomycin E-test minimum inhibitory concentration (MIC) of the infecting isolate.
| Characteristic | MIC of vancomycina, mg/L
|
P | ||
|---|---|---|---|---|
| ≤ 1.0 (n = 28) | 1.5 (n = 31) | 2.0 (n = 6) | ||
| Patient Characteristics : | ||||
| Male sex | 18 (64.3) | 15 (48.4) | 2 (33.3) | 0.269 |
| Age, years, median (25th–75th percentiles) | 67.5 (58.4–75.9) | 65.6 (45.4–74.8) | 67.6 (66.5–76.6) | 0.554 |
| Predisposing conditions : | ||||
| Diabetes mellitus | 11 (40.7) | 13 (41.9) | 4 (66.7) | 0.570 |
| Hemodialysis dependent | 5 (17.9) | 9 (29.0) | 1 (16.7) | 0.623 |
| HIV positive | 0 (0.0) | 1 (3.2) | 0 (0.0) | 1.00 |
| History of cancer | 5 (17.9) | 3 (9.7) | 1 (16.7) | 0.569 |
| Chronic immunosuppressive therapy | 5 (18.5) | 6 (19.3) | 0 (0.0) | 0.785 |
| History of infective endocarditis | 1 (3.5) | 4 (12.9) | 1 (16.6) | 0.328 |
| Other chronic disease | 19 (67.9) | 22 (71.0) | 3 (50) | 0.679 |
| Recent Invasive procedures | 12 (42.9) | 13 (41.9) | 3 (50) | 0.457 |
| History of congenital heart disease | 3 (10.7) | 2 (6.7) | 1 (16.7) | 0.443 |
| Presumed place of acquisition : | 0.547 | |||
| Nosocomial | 12 (42.9) | 13 (41.9) | 5 (83.3) | 0.187 |
| Non-nosocomial health care-associated | 10 (35.7) | 12 (38.7) | 1 (16.6) | 0.697 |
| Community-acquired | 6 (21.4) | 6 (19.3) | 0 (0.0) | 0.717 |
| Patient outcome : | ||||
| Cardiac surgery during hospitalization | 7 (25.0) | 8 (25.8) | 2 (33.3) | 0.845 |
| Stroke | 6 (21.4) | 4 (12.9) | 3 (50.0) | 0.124 |
| Congestive heart failure | 7 (25.0) | 7 (22.6) | 4 (66.7) | 0.107 |
| Intracardiac abscess | 4 (14.3) | 2 (6.5) | 1 (16.7) | 0.398 |
| Persistent bacteremia | 12 (42.9) | 13 (41.9) | 5 (83.3) | 0.187 |
| New conduction abnormalities | 2/27 (7.4) | 2/30 (6.7) | 1 (16.7) | 0.616 |
| In-hospital death | 11 (39.3) | 9 (29.0) | 4 (66.7) | 0.242 |
NOTE. Data are no. (%) of patients, unless otherwise indicated.
MIC values defined according to E-test.
DISCUSSION
Over one-quarter of the isolates in this study exhibited the hVISA phenotype. This rate -approximately three times higher than those reported in two recent reports in patients with primarily non-IE infections [6, 26] - may reflect the fact that IE is a high-inoculum infection, with valvular vegetations serving as “sanctuary sites” for the bacteria. Penetration of glycopeptides into these vegetations is limited [27], leading to the potential for prolonged bacterial exposure to sub-therapeutic glycopeptide levels. The presence of hVISA related to prolonged sub-therapeutic glycopeptide exposure seems particularly possible among MRSA isolates with higher vancomycin MICs [28]. In support of this possibility is the observation that over 80% of the IE isolates in the present investigation with a vancomycin MIC of 2 mg/L exhibited the hVISA phenotype.
hVISA-infected patients had more IE complications - including persistent bacteremia and congestive heart failure - than patients with VSSA IE. Congestive heart failure is a leading cause of death in patients with IE [29, 30], and its presence has the greatest impact on prognosis of all IE complications [31, 32]. The association between persistent bacteremia and hVISA has been previously reported among patients with S. aureus infections of multiple types [7, 10, 11], but was confounded by the issue of whether the persistent bacteremia was simply due to the presence of IE. The current study addresses this issue by demonstrating that, even among patients with IE, persistent bacteremia and hVISA are associated. On the other hand, it should be acknowledged that persistent bacteremia per se does not equal failure, particularly as the mortality difference between VSSA- and hVISA-infected subjects (35% vs. 42%) did not achieve statistical significance. Because this observation is consistent with previous reports [7, 10], it is possible that some infections due to hVISA may eventually be treated successfully with vancomycin. However, the recent finding by Rose et al. that daily vancomycin doses of up to 10 grams were ineffective against an in vitro model of a high inoculum hVISA infection argues against this possibility [33]. Thus, our failure to demonstrate a statistically significant difference in mortality between hVISA- and VSSA-infected patients may simply be due to an insufficient sample size. However, the fact that almost 40% of the entire cohort died underscores the need for alternative therapies for MRSA IE.
