Abstract
PROBLEM
Spontaneous labor at term involves leukocyte recruitment and infiltration into the choriodecidua; yet, characterization of these leukocytes and their immunological mediators is incomplete. The purpose of this study was to characterize the immunophenotype of choriodecidual leukocytes as well as the expression of inflammatory mediators in human spontaneous parturition at term.
METHOD OF STUDY
Choriodecidual leukocytes were analyzed by FACS, immunohistochemistry, and RT-PCR in three different groups: (i) preterm gestation delivered for medical indications without labor; (ii) term pregnancy without labor; and (iii) term pregnancy after spontaneous labor.
RESULTS
Two T-cell subsets of memory-like T cells (CD3+CD4+CD45RO+ and CD3+CD4−CD8−CD45RO+ cells) were identified in the choriodecidua of women who had spontaneous labor. Evidence for an extensive immune signaling network composed of chemokines (CXCL8 and CXCL10), chemokine receptors (CXCR1-3), cytokines (IL-1β and TNF-α), cell adhesion molecules, and MMP-9 was identified in these cells during spontaneous labor at term.
CONCLUSIONS
The influx of memory-like T cells in the choriodecidua and the evidence that they are active by producing chemokines and cytokines, and expressing chemokine receptors, cell adhesion molecules, and a matrix-degrading enzyme provides support for the participation of the adaptive immune system in the mechanisms of spontaneous parturition at term.
Keywords: Chemokines, choriodecidua, chorion, cytokines, decidua, labor, leukocytes, memory T cells, pregnancy, T cells
INTRODUCTION
Human parturition is characterized by an inflammatory response that has been demonstrated in the cervix,1–11 myometrium,9,10,12–14 and choriodecidua.9,10,12,15–22 Indeed, leukocyte infiltration has been demonstrated in all these tissues in humans.1–22 Moreover, an unbiased analysis of gene expression (transcriptome) of these tissues has also demonstrated that labor at term is associated with an inflammatory signature, as there is enrichment of gene ontology categories associated with inflammation.23–32 Importantly, neutrophil chemokines and other inflammatory mediators are over-expressed in the chorioamniotic membranes, even in the absence of leukocyte infiltration (histologic chorioamnionitis).23,24,32 Similarly, an inflammatory signature has been observed in the myometrium of women in early labor without histologic evidence of inflammation of the chorioamniotic membranes.31 In contrast, in the uterine cervix, degradation of extracellular matrix appears to be the key process for ripening25,26,29,30 and inflammation is involved in cervical dilatation during labor after ripening has occurred, as well as postpartum repair.27,33,34
The choriodecidua is strategically located, as it represents an area of direct contact between maternal (decidua) and fetal tissues (chorion or trophoblast). The areas of contact are (i) the decidua parietalis, which lines the uterine cavity not covered by the placenta, and which is in juxtaposed to the chorion laeve, and (ii) the decidua basalis, which is in the basal plate of the placenta and is invaded by interstitial trophoblast.35 The intimacy of these areas of contact creates the conditions for fetal antigenic exposure to the maternal immune system.36–43 Tolerance of the fetal semi-allograft requires modulation of the local immune response for successful reproduction.39–42,44–57 Rejection of the semi-allograft has been implicated as a mechanism of disease in pregnancy complications, such as recurrent spontaneous abortion, preterm labor, preeclampsia, and fetal death58–69; however, the precise mechanisms for both tolerance and maternal anti-fetal rejection are poorly understood.
The decidua is composed of typical stromal-type cells, glandular cells and leukocytes.70–73 The phenotype of decidual leukocytes during spontaneous labor at term has not been completely characterized, and the emphasis has been on the characterization of the cells of the innate limb of the immune response [neutrophils and macrophages].74–79 There is a paucity of information about cells of the adaptive immune response (T and B cells) in the decidua during labor. Previous studies conducted by our group and others have demonstrated the importance of the choriodecidual