Abstract
Objective
Intra-amniotic and systemic infection/inflammation have been causally linked to preterm parturition and fetal injury. An emerging theme is that adipose tissue can orchestrate a metabolic response to insults, but also an inflammatory response via the production of adipocytokines, and that these two phenomenon are interrelated. Adiponectin, an insulin-sensitizing, anti-inflammatory adipocytokine, circulates in multimeric complexes including low-molecular-weight (LMW) trimers, medium-molecular-weight (MMW) hexamers and high-molecular-weight (HMW) isoforms. Each of these complexes can exert differential biological effects. The aim of this study was to determine whether spontaneous preterm labor (PTL) with intact membranes and intra-amniotic infection/inflammation (IAI) is associated with changes in maternal serum circulating adiponectin multimers.
Study design
This cross-sectional study included patients in the following groups: 1) normal pregnant women (n=158); 2) patients with an episode of preterm labor and intact membranes without IAI who delivered at term (n=41); 3) preterm labor without IAI who delivered preterm (n=27); and 4) preterm labor with IAI who delivered preterm (n=36). Serum adiponectin multimers (total, HMW, MMW and LMW) concentrations were determined by ELISA. Non-parametric statistics were used for analyses.
Results
1) Preterm labor leading to preterm delivery or an episode of preterm labor which does not lead to preterm delivery, was associated with a lower median maternal serum concentration of total and HMW adiponectin, a lower median HMW/total adiponectin ratio, and a higher median LMW/total adiponectin ratio than normal pregnancy; 2) among patients with preterm labor, those with IAI had the lowest median concentration of total and HMW adiponectin, as well as the lowest median HMW/total adiponectin ratio; 3) The changes in maternal adiponectin and adiponectin multimers remained significant after adjusting for confounding factors such as maternal age, BMI, gestational age at sampling, and parity.
Conclusion
1) Preterm labor is characterized by a change in the profile of adiponectin multimers concentrations and their relative isoforms. These changes were observed in patients with an episode of preterm labor not leading to preterm delivery, in patients with intra-amniotic inflammation, or in those without evidence of intra-amniotic inflammation; 2) The changes in adiponectin multimer concentrations reported in preterm labor are different from those previously reported in spontaneous labor at term, suggesting that there is a fundamental difference between preterm labor and labor at term; 3) The findings reported herein, provide the first evidence for the participation of adiponectin multimer in preterm parturition. We propose that adiponectins and adipokines in general provide a mechanism to organize the metabolic demands generated by the process of preterm parturition regardless of the nature of the insult (intra-amniotic inflammation or not).
Keywords: Adiponectin, Adipokines, Pregnancy, High molecular weight (HMW), Medium molecular weight (MMW), Low molecular weight (LMW), Preterm labor, Intra-amniotic infection, Inflammation, Chorioamnionitis, Preterm delivery, Energy Requirements, Energy Expenditure, Preterm Birth, Metabolism, Metaflammation
Introduction
Infection and/or inflammation has been implicated as a mechanism of disease responsible for preterm parturition and fetal injury,1–12 indeed, infection and/or inflammation are the only pathologic processes for which a firm causal link with preterm parturition has been established and a molecular pathophysiology has been defined.8;13
In addition to the unequivocal experimental, epidemiologic and clinical evidence causally linking intra-amniotic infection and/or inflammation (IAI) and preterm parturition,1–4;6;8;9;13–52 there are several lines of evidence supporting a cause and effect relationship between systemic infection/inflammation and preterm parturition: 1) systemic administration of microbial products to pregnant animals can result in spontaneous preterm labor and preterm birth;29;53 2) administration of IL-1, a potent pro-inflammatory cytokine, to pregnant mice induces preterm labor and preterm birth.54 Furthermore, exposure of these mice to the natural antagonist of IL-1 (IL-1 receptor antagonist) abrogates parturition;55 3) extra uterine infection resulting in systemic inflammation such as malaria,56;57 pyelonephritis,58–60 pneumonia61;62 and periodontal disease63;64 have been associated with preterm birth; and 4) non-infectious chronic inflammatory disorders like systemic lupus erythematosus,65;66 inflammatory bowel diseases,67–69 asthma,70;71 and morbid obesity72–74 have been associated with preterm parturition.
Adipose tissue has emerged as a highly active endocrine organ75–77 that can orchestrate a metabolic response to insults, but also an inflammatory response via the production of soluble factors known as adipocytokines. Adipocytokine is a term used to describe cytokines that are produced mainly, but not necessarily exclusively, by adipose tissue. A wide range of highly active molecules are members of the adipocytokine family: Interleukin (IL)-6,78;79 tumor necrosis factor (TNF)-α,80 leptin,81;82 adiponectin,83–86 resistin,87–89 visfatin,90–92 and others.93;94 Adipocytokines have been implicated in the pathophysiology of inflammatory disorders such as asthma,95 inflammatory bowel disease,96 rheumatoid arthritis,97;98 multiple sclerosis99;100 and obesity.101–109 Furthermore, several adipocytokines such as resistin110;111 and visfatin51;112;113 have an immunoregulatory effect on the innate immune response. Others, like leptin, have been shown to regulate both the innate114;115 and the adaptive immune pathways.116;117 Collectively these finding support an important role for adipocytokines in the regulation of the inflammatory response.
Adiponectin is the most abundant gene (AMP1) product of adipose tissue83;118–120 and has a wide range of biological activities. Indeed, adiponectin has been implicated in the pathophysiology of the metabolic syndrome including insulin resistance,121–124 atherosclerosis,125;126 hypertension,127 and dyslipidemia.128 In addition to its well-characterized metabolic effects, a solid body of evidence strongly suggests that adiponectin is an important mediator of inflammatory responses. Adiponectin circulates in humans in distinct forms: 1) low-molecular-weight (LMW) trimers; 2) medium-molecular-weight (MMW) hexamers; and 3) high-molecular-weight (HMW) oligomers (12 to 18 subunits).129;130 These adiponectin multimeric complexes have differential biological effects,130–133 and it has been proposed that elucidation of the putative physiologic and pathologic role of adiponectin can not be discerned without the investigation of the profile of adiponectin multimer concentrations and their relative distribution.
Recently, we have suggested that intra-amniotic adipocytokines may play a role in the innate immune response against intra-amniotic infection/inflammation.51;111 However, to date there are no studies about primary adipokines in preterm labor. Thus, the aim of this study was to determine whether there are changes in adiponectin multimers in patients with preterm labor (PTL) with and without IAI.
Materials and methods
Study design and population
A cross-sectional study was designed by searching our clinical database and bank of biological samples, including 262 patients in the following groups: 1) normal pregnant women (n=158); 2) patients with an episode of preterm labor and intact membranes without IAI who delivered at term (n=41); 3) preterm labor without IAI who delivered preterm (<37 weeks gestation) (n=27); and 4) preterm labor with IAI who delivered preterm (n=36).