The prevalence of hVISA among MRSA IE isolates differed by geographic region in our study. IE isolates from Oceania or Europe were more likely to exhibit the hVISA phenotype than isolates from North America. Although the absolute number of isolates and study sites from each region was relatively small, the findings were striking: over three-quarters of MRSA IE isolates from Australia or New Zealand exhibited the hVISA phenotype on PAP analysis. Previous studies evaluating hVISA prevalence have used a variety of laboratory definitions, testing strategies, and patient populations [6, 10, 34–39] (table 5), thereby limiting comparisons. The current investigation overcomes these limitations to make a key point: even in a “high-risk” infection type such as IE, the prevalence of hVISA varies significantly and is potentially associated with non-clinical factors such as geography. Although the reasons for the apparently higher hVISA prevalence in Oceania and Europe are unknown, it is intriguing to note that teicoplanin, a glycopeptide that has been associated with development of the hVISA phenotype [40, 41] is used in both of these regions, but not the US. Further studies will be required to substantiate this interesting speculation.
Table 5.
Prevalence of heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) in previous studies.
| Author (reference) | Study period | Geographic region | Design | MRSA, N | hVISA, N | % hVISA | Study population | Screening tests | Confirmatory test |
|---|---|---|---|---|---|---|---|---|---|
| Hiramatsu et al. [35] | 1996~1997 | Japan | M | 1,149 | 35 | 3.05 | All clinical isolates | sPAP-BHIA-V4 | Broth Microdilution |
| Kim et al. [36] | 1999 | South Korea | S | 3,363 | 24 | 0.71 | All clinical isolates | sPAP-BHIA-V4 | PAP |
| Sancak et al. [39] | 1998~2001 | Turkey | S | 256 | 46 | 17.97 | Blood (41%), pus (39.8%), tracheal aspiration/bronchoalveolar fluid (18.4%) | BHIA-V4, MET | PAP |
| Charles et al. [10] | 2001~2002 | Australia | S | 53 | 5 | 9.4 | Bloodstream isolates | None | mPAP |
| Garnier et al. [34] | 2001~2002 | France | S | 2,300 | 255 | 11.1 | All clinical isolates | BHIA-T4, MET | mPAP |
| Maor et al. [38] | 2003~2004 | Israel | S | 246 | 16 | 6.5 | Bloodstream isolates | None | MET |
| Kosowska-Shick et al. [37] | 2006~2007 | US | S | 982 | 2 | 0.2 | All clinical isolates: wound (60%), respiratory tract (20.7%), blood (9.7%), etc. | BHIA-V3, MET | PAP |
| Rybak et al. [6] | 1986~2007 | US | M | 1,499 | 112 | 7.5 | Clinical isolates from surveillance studies: blood (60%), lung (21%), skin (14%), etc. | MET | mPAP |
NOTE. M = multicenter trial; S = single center trial; sPAP-BHIA-V4 = simplified population analysis profile on brain heart infusion agar with 4 mg/L vancomycin; BHIA-T4 = Brain heart infusion agar with 4 mg/L teicoplanin; BHIA-V3 = Brain heart infusion agar with 3 mg/L vancomycin; MET = Macrodilution E-test; PAP = Population analysis profile by Hiramatsu et al. [35]; mPAP = Modified popultion analysis profile by Wooton et al [19]
Detection of the hVISA phenotype in S. aureus is difficult. PAP-AUC is time-consuming, labor-intensive and unsuitable for clinical laboratories. Several other laboratory methods have been considered [42–44], but also have limitations as simple screening tests. Thus, a clinically directed approach will likely be required before hVISA testing can be routinely considered. One readily available strain characteristic that may identify bacteria at higher risk for hVISA is the presence of a vancomycin MIC of 2.0 mg/L. Although only ~ 10% of the cohort had a vancomycin MIC of 2.0 mg/L on E-test, over 80% of these isolates exhibited the hVISA phenotype on PAP testing. Thus, discovery of a high vancomycin MIC MRSA isolate from an appropriate clinical setting (e.g., MRSA IE with persistent bacteremia) could identify situations in which hVISA testing might be helpful. However, any such screening strategy should be properly validated before widespread adoption into clinical practice.
Using PFGE, MLST, and PCR for a large number of putative virulence genes, hVISA isolates were genotypically similar to VSSA isolates. This finding conflicts with a previous report suggesting an association between agr type II polymorphism and the development of vancomycin heteroresistance [45]. Our failure to identify a single clonotype associated with hVISA also suggests that the emergence of hVISA may be due more to the influence of ubiquitous forces – such as widespread glycopeptide use - acting simultaneously upon multiple different bacterial clones in different regions of the world than the global dissemination of a limited number of bacterial clones exhibiting hVISA. This finding also suggests that bacterial genotype is unlikely to be a useful method to target selected clinical isolates for hVISA testing. However, recent studies linking point mutations in graSR and vraSR to the stepwise development of the VISA phenotype [46] increase the possibility of the future development of molecular methods for detection of hVISA strains.
Using both E-test and broth microdilution techniques, our study found an association between higher vancomycin MIC values and presence of the hVISA phenotype. This finding is consistent with a recent report from Detroit [6]. Several studies have reported higher rates of treatment failure [47–49] and mortality [50] among patients with infections caused by MRSA isolates with higher vancomycin MIC values. In the current study, the mortality of infections caused by isolates with vancomycin MIC of 2 mg/L by E-test was particularly high (66%), although the difference did not achieve statistical significance. This finding agrees with a recent report demonstrating suboptimal cure rates of vancomycin in an animal model of high vancomycin MIC (2 mg/L) MRSA aortic endocarditis [8].
The present study has limitations. Our sample size was relatively small, limiting our ability to detect significant differences betwen VSSA and hVISA infections, and between infections with different vancomycin MICs. Next, we had no information regarding timing of complications, antibiotic dosing, trough serum concentration monitoring, prior antibiotic therapy before the onset of MRSA bacteremia, or the ultimate duration of bacteremia after therapy was initiated. We were also unable to consider why individual patients did or did not undergo cardiac surgery, or why patients with VSSA infection underwent cardiac surgery twice as frequently as did those with hVISA (30.4% vs. 15.8%; P=.353). Standard practice in the ICE-PCS Microbiological Repository is to evaluate the first available bloodstream isolate. However, patients transferred from other facilities may have had bacteremia for extended periods of time prior to arrival at the ICE study center. Finally, because serial isolates from the same patient were not routinely available, we were unable to further evaluate the association between persistent bacteremia and the hVISA phenotype.
In summary, this study makes several key observations. The hVISA phenotype occurred in over one-quarter of MRSA IE isolates, and was associated with a higher frequency of IE complications such as persistent bacteremia and heart failure. hVISA was geographically distributed, with highest rates being encountered in MRSA IE isolates from Europe or Oceania. hVISA and VSSA isolates were genotypically similar in this study. Vancomycin MIC values of 2 mg/L were relatively uncommon, but associated with hVISA. Future studies are required to validate the findings of this investigation, to better understand specific risk factors for the emergence of hVISA, and to identify practical, effective screening tests to detect hVISA in the clinical setting.
Acknowledgments
Financial Support:
Multiplex PCR and PFGE were funded by a grant from Cubist Pharmaceuticals (to CWW). Population analyses were funded by an MRSA Center of Excellence Grant from Astellas and administered by Fallon Medica LLC. (to VGF). Multilocus sequence typing was funded by R01-AI059111 (to VGF).
Footnotes
Dr. Bae, Rude, Newton, Park, Dr. Marco and Dr. Garcia-de-la-Maria did not have any conflict of interest.
This work has been presented in part at the 48th Interscience Conference on Antimicrobial Agent and Chemotherapy (ICAAC)/Infectious Disease Society of America (IDSA) 46th. Annual Meeting, Washington D.C., USA, 2008; Abstract C1-197.