All women provided written informed consent prior to enrollment and the collection of blood and amniotic fluid. The collection and utilization of blood and amniotic fluid for research purposes was approved by the Institutional Review Boards of the Sotero del Rio Hospital (Chile), the Wayne State University (Detroit, Michigan, USA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (Bethesda, Maryland, USA). Many of these samples have previously been used to study the biology of inflammation, hemostasis, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Clinical Definitions
Patients were considered to have a normal pregnancy or spontaneous preterm labor as previousely defined.51;111 Intra-amniotic infection was defined as a positive amniotic fluid culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an amniotic fluid IL-6 concentration ≥2.6 ng/mL.134
Amniotic fluid collection
Amniotic fluid samples were obtained by trans-abdominal amniocentesis. Samples of amniotic fluid were cultured for aerobic/anaerobic bacteria and genital mycoplasmas. An amniotic fluid white blood cell (WBC) count, glucose concentration and Gram-stain were also performed shortly after collection as previously described.135–137
Maternal serum sample collection and quantitative determination of adiponectin multimeric forms
Maternal blood samples were collected immediately before or after the amniocentesis into vacutainer tubes. Samples were centrifuged and the sera were stored at −80°C until analysis. Sensitive enzyme-linked immunoassays were used to determine the concentrations of adiponectin multimeric forms in maternal serum. Immunoassays were purchased from ALPCO Diagnostics (Salem, NH, USA). The assays were run according to the recommendations of the manufacturer. To detect HMW adiponectin, serum samples were pretreated with a specific protease that selectively digested MMW and LMW adiponectin. We were also able to determine the combined HMW and MMW adiponectin concentrations by pretreating the samples with a protease that specifically digested LMW adiponectin. Maternal serum samples were assayed directly to determine total adiponectin concentrations. Briefly, untreated and pretreated maternal serum samples were incubated in duplicate wells of the micro titer plates, which had been pre-coated with a monoclonal antibody specific for adiponectin. During this incubation any adiponectin present in the standards and untreated or pretreated maternal serum samples was bound by the immobilized antibodies. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for adiponectin was added to the wells. Unbound materials were removed with repeated washing and a substrate solution was added to the wells and color developed in proportion to the amount of adiponectin bound in the initial step. The color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentration of adiponectin in untreated and treated maternal serum samples was determined by interpolation from individual standard curves composed of human adiponectin. Total, HMW, and HMW-MMW adiponectin concentrations were derived directly from the assay plates. MMW adiponectin concentrations were obtained by subtracting HMW adiponectin value from the combined HMW-MMW value. Finally, the LMW adiponectin value was computed by subtracting HMW and MMW adiponectin values from the total adiponectin values. The calculated inter- and intra-assay coefficients of variation for adiponectin multimers immunoassays in our laboratory were 2.2% and 4.2%, respectively. The sensitivity was calculated to be 0.04 ng/mL.
Statistical analysis
Normality of the data was tested using the Shapiro-Wilk or Kolmogorov-Smirnov tests. Kruskal–Wallis tests with post-hoc analysis by Mann-Whitney U tests were used for comparisons of continuous variables. Multiple linear regression analysis was used to determine which factors were significantly and independently associated with maternal serum adiponectin isoforms as well as their relative distribution (after log transformation). The following parameters were included in the model: maternal age, gestational age at sampling, birth weight, first trimester body mass index (BMI) and the presence of preterm labor. A p value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 14 (SPSS Inc., Chicago, IL, USA).
Results
The demographic and clinical characteristics of women with a normal pregnancy and those with preterm labor are displayed in Table I. There was no significant difference in the median first trimester BMI or parity between the four groups. Women with a normal pregnancy had a lower gestational age at sampling than those with preterm labor without IAI who delivered either at term (p=0.003) or preterm (p<0.001). The median gestational age at sampling did not differ significantly between women with a normal pregnancy and those with preterm labor with IAI (p=0.08). Women with a normal pregnancy had a higher median maternal age than those with preterm labor without IAI who delivered either at term (p=0.003) or preterm (p=0.01). The median maternal age did not differ significantly between women with a normal pregnancy and those with preterm labor and IAI (p=0.06). Comparison of the demographics and clinical characteristics among patients with preterm labor is presented in Table I.
Table I.
Clinical and demographic characteristics of the study population
| Normal Pregnancy (n=158) | PTL without IAI Term delivery (n=41) | p1 | PTL without IAI Preterm delivery (n=27) | p2 | PTL with IAI Preterm delivery (n=36) | p3 | |
|---|---|---|---|---|---|---|---|
| Maternal age (years) | 26 (21–31) | 23 (19–27) | NS | 22 (20–29) | NS | 24 (20–28) | NS |
| Parity | 1 (0–2) | 2 (1–2) | NS | 2 (1–3) | NS | 2 (1–3) | NS |
| First trimester BMI (kg/m2) | 23.1 (21.3–25.9) | 23.6 (20.3–29.2) | NS | 25.5 (22.3–29.0) | NS | 26.6 (23.4–31.1) | NS |
| GA at blood sampling (weeks) | 27.7 (25.3–29.0) | 30.0 (26.1–32.2) | NS | 31.0 (28.0–32.9) | <0.01 | 25.4 (24.3–30.8) | <0.05 |
| GA at delivery (weeks) | 40.0 (39.0–40.4) | 38.3 (37.3–39.4) | <0.01 | 34.6 (33.7–35.3) | <0.01 | 26.3 (24.5–31.0) | <0.01 |
| Birth weight (grams) | 3465 (3210–3702) | 2903 (2697–3267) | <0.01 | 1940 (1760–2310) | <0.01 | 815 (628–1645) | <0.01 |
p1: comparison between preterm labor who delivered at term and preterm labor without IAI
p2: comparison between preterm labor who delivered preterm without IAI and preterm labor with IAI
p3: comparison between preterm labor who delivered at term and preterm labor with IAI
Values are expressed as median and interquartile (IQR) range; PTL: preterm labor; GA: gestational age; BMI: body mass index; IAI: intra-amniotic infection/inflammation NS: not significant
Total Adiponectin concentrations in preterm labor vs. normal pregnancy
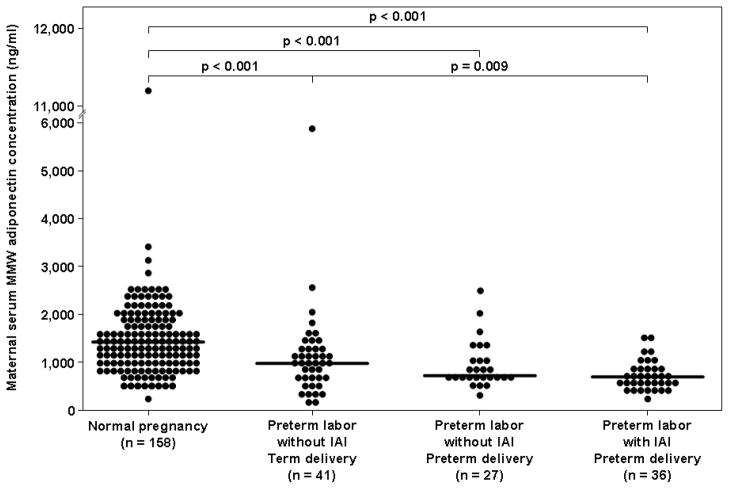
The median maternal serum total adiponectin concentration was lower in patients with preterm labor with IAI than in those with preterm labor without IAI who delivered either preterm (median: 3,076 ng/mL, interquartile range [IQR] 2,082–3,391 vs. 3,411 ng/mL, IQR 2,937–4,586; p=0.01, Figure 1) or at term (4,115 ng/mL, IQR 2,977–5,596; p<0.001, Figure 1). Similarly, patients with preterm labor and IAI had a lower median maternal serum total adiponectin concentration than those with a normal pregnancy (3,076 ng/mL, IQR: 2,082–3,391 vs. 6,551 ng/mL, IQR 4,790–8,635; p<0.001, Figure 1).
Figure 1. Comparison of the median maternal serum total adiponectin concentrations between women with normal pregnancies and patients with spontaneous PTL.
The median maternal serum concentration of total adiponectin was lower in patients with PTL than in those with a normal pregnancy. Among women with PTL, patients with IAI had the lowest median maternal total adiponectin.
The median maternal serum total adiponectin concentration was lower in patients with preterm labor without IAI who delivered either preterm or at term than in those with a normal pregnancy (p<0.001 for both comparisons, Figure 1). Among patients with preterm labor without IAI, the median maternal serum total adiponectin did not differ significantly between those who delivered preterm and those who delivered at term (p=0.2).
HMW Adiponectin concentrations in preterm labor vs. normal pregnancy
The median maternal serum HMW adiponectin concentration was lower in patients with preterm labor and IAI than in those with preterm labor without IAI who delivered either preterm (1,372 ng/mL, IQR 756–1,557 vs. 1,783 ng/mL, IQR 928–2,438; p=0.01, Figure 2) or at term (1,936 ng/mL, IQR 1,209–2,827; p<0.001, Figure 2). Similarly, patients with preterm labor and IAI had a lower median HMW adiponectin concentration than those with a normal pregnancy (1,372 ng/mL, IQR 756–1,557 vs. 3,660 ng/mL, IQR 2,444–5,418; p<0.001, Figure 2).
Figure 2. Comparison of the median maternal serum HMW adiponectin concentrations between women with normal pregnancies and patients with spontaneous PTL.
The median maternal serum concentration of HMW adiponectin was lower in patients with PTL than in those with a normal pregnancy. Among women with PTL, patients with IAI had the lowest median maternal HMW adiponectin.
The median maternal serum HMW adiponectin concentration was lower in patients with preterm labor without IAI who delivered either preterm or at term than in those with a normal pregnancy (p<0.001 for both comparisons, Figure 2). There was no significant difference in the median maternal serum HMW adiponectin concentration between patients with preterm labor without IAI who delivered preterm and those with preterm labor without IAI who delivered at term (p=0.4)
MMW Adiponectin concentrations in preterm labor vs. normal pregnancy
The median maternal serum MMW adiponectin concentration was lower in patients with preterm labor and IAI than in those with preterm labor without IAI who delivered at term (683 ng/mL, IQR 544–9467 vs. 977 ng/mL, IQR 660–1,292; p=0.009, Figure 3) but comparable with those with preterm labor without IAI who delivered preterm (654 ng/mL, IQR 481–745; p=0.13) Patients with preterm labor and IAI had a lower median MMW adiponectin concentration than those with a normal pregnancy (683 ng/mL, IQR 544–9467 vs. 1,437 ng/mL, IQR 965–1,804; p<0.001, Figure 3).
Figure 3. Comparison of the median maternal serum MMW adiponectin concentration between women with normal pregnancies and patients with spontaneous PTL.
The median maternal serum concentration of MMW adiponectin was lower in patients with PTL than in those with a normal pregnancy. Among women with PTL, patients with IAI had lower median maternal MMW adiponectin than patients with PTL who delivered at term.
The median maternal serum MMW adiponectin concentration was lower in patients with preterm labor without IAI who delivered either preterm or at term than in those with a normal pregnancy (p<0.001 for both comparisons, Figure 3). There was no significant difference in the median maternal serum MMW adiponectin concentration between patients with preterm labor without IAI who delivered preterm and those with preterm labor without IAI who delivered at term (p=0.3).
LMW Adiponectin concentrations in preterm labor vs. normal pregnancy
The median maternal serum LMW adiponectin concentration was lower in patients with preterm labor and IAI than in those with preterm labor without IAI who delivered at term (975 ng/mL, IQR 797–1,105 vs. 1,232 ng/mL, IQR 928–1,641; p=0.001, Figure 4) but comparable with those with preterm labor without IAI who delivered preterm (1,071 ng/mL, IQR 612–1,426; p=0.2) Patients with preterm labor and IAI had a lower median LMW adiponectin concentration than those with a normal pregnancy (975 ng/mL, IQR 797–1,105 vs. 1,195 ng/mL, IQR 886–1,672; p<0.001, Figure 4).
Figure 4. Comparison of the median maternal serum LMW adiponectin concentrations between women with normal pregnancies and patients with spontaneous preterm labor.
The median maternal serum concentration of LMW adiponectin was lower in patients with preterm labor and IAI than in those with a normal pregnancy and than that of those with preterm labor who delivered at term.
The median maternal serum LMW adiponectin concentration did not differ significantly between patients with preterm labor without IAI who delivered either preterm or at term than in those with a normal pregnancy (p=0.07 and p=0.98, respectively; Figure 4). Similarly, there was no significant difference in the median maternal serum LMW adiponectin concentration between patients with preterm labor without IAI who delivered preterm and those with preterm labor without IAI who delivered at term (p=0.1).
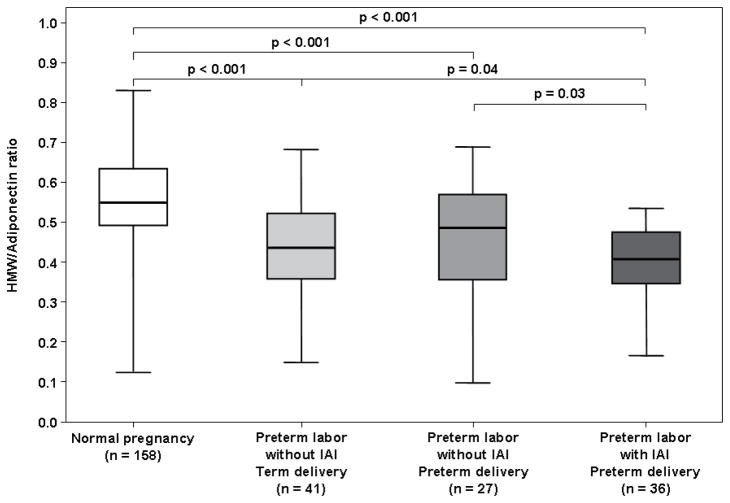
HMW/total adiponectin ratio in preterm labor vs. normal pregnancy
The median maternal HMW/total adiponectin ratio was lower in patients with preterm labor and IAI than in those with preterm labor without IAI who delivered either preterm (0.41, IQR 0.34–0.48 vs. 0.49, IQR 0.36–0.57; p=0.03, Figure 5) or at term (0.44, IQR 0.36–0.52; p=0.04, Figure 5). Similarly, patients with preterm labor and IAI had a lower median HMW/total adiponectin ratio than those with a normal pregnancy (0.41, IQR 0.34–0.48 vs. 0.56, IQR 0.49–0.64; p<0.001, Figure 5).
Figure 5. Comparison of the median maternal serum HMW/Total adiponectin ratios between women with normal pregnancies and patients with spontaneous preterm labor.