Potential Conflict of Interest: Dr. Fowler has served as a consultant for Astellas, Cubist, Inhibitex, Merck, Johnson & Johnson, Leo Pharmaceuticals; reports having received grant or research support from Astellas, Cubist, Merck, Theravance, Inhibitex, Cerexa, National Institute of Health; reports having received honoraria from Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Ortho-McNeil; has served as a membership on advisory committee for Cubist; has served as a speaker’s bureau for Cubist. Dr. Corey serves as a consultant for (money for these meetings is donated to the Global Health Fund at Duke University) Theravance, Cubist, AstraZeneca Astellas, Cerexa, Merck, Cempra, Pfizer, Arpida, GSK, Inimex, Targanta, and Trius; receives research contracts/grant (these relationships result in funds being provided through Duke University to support research or salary) from Theravance, Innocoll, Cerexa, Cempra. Dr. Kanj is on the advisory board for Sanofi Aventis. Pharm.D. Rybak is a speaker for Cubist, Wyeth, Pfizer, Astellas, Theravance Forest, Ortho-McNeil, and Targanta. Dr. Woods has served as a consultant for Roche Molecular; reports having received grant or research support from Cubist, Roche Molecular; has served as a membership on advisory committee for BioMerieux, Astellas; involved clinical trial of BioMerieux and Cepheid.
References
- 1.Deresinski S. Vancomycin heteroresistance and methicillin-resistant Staphylococcus aureus. J Infect Dis. 2009;199:605–9. doi: 10.1086/596630. [DOI] [PubMed] [Google Scholar]
- 2.Tenover FC, Moellering RC., Jr The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–15. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 3.Fridkin SK, Hageman J, McDougal LK, et al. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin Infect Dis. 2003;36:429–39. doi: 10.1086/346207. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother. 2003;47:3040–5. doi: 10.1128/AAC.47.10.3040-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50:3039–47. doi: 10.1128/AAC.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rybak MJ, Leonard SN, Rossi KL, Cheung CM, Sader HS, Jones RN. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007) J Clin Microbiol. 2008;46:2950–4. doi: 10.1128/JCM.00582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maor Y, Hagin M, Belausov N, Keller N, Ben-David D, Rahav G. Clinical features of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia versus those of methicillin-resistant S. aureus bacteremia. J Infect Dis. 2009;199:619–24. doi: 10.1086/596629. [DOI] [PubMed] [Google Scholar]
- 8.Marco F, de la Maria CG, Armero Y, et al. Daptomycin is effective in treatment of experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52:2538–43. doi: 10.1128/AAC.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens DL. The role of vancomycin in the treatment paradigm. Clin Infect Dis. 2006;42 (Suppl 1):S51–7. doi: 10.1086/491714. [DOI] [PubMed] [Google Scholar]
- 10.Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38:448–51. doi: 10.1086/381093. [DOI] [PubMed] [Google Scholar]
- 11.Howden BP, Ward PB, Charles PG, et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis. 2004;38:521–8. doi: 10.1086/381202. [DOI] [PubMed] [Google Scholar]
- 12.Fowler VG, Jr, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–21. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 13.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–73. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200–9. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 15.Adams HP, Jr, Brott TG, Crowell RM, et al. Guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the Stroke Council. American Heart Association Stroke. 1994;25:1901–14. doi: 10.1161/01.str.25.9.1901. [DOI] [PubMed] [Google Scholar]
- 16.Bonow RO, Carabello B, de Leon AC, et al. ACC/AHA Guidelines for the Management of Patients With Valvular Heart Disease. Executive Summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease) J Heart Valve Dis. 1998;7:672–707. [PubMed] [Google Scholar]
- 17.Friedman ND, Kaye KS, Stout JE, et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standard Institute. Approved standard M7-A7. Clinical and Laboratory Standards Institute; Wayne, PA: 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. [Google Scholar]
- 19.Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother. 2001;47:399–403. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]
- 20.Campbell SJ, Deshmukh HS, Nelson CL, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008;46:678–84. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peacock SJ, Moore CE, Justice A, et al. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun. 2002;70:4987–96. doi: 10.1128/IAI.70.9.4987-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2155–61. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler VG, Jr, Nelson CL, McIntyre LM, et al. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis. 2007;196:738–47. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 26.Lalani T, Federspiel JJ, Boucher HW, et al. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J Clin Microbiol. 2008;46:2890–6. doi: 10.1128/JCM.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cremieux AC, Maziere B, Vallois JM, et al. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J Infect Dis. 1989;159:938–44. doi: 10.1093/infdis/159.5.938. [DOI] [PubMed] [Google Scholar]