The median maternal serum HMW/Total adiponectin ratio was lower in patients with preterm labor than in those with a normal pregnancy. Among women with preterm labor, patients with IAI had the lowest median maternal HMW/Total adiponectin ratio.
The median maternal HMW/total adiponectin ratio was lower in patients with preterm labor without IAI who delivered either preterm or at term than in those with a normal pregnancy (p<0.001 for both comparisons, Figure 5). There was no significant difference in the median maternal HMW/total total adiponectin ratio between patients with preterm labor without IAI who delivered preterm and those with preterm labor without IAI who delivered at term (p=0.46).
MMW/total adiponectin ratio in preterm labor vs. normal pregnancy
The median maternal MMW/total adiponectin ratio did not differ significantly between patients with preterm labor and IAI and those with preterm labor without IAI who delivered either preterm (0.24, IQR 0.20–0.31 vs. 0.26, IQR 0.17–0.29; p=0.8, Figure 6) or at term (0.23, IQR 0.16–0.27; p=0.3, Figure 6). In contrast, patients with preterm labor and IAI had a higher median MMW/total adiponectin ratio than those with a normal pregnancy (0.24, IQR 0.20–0.31vs. 0.22, IQR 0.17–0.26; p=0.01, Figure 6).
Figure 6. Comparison of the median maternal serum MMW/Total adiponectin ratio between women with normal pregnancies and patients with spontaneous preterm labor.
The median maternal serum MMW/Total adiponectin ratio was higher in patients with preterm labor and IAI than in those with a normal pregnancy. The MMW/Total adiponectin ratio did not differ significant among the preterm labor groups.
The median maternal MMW/total adiponectin ratio did not differ significantly between patients with preterm labor without IAI who delivered either preterm or at term than in those with a normal pregnancy (p=0.1 and p=0.4, respectively; Figure 6). There was no significant difference in the median maternal MMW/total total adiponectin ratio between patients with preterm labor without IAI who delivered preterm and those with preterm labor without IAI who delivered at term (p=0.4).
LMW/total adiponectin ratio in preterm labor vs. normal pregnancy
The median maternal LMW/total adiponectin ratio did not differ significantly between patients with preterm labor and IAI than in those with preterm labor without IAI who delivered either preterm (0.32, IQR 0.25–0.37 vs. 0.28, IQR 0.23–0.38; p=0.3, Figure 7) or at term (0.30, IQR 0.20–0.43; p=0.4, Figure 7). In contrast, patients with preterm labor and IAI had a higher median LMW/total adiponectin ratio than those with a normal pregnancy (0.32, IQR 0.25–0.37 vs. 0.20, IQR 0.13–0.28; p<0.001, Figure 7).
Figure 7. Comparison of the median maternal serum LMW/Total adiponectin ratio between women with normal pregnancies and patients with spontaneous preterm labor.
The median maternal serum LMW/Total adiponectin ratio was higher in patients with preterm labor than in those with a normal pregnancy. LMW/Total adiponectin ratio did not differ significant among the preterm labor groups.
The median maternal LMW/total adiponectin ratio was higher in patients with preterm labor without IAI who delivered either preterm or at term than that of those with a normal pregnancy (p<0.001 for both comparisons, Figure 7). There was no significant difference in the median maternal LMW/total total adiponectin ratio between patients with preterm labor without IAI who delivered preterm and those with preterm labor without IAI who delivered at term (p=0.6).
Multiple regression analysis was employed to examine the relationship between the serum concentrations of adiponectin isoforms and preterm labor while adjusting for maternal age, maternal BMI at the first trimester, gestational age at blood sampling, and birthweight. The final regression model suggested that an episode of preterm labor and first trimester BMI was independently associated with a lower maternal serum total adiponectin (p<0.001 and p=0.005, respectively), and HMW adiponectin concentrations (p<0.001 and p=0.001, respectively), as well as with a lower HMW/total adiponectin ratio (p<0.001 and p=0.008, respectively). In addition, only the presence of preterm labor was independently associated with higher maternal LMW/total adiponectin ratio (p<0.001) compared with normal pregnancies.
Discussion
Principal findings of the study
1) Preterm labor leading to preterm delivery or an episode of preterm labor which does not lead to preterm delivery, was associated with a lower median maternal serum concentration of total and HMW adiponectin, a lower median HMW/total adiponectin ratio, and a higher median LMW/total adiponectin ratio than normal pregnancy; 2) among patients with preterm labor, those with IAI had the lowest median concentration of total and HMW adiponectin, as well as the lowest median HMW/total adiponectin ratio; 3) The changes in adiponectin and adiponectin multimers remained significant after adjusting for confounding factors such as maternal age, BMI, gestational age at sampling, and parity.
Metaflammation - the intricate interface between metabolism and inflammation
An emerging theme in modern biology is that adipose tissue can orchestrate metabolic responses to injury, and also inflammatory responses. The first description of a molecular link between adipose tissue and inflammation was made by Hotamisligil et al.138 who demonstrated an over-expression of TNF-α, a pro-inflammatory cytokine, in obese rodents. Subsequently, unequivocal experimental, clinical and epidemiological evidence has strongly supported a causal link between adipose tissue and the inflammatory response: 1) adipose tissue is a crucial site for the production of inflammatory mediators such as TNF-α,139 IL-6,140 monocyte chemoattractant protein (MCP)-1,141;142 C-reactive protein (CRP),143;144 serum amyloid A145 and plasminogen activator inhibitor-1 (PAI-1);146 2) adipocytokines such as resistin,111;147 visfatin,51;112;113 and adipsin148 have been implicated in the regulation of the innate immune responses. Other adipocytokines such as leptin117;149 and adiponectin150;151 have been shown to have an effect on both the innate and adaptive limbs of the immune system; 3) knockout mice for IL-6,152 TNF-α,153 PAI-1,154 IL-18,155 IL-1α,156 MCP-1,157 JNK1158 are often obese or have a metabolic phenotype related to obesity (e.g. insulin resistance or improved insulin sensitivity); 4) adipose tissue in obese individuals is characterized by macrophage infiltration.159;160 Moreover, adipose tissue-resident macrophages are a source of pro-inflammatory mediators that can regulate the secretory activity of adipocytes;161 and 5) obese patients have higher circulating pro-inflammatory and acute phase reactant adipocytokines such as TNF-α,162 IL-6,163 and CRP140 than non-obese individuals. Furthermore, weight loss is associated with low serum CRP,164;165 IL-6166 and circulating serum amyloid A.145
It is important to note that the classic features of inflammation: calor (heat), dolor (pain), rubor, (redness) tumor (swelling) and function laesa (impaired function)167 are not necessarily present in the inflammatory response observed with an increased deposition of fat or adipose tissue. Indeed, the term “Metaflammation”168 (metabolically triggered inflammation) was coined to note the unique characteristics of the inflammatory response associated with metabolic derangements, such as obesity or insulin resistance. In contrast to the often short-term, adaptive response of “classical” inflammation, which is crucial for tissue repair, metaflammation is chronic, triggered by nutrient surplus and has detrimental long-term effects. Importantly, both processes engage a similar set of molecules and signaling pathways.161;169 The terms “subclinical,” “low-grade,” and “chronic” inflammation are being used interchangeably in the context of obesity.
The two major roles of adiponectin: regulation of metabolic responses and/or inflammation
While a large body of evidence supports a causal relationship between obesity and inflammation, the precise mechanisms through which this regulation occurs have not been yet completely characterized. One plausible mechanism involves adipocytokines.94;151;170;171 Adiponectin is the adipocytokine83;118–120 that circulates at the highest concentrations.101;118;172–177 In addition to its well-described role in governing energy homeostasis,122–124;178;179 adiponectin has anti-inflammatory properties. Several lines of evidence support the immunoregulatory and anti-inflammatory effects of adiponectin: 1) adiponectin suppresses macrophage production of pro-inflammatory cytokines such as TNF-α,151;180 IFN-γ,150 and IL-6;181 2) adiponectin induces production of anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonist150;180;182 by human monocytes, macrophages and dendritic cells; 3) exposure of cultured macrophages to adiponectin results in inhibition of their phagocytic activity in response to stimulation with LPS;151 4) the presence of adiponectin in T-cell proliferation assays results in a decreased ability to evoke an allogenic T-cell response;150 5) adiponectin inhibits activation of the nuclear transcription factor NF-κB in endothelial cells;171 6) adiponectin prevents LPS-induced hepatic injury by inhibiting the synthesis and/or release of TNF-α;183 7) adiponectin knockout mice have higher levels of TNF-α mRNA expression in adipose tissue, as well as higher circulating TNF-α concentrations than adiponectin-sufficient mice;179 8) alterations in adiponectin concentrations have been reported in the presence of systemic inflammatory conditions such as in the serum of overweight/obese individuals,84;104;118 those with insulin resistance, 124;178 systemic lupus erythematosus,184;185 in the intestinal adipose tissue of patients with Crohn’s disease,186;187 and in the synovial fluid97 and circulation188 of patients with rheumatoid arthritis.
Adiponectin multimers: a new paradigm for hormonal regulation
Adiponectin undergoes post-translational modifications before its secretion by adipocytes. Distinct multimeric forms include: LMW trimers, MMW hexamers, and HMW oligomers (12 to 18 subunits).129;130;189 These isoforms have different biological effects, and it has been proposed that the concentrations of each multimeric isoforms, as well as their relative abundance, regulate the pleiotropic effects of adiponectin. This view is supported by the following findings: 1) in vitro, HMW and MMW adiponectin induce increased secretion of the pro-inflammatory cytokines, IL-6190 and IL-8190;191 by monocytes; 2) in contrast, exposure of monocyte to LMW adiponectin results in increased secretion of the anti-inflammatory cytokine IL-10 192 and a decreased release of IL-6;180;181 3) treatment with HMW adiponectin, but not with LMW adiponectin, results in a dose dependent reduction in serum glucose concentrations193 in adiponectin knockout mice; 4) weight reduction,194 refeeding of patients with anorexia nervosa177 and treatment with thiazolidineone193 are characterized by improved insulin resistance and by marked and specific elevation of HMW; and 5) LMW adiponectin activates AMP-activated protein kinase (AMPK),132 whereas HMW and MMW activate NF-κB.132 Collectively, these findings suggest a differential biological effect for adiponectin multimeric complexes. Moreover, these reports highlight the importance of evaluating adiponectin multimers and their relative distribution in order to elucidate the role of adiponectin in physiologic and pathologic conditions.
Adipokines, inflammation and human pregnancy
Several lines of evidence suggest that adipokines play an important role in normal pregnancy and in pregnancy complications: 1) normal pregnancy is associated with alterations in circulating adiponectin,104;107;173–176 resistin,105 visfatin109 and other adipocytokines; 2) circulating maternal adiponectin correlates with insulin resistance indices during pregnancy;77;195–197 3) gestational diabetes mellitus (GDM) is associated with higher maternal concentrations of leptin,198 CRP,197 TNF-α,199 resistin200 and visfatin108;201;202 than non diabetic pregnant women. In addition, patients with GDM have lower concentrations of adiponectin than normal pregnant women;196;203–207 4) overweight pregnant women have a lower plasma concentration of adiponectin104;107 and a higher concentration of leptin208 than non-obese pregnant women; 5) preeclampsia is associated with higher concentrations of leptin,209 visfatin,210 and TNF-α,211 as well as lower concentrations of resistin212 and altered maternal circulating adiponectin;106;213–215 and 6) IAI is associated with higher amniotic fluid concentrations of visfatin51 and resistin.111 The aforementioned reports support for a role for adipocytokines in metabolic- and inflammatory phenomenon observed in complications of pregnancy.
Preterm labor is characterized by quantitative and qualitative changes in adiponectin multimers
Preterm labor was associated with low maternal concentrations of total adiponectin, HMW adiponectin, MMW adiponectin, as well as a low HMW/total adiponectin ratio and a high LMW/total adiponectin ratio. Of note, these findings were consistent across all preterm labor groups regardless of preterm delivery or the presence of IAI. To date, there is no data concerning the association between primary adipocytokines (those who are produced primarily by adipose tissue) and preterm labor. We have previously reported higher HMW adiponectin concentrations and higher HMW/total adiponectin ratios in normal pregnant women in labor.107 Thus, the lower serum concentration of adiponectin multimers in patients with preterm labor may be attributed to the process of preterm parturition, and not labor per se. In this regard, term and preterm labor would appear to be different.
Adipocytes control the production of adiponectin multimers. Hence, the findings of the current study indicate that even a single episode of preterm labor which does not result in preterm delivery is associated with changes in the profile of maternal adipocytokines. Similar findings were observed in preterm labor which leads to preterm delivery regardless of the presence or absence of intra-amniotic inflammation/infection. The explanation for the observations reported in this study requires further investigation.
Why is preterm labor a state of dysregulation of adiponectin multimers?
Patients with preterm labor had a significantly lower median maternal serum concentration of total adiponectin than that of women with normal pregnancies. This finding is due to a selective lowering in the serum concentrations of HMW and MMW isoforms (indeed, LMW/total adiponectin ratio was higher). This observation is important because some metabolic effects of adiponectin, such as the insulin sensitizing effect, are mediated by the HMW isoform.
Whether these changes in serum adiponectin isoforms concentrations are a cause or a consequence of preterm labor cannot be discerned by this study because of its cross-sectional nature. One possibility is that an episode of preterm labor imposes metabolic challenges to both hosts (mother and/or fetus). For example, the mother requires more fuel to maintain increased uterine contractility and the fetus may have its own increased requirements in preparation for birth. The changes in maternal serum adiponectin multimers reported herein favor a state of insulin resistance which enhances the availability of glucose, the major metabolic fuel.
Differences between spontaneous labor at term and preterm labor: why?
Spontaneous labor at term is characterized by higher maternal serum concentrations of the high molecular weight and HMW/total adiponectin ratio.107 The opposite is the case for preterm labor. What is the explanation for this paradox?
One view of pregnancy is that energy must be employed first to support the growth of the conceptus, and then when the fetus reaches maturity and cannot be sustained in utero by the placenta and/or mother, energy utilization is shifted towards the execution of parturition. In contrast, preterm parturition is the result of a pathologic process which may affect adipose tissue function, directly or indirectly. The response of adipose tissue to such a pathological insult is translated by a change in the concentrations of adiponectin multimers, but such a change is not observed in normal labor because there is no adipose tissue dysfunction in normal labor.