- 28.Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. 2001;1:147–55. doi: 10.1016/S1473-3099(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 29.Miro JM, Anguera I, Cabell CH, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507–14. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- 30.Vikram HR, Buenconsejo J, Hasbun R, Quagliarello VJ. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. JAMA. 2003;290:3207–14. doi: 10.1001/jama.290.24.3207. [DOI] [PubMed] [Google Scholar]
- 31.Chu VH, Cabell CH, Benjamin DK, Jr, et al. Early predictors of in-hospital death in infective endocarditis. Circulation. 2004;109:1745–9. doi: 10.1161/01.CIR.0000124719.61827.7F. [DOI] [PubMed] [Google Scholar]
- 32.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 33.Rose WE, Leonard SN, Rossi KL, Kaatz GW, Rybak MJ. Impact of inoculum size and heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) on vancomycin activity and emergence of VISA in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2009;53:805–7. doi: 10.1128/AAC.01009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garnier F, Chainier D, Walsh T, et al. A 1 year surveillance study of glycopeptide-intermediate Staphylococcus aureus strains in a French hospital. J Antimicrob Chemother. 2006;57:146–9. doi: 10.1093/jac/dki413. [DOI] [PubMed] [Google Scholar]
- 35.Hiramatsu K, Aritaka N, Hanaki H, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–3. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 36.Kim MN, Hwang SH, Pyo YJ, Mun HM, Pai CH. Clonal spread of Staphylococcus aureus heterogeneously resistant to vancomycin in a university hospital in Korea. J Clin Microbiol. 2002;40:1376–80. doi: 10.1128/JCM.40.4.1376-1380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosowska-Shick K, Ednie LM, McGhee P, et al. Incidence and characteristics of vancomycin nonsusceptible strains of methicillin-resistant Staphylococcus aureus at Hershey Medical Center. Antimicrob Agents Chemother. 2008;52:4510–3. doi: 10.1128/AAC.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maor Y, Rahav G, Belausov N, Ben-David D, Smollan G, Keller N. Prevalence and characteristics of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia in a tertiary care center. J Clin Microbiol. 2007;45:1511–4. doi: 10.1128/JCM.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sancak B, Ercis S, Menemenlioglu D, Colakoglu S, Hascelik G. Methicillin-resistant Staphylococcus aureus heterogeneously resistant to vancomycin in a Turkish university hospital. J Antimicrob Chemother. 2005;56:519–23. doi: 10.1093/jac/dki272. [DOI] [PubMed] [Google Scholar]
- 40.Kaatz GW, Seo SM, Dorman NJ, Lerner SA. Emergence of teicoplanin resistance during therapy of Staphylococcus aureus endocarditis. J Infect Dis. 1990;162:103–8. doi: 10.1093/infdis/162.1.103. [DOI] [PubMed] [Google Scholar]
- 41.Shlaes DM, Shlaes JH. Teicoplanin selects for Staphylococcus aureus that is resistant to vancomycin. Clin Infect Dis. 1995;20:1071–3. doi: 10.1093/clinids/20.4.1071. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgibbon MM, Rossney AS, O’Connell B. Investigation of reduced susceptibility to glycopeptides among methicillin-resistant Staphylococcus aureus isolates from patients in Ireland and evaluation of agar screening methods for detection of heterogeneously glycopeptide-intermediate S. aureus. J Clin Microbiol. 2007;45:3263–9. doi: 10.1128/JCM.00836-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wootton M, MacGowan AP, Walsh TR, Howe RA. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J Clin Microbiol. 2007;45:329–32. doi: 10.1128/JCM.01508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonard SN, Rossi KL, Newton KL, Rybak MJ. Evaluation of the E-test GRD for the detection of Staphylococcus aureus with reduced susceptibility to glycopeptides. J Antimicrob Chemother. 2009;63:489–92. doi: 10.1093/jac/dkn520. [DOI] [PubMed] [Google Scholar]
- 45.Sakoulas G, Eliopoulos GM, Moellering RC, Jr, et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002;46:1492–502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui L, Neoh HM, Shoji M, Hiramatsu K. Contribution of vraSR and graSR point mutations to vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:1231–4. doi: 10.1128/AAC.01173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52:3315–20. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering RC., Jr Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis. 2004;38:1700–5. doi: 10.1086/421092. [DOI] [PubMed] [Google Scholar]
- 49.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]