In conclusion, the present study is the first to examine maternal serum adiponectin multimers concentrations in patients with preterm labor. Since adiponectin multimers are exclusively produced by adipocytes, the findings reported herein indicating a change in adiponectin multimers in maternal serum lead us to suggest that a form of adipose tissue dysregulation occurs in preterm labor. This observation is novel because it suggests that adipokines may play a role in preterm parturition, and provides a molecular basis for the excess rate of spontaneous preterm birth in extremely lean and obese patients. The implication of this is that changes in lifestyle and pharmacologic interventions which target adipose tissue may be of value in the prevention of preterm birth, in a subset of patients, and that there may be molecular markers to assist with monitoring response. Further studies are required to test the set of hypotheses that derive from our observations.
Acknowledgments
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 2.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 6.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 7.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. 2004:28–60. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113 (Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F, Mittal P, Than NG, Espinoza J, Hassan SS. Recurrent preterm birth. Semin Perinatol. 2007;31:142–158. doi: 10.1053/j.semperi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, Davis JK. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10:294–302. [PubMed] [Google Scholar]
- 15.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol. 1987;157:815–819. doi: 10.1016/s0002-9378(87)80061-3. [DOI] [PubMed] [Google Scholar]
- 16.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, Hobbins JC. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–666. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 18.Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, Mazor M, Brekus CA, Hobbins JC. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990;163:757–761. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann NY Acad Sci. 1991;622:355–375. doi: 10.1111/j.1749-6632.1991.tb37880.x. [DOI] [PubMed] [Google Scholar]
- 20.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 21.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111–117. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell MC, Cotton DB. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–1091. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, Parra M, Behnke E, Montiel F, Cassell GH. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–133. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, Bracken MB. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 25.Saito S, Kato Y, Ishihara Y, Ichijo M. Amniotic fluid granulocyte colony-stimulating factor in preterm and term labor. Clin Chim Acta. 1992;208:105–109. doi: 10.1016/0009-8981(92)90027-n. [DOI] [PubMed] [Google Scholar]
- 26.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–210. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 28.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995;172:1598–1603. doi: 10.1016/0002-9378(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med. 1995;40:375–379. [PubMed] [Google Scholar]
- 31.Mazor M, Hershkovitz R, Ghezzi F, Maymon E, Horowitz S, Leiberman JR. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand. 1996;75:624–627. doi: 10.3109/00016349609054686. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. 1997:29–49. [Google Scholar]
- 33.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 35.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, Janisse J, Mazor M. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 36.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 37.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, Jun JK. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–1137. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH, Berry SM. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol. 2000;183:1070–1077. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 39.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 40.Challis JR, Lye SJ, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann NY Acad Sci. 2001;943:225–234. doi: 10.1111/j.1749-6632.2001.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 41.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, Jun JK. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–1167. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 42.Gibbs RS, Davies JK, McDuffie RS, Jr, Leslie KK, Sherman MP, Centretto CA, Wolf DM. Chronic intrauterine infection and inflammation in the preterm rabbit, despite antibiotic therapy. Am J Obstet Gynecol. 2002;186:234–239. doi: 10.1067/mob.2002.119640. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 44.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, Jun JK. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 45.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA, Mitchell MD. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biology of Reproduction. 2004;70:253–259. doi: 10.1095/biolreprod.103.016204. [DOI] [PubMed] [Google Scholar]
- 47.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil. Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 48.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, Adashi EY. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 50.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 51.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, Than NG, Nhan-Chang CL, Hamill N, Vaisbuch E, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008;36:485–496. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fidel PL, Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 55.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 56.Gilles HM, Lawson JB, Sibelas M, Voller A, Allan N. Malaria, anaemia and pregnancy. Ann Trop Med Parasitol. 1969;63:245–263. doi: 10.1080/00034983.1969.11686625. [DOI] [PubMed] [Google Scholar]
- 57.Herd N, Jordan T. An investigtion of malaria during pregnancy in Zimbabwe. Afr J Med. 1981;27:62. [PubMed] [Google Scholar]
- 58.Wren BG. Subclinical renal infection and prematurity. Med J Aust. 1969;2:596–600. doi: 10.5694/j.1326-5377.1969.tb107290.x. [DOI] [PubMed] [Google Scholar]
- 59.Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: A clinical review. Obstet Gynecol. 1973;42:112–117. [PubMed] [Google Scholar]
- 60.Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun. 1999;67:5958–5966. doi: 10.1128/iai.67.11.5958-5966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benedetti TJ, Valle R, Ledger WJ. Antepartum pneumonia in pregnancy. Am J Obstet Gynecol. 1982;144:413–417. doi: 10.1016/0002-9378(82)90246-0. [DOI] [PubMed] [Google Scholar]
- 62.Madinger NE, Greenspoon JS, Ellrodt AG. Pneumonia during pregnancy: has modern technology improved maternal and fetal outcome? Am J Obstet. Gynecol. 1989;161:657–662. doi: 10.1016/0002-9378(89)90373-6. [DOI] [PubMed] [Google Scholar]
- 63.Khader YS, Ta’ani Q. Periodontal diseases and the risk of preterm birth and low birth weight: A meta-analysis. Journal of Periodontology. 2005;76:161–165. doi: 10.1902/jop.2005.76.2.161. [DOI] [PubMed] [Google Scholar]
- 64.Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, Mauriello SM, Moss KL, Beck JD. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107:29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- 65.Johnson MJ, Petri M, Witter FR, Repke JT. Evaluation of preterm delivery in a systemic lupus erythematosus pregnancy clinic. Obstet Gynecol. 1995;86:396–399. doi: 10.1016/0029-7844(95)00186-U. [DOI] [PubMed] [Google Scholar]
- 66.Le HD, Wechsler B, Vauthier-Brouzes D, Seebacher J, Lefebvre G, Bletry O, Darbois Y, Godeau P, Piette JC. Outcome of planned pregnancies in systemic lupus erythematosus: a prospective study on 62 pregnancies. Br J Rheumatol. 1997;36:772–777. doi: 10.1093/rheumatology/36.7.772. [DOI] [PubMed] [Google Scholar]
- 67.Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol. 2002;97:641–648. doi: 10.1111/j.1572-0241.2002.05543.x. [DOI] [PubMed] [Google Scholar]
- 68.Norgard B, Fonager K, Pedersen L, Jacobsen BA, Sorensen HT. Birth outcome in women exposed to 5-aminosalicylic acid during pregnancy: a Danish cohort study. Gut. 2003;52:243–247. doi: 10.1136/gut.52.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elbaz G, Fich A, Levy A, Holcberg G, Sheiner E. Inflammatory bowel disease and preterm delivery. Int J Gynaecol Obstet. 2005;90:193–197. doi: 10.1016/j.ijgo.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Kallen B, Rydhstroem H, Aberg A. Asthma during pregnancy--a population based study. Eur J Epidemiol. 2000;16:167–171. doi: 10.1023/a:1007678404911. [DOI] [PubMed] [Google Scholar]
- 71.Dombrowski MP, Schatz M, Wise R, Momirova V, Landon M, Mabie W, Newman RB, McNellis D, Hauth JC, Lindheimer M, et al. Asthma during pregnancy. Obstet Gynecol. 2004;103:5–12. doi: 10.1097/01.AOG.0000103994.75162.16. [DOI] [PubMed] [Google Scholar]
- 72.Adams MM, Sarno AP, Harlass FE, Rawlings JS, Read JA. Risk factors for preterm delivery in a healthy cohort. Epidemiology. 1995;6:525–532. doi: 10.1097/00001648-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Nohr EA, Bech BH, Vaeth M, Rasmussen KM, Henriksen TB, Olsen J. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2007;21:5–14. doi: 10.1111/j.1365-3016.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 74.Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of Body Mass Index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168. doi: 10.1186/1471-2458-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann NY Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 76.Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 77.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, Hauguel-De MS. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 78.Vidal H. Gene expression in visceral and subcutaneous adipose tissues. Ann Med. 2001;33:547–555. doi: 10.3109/07853890108995965. [DOI] [PubMed] [Google Scholar]
- 79.Wang B, Jenkins JR, Trayhurn P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E731–E740. doi: 10.1152/ajpendo.00475.2004. [DOI] [PubMed] [Google Scholar]
- 80.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 81.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 82.Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, Jebb SA, Lip GY, O’Rahilly S. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–35. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 83.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 84.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 85.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 86.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 87.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 89.Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O’Rahilly S. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 90.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 91.Hug C, Lodish HF. Medicine. Visfatin: a new adipokine Science. 2005;307:366–367. doi: 10.1126/science.1106933. [DOI] [PubMed] [Google Scholar]
- 92.Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol. Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 95.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114:254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 96.Tuzun A, Uygun A, Yesilova Z, Ozel AM, Erdil A, Yaman H, Bagci S, Gulsen M, Karaeren N, Dagalp K. Leptin levels in the acute stage of ulcerative colitis. J Gastroenterol Hepatol. 2004;19:429–432. doi: 10.1111/j.1440-1746.2003.03300.x. [DOI] [PubMed] [Google Scholar]
- 97.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Scholmerich J, Muller-Ladner U, Gay S. Adipocytokines in synovial fluid. JAMA. 2003;290:1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 98.Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C. Delayed resolution of acute inflammation during zymosan-induced arthritis in leptin-deficient mice. Arthritis Res Ther. 2004;6:R256–R263. doi: 10.1186/ar1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matarese G, Sanna V, Di GA, Lord GM, Howard JK, Bloom SR, Lechler RI, Fontana S, Zappacosta S. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol. 2001;31:1324–1332. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 100.Batocchi AP, Rotondi M, Caggiula M, Frisullo G, Odoardi F, Nociti V, Carella C, Tonali PA, Mirabella M. Leptin as a marker of multiple sclerosis activity in patients treated with interferon-beta. J Neuroimmunol. 2003;139:150–154. doi: 10.1016/s0165-5728(03)00154-1. [DOI] [PubMed] [Google Scholar]
- 101.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 102.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 103.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 104.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J Perinat Med. 2007;35:522–531. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Pineles BL, Friel LA, Espinoza J, Goncalves L, et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J Perinat Med. 2007;35:513–521. doi: 10.1515/JPM.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Adiponectin in severe preeclampsia. J Perinat Med. 2007;35:503–512. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, Mittal P, Than GN, Nhan-Chang C, Chaiworapongsa T, et al. Adiponectin multimers in maternal plasma. J Matern Fetal Neonatal Med. 2008;21:796–815. doi: 10.1080/14767050802266881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than GN, Chaiworapongsa T, Nhan-Chang C, Pacora P, Gotsch F, et al. Maternal visfatin concentration in normal pregnancy. J Perinat Med. 2009;37:206–217. doi: 10.1515/JPM.2009.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than GN, Chaiworapongsa T, Nhan-Chang C, Pacora P, Gotsch F, et al. Visfatin in human pregnancy: maternal gestational diabetes vis-a-vis neonatal birthweight. J Perinat Med. 2009;37:218–231. doi: 10.1515/JPM.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 111.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 113.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 114.Zhao T, Hou M, Xia M, Wang Q, Zhu H, Xiao Y, Tang Z, Ma J, Ling W. Globular adiponectin decreases leptin-induced tumor necrosis factor-alpha expression by murine macrophages: involvement of cAMP-PKA and MAPK pathways. Cell Immunol. 2005;238:19–30. doi: 10.1016/j.cellimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 116.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 118.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 119.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem (Tokyo) 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 120.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 121.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 123.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 124.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 125.Okamoto Y, Arita Y, Nishida M, Muraguchi M, Ouchi N, Takahashi M, Igura T, Inui Y, Kihara S, Nakamura T, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 126.Kazumi T, Kawaguchi A, Sakai K, Hirano T, Yoshino G. Young men with high-normal blood pressure have lower serum adiponectin, smaller LDL size, and higher elevated heart rate than those with optimal blood pressure. Diabetes Care. 2002;25:971–976. doi: 10.2337/diacare.25.6.971. [DOI] [PubMed] [Google Scholar]
- 127.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 128.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 129.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 130.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 131.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 132.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 134.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 135.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 136.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 137.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 138.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 140.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 142.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL, Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 143.Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ. 1996;312:1061–1065. doi: 10.1136/bmj.312.7038.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii S. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation. 1996;93:106–110. doi: 10.1161/01.cir.93.1.106. [DOI] [PubMed] [Google Scholar]
- 146.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 147.White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, Flier JS, Spiegelman BM. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267:9210–9213. [PubMed] [Google Scholar]
- 148.Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci USA. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 150.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 151.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 152.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 153.Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 154.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van KH, Kim SH, Stalenhoef AF, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 155.Matsuki T, Horai R, Sudo K, Iwakura Y. IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J Exp Med. 2003;198:877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 158.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 161.Dandona P, Weinstock R, Thusu K, bdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 162.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 163.Kopp HP, Krzyzanowska K, Mohlig M, Spranger J, Pfeiffer AF, Schernthaner G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (Lond) 2005;29:766–771. doi: 10.1038/sj.ijo.0802983. [DOI] [PubMed] [Google Scholar]
- 164.McLaughlin T, Abbasi F, Lamendola C, Liang L, Reaven G, Schaaf P, Reaven P. Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation. 2002;106:2908–2912. doi: 10.1161/01.cir.0000041046.32962.86. [DOI] [PubMed] [Google Scholar]
- 165.Kopp HP, Kopp CW, Festa A, Krzyzanowska K, Kriwanek S, Minar E, Roka R, Schernthaner G. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23:1042–1047. doi: 10.1161/01.ATV.0000073313.16135.21. [DOI] [PubMed] [Google Scholar]
- 166.Ley K. Physiology of Inflammation. 2001:1–10. [Google Scholar]
- 167.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 168.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 169.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 170.Bradley RL, Cleveland KA, Cheatham B. The adipocyte as a secretory organ: mechanisms of vesicle transport and secretory pathways. Recent Prog Horm Res. 2001;56:329–358. doi: 10.1210/rp.56.1.329. [DOI] [PubMed] [Google Scholar]
- 171.Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr Diab Rep. 2005;5:278–281. doi: 10.1007/s11892-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 172.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Wiser A, Schiff E, Sivan E. Maternal serum adiponectin levels during human pregnancy. J Perinatol. 2007;27:77–81. doi: 10.1038/sj.jp.7211639. [DOI] [PubMed] [Google Scholar]
- 173.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, Shoham A, Sivan E. Determining the source of fetal adiponectin. J Reprod Med. 2007;52:774–778. [PubMed] [Google Scholar]
- 174.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, Kanety H. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88:5656–5660. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 175.Modan-Moses D, Stein D, Pariente C, Yaroslavsky A, Ram A, Faigin M, Loewenthal R, Yissachar E, Hemi R, Kanety H. Modulation of adiponectin and leptin during refeeding of female anorexia nervosa patients. J Clin Endocrinol Metab. 2007;92:1843–1847. doi: 10.1210/jc.2006-1683. [DOI] [PubMed] [Google Scholar]
- 176.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 178.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 179.Schober F, Neumeier M, Weigert J, Wurm S, Wanninger J, Schaffler A, Dada A, Liebisch G, Schmitz G, Aslanidis C, et al. Low molecular weight adiponectin negatively correlates with the waist circumference and monocytic IL-6 release. Biochem Biophys Res Commun. 2007;361:968–973. doi: 10.1016/j.bbrc.2007.07.106. [DOI] [PubMed] [Google Scholar]
- 180.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 181.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 182.Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, Yung YC, Nagaraja HN. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–1833. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 183.Sada KE, Yamasaki Y, Maruyama M, Sugiyama H, Yamamura M, Maeshima Y, Makino H. Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J Rheumatol. 2006;33:1545–1552. [PubMed] [Google Scholar]
- 184.Paul G, Schaffler A, Neumeier M, Furst A, Bataillle F, Buechler C, Muller-Ladner U, Scholmerich J, Rogler G, Herfarth H. Profiling adipocytokine secretion from creeping fat in Crohn’s disease. Inflamm Bowel Dis. 2006;12:471–477. doi: 10.1097/00054725-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 185.Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, Ito T, Nezu R, Tsutsui S, Miyagawa JI, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut. 2005;54:789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, Okuno T, Inoue T, Node K, Tobe T, et al. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954–1960. doi: 10.2337/db05-1525. [DOI] [PubMed] [Google Scholar]
- 188.Abke S, Neumeier M, Weigert J, Wehrwein G, Eggenhofer E, Schaffler A, Maier K, Aslanidis C, Scholmerich J, Buechler C. Adiponectin-induced secretion of interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1, CCL2) and interleukin-8 (IL-8, CXCL8) is impaired in monocytes from patients with type I diabetes. Cardiovasc Diabetol. 2006;5:17. doi: 10.1186/1475-2840-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rovin BH, Song H. Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol. 2006;120:99–105. doi: 10.1016/j.clim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 190.Neumeier M, Weigert J, Schaffler A, Wehrwein G, Muller-Ladner U, Scholmerich J, Wrede C, Buechler C. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 191.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 192.Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert MO, Mohlig M, Pfeiffer AF, Spranger J. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 193.Lopez-Bermejo A, Fernandez-Real JM, Garrido E, Rovira R, Brichs R, Genaro P, Bach C, Cabrero D, Kihara S, Funahashi T, et al. Maternal soluble tumour necrosis factor receptor type 2 (sTNFR2) and adiponectin are both related to blood pressure during gestation and infant’s birthweight. Clin Endocrinol (Oxf) 2004;61:544–552. doi: 10.1111/j.1365-2265.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- 194.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 195.McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22:131–138. doi: 10.1002/dmrr.591. [DOI] [PubMed] [Google Scholar]
- 196.Kautzky-Willer A, Pacini G, Tura A, Bieglmayer C, Schneider B, Ludvik B, Prager R, Waldhausl W. Increased plasma leptin in gestational diabetes. Diabetologia. 2001;44:164–172. doi: 10.1007/s001250051595. [DOI] [PubMed] [Google Scholar]
- 197.Kirwan JP, Hauguel-De MS, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 198.Chen D, Fang Q, Chai Y, Wang H, Huang H, Dong M. Serum resistin in gestational diabetes mellitus and early postpartum. Clin Endocrinol (Oxf) 2007;67:208–211. doi: 10.1111/j.1365-2265.2007.02862.x. [DOI] [PubMed] [Google Scholar]
- 199.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, Schernthaner G. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 200.Lewandowski KC, Stojanovic N, Press M, Tuck SM, Szosland K, Bienkiewicz M, Vatish M, Lewinski A, Prelevic GM, Randeva HS. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia. 2007;50:1033–1037. doi: 10.1007/s00125-007-0610-7. [DOI] [PubMed] [Google Scholar]
- 201.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–347. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 202.Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knofler M, Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191:2120–2124. doi: 10.1016/j.ajog.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 203.Thyfault JP, Hedberg EM, Anchan RM, Thorne OP, Isler CM, Newton ER, Dohm GL, deVente JE. Gestational diabetes is associated with depressed adiponectin levels. J Soc Gynecol Investig. 2005;12:41–45. doi: 10.1016/j.jsgi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 204.Tsai PJ, Yu CH, Hsu SP, Lee YH, Huang IT, Ho SC, Chu CH. Maternal plasma adiponectin concentrations at 24 to 31 weeks of gestation: negative association with gestational diabetes mellitus. Nutrition. 2005;21:1095–1099. doi: 10.1016/j.nut.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 205.Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, Cetin I, Cortelazzi R, Beck-Peccoz P, Spada A. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf) 2007;66:447–453. doi: 10.1111/j.1365-2265.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 206.Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, Cotton DB. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol. 2005;193:979–983. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 207.McCarthy JF, Misra DN, Roberts JM. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. Am J Obstet Gynecol. 1999;180:731–736. doi: 10.1016/s0002-9378(99)70280-2. [DOI] [PubMed] [Google Scholar]
- 208.Fasshauer M, Waldeyer T, Seeger J, Schrey S, Ebert T, Kratzsch J, Lossner U, Bluher M, Stumvoll M, Faber R, et al. Serum levels of the adipokine visfatin are increased in preeclampsia. Clin Endocrinol (Oxf) 2008;69:69–73. doi: 10.1111/j.1365-2265.2007.03147.x. [DOI] [PubMed] [Google Scholar]
- 209.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 210.Chen D, Dong M, Fang Q, He J, Wang Z, Yang X. Alterations of serum resistin in normal pregnancy and pre-eclampsia. Clin Sci (Lond) 2005;108:81–84. doi: 10.1042/CS20040225. [DOI] [PubMed] [Google Scholar]
- 211.Haugen F, Ranheim T, Harsem NK, Lips E, Staff AC, Drevon CA. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab. 2006;290:E326–E333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- 212.Ramsay JE, Jamieson N, Greer IA, Sattar N. Paradoxical elevation in adiponectin concentrations in women with preeclampsia. Hypertension. 2003;42:891–894. doi: 10.1161/01.HYP.0000095981.92542.F6. [DOI] [PubMed] [Google Scholar]
- 213.Kajantie E, Kaaja R, Ylikorkala O, Andersson S, Laivuori H. Adiponectin concentrations in maternal serum: elevated in preeclampsia but unrelated to insulin sensitivity. J Soc Gynecol Investig. 2005;12:433–439. doi: 10.1016/j.jsgi.2005.04.006. [DOI] [PubMed] [Google Scholar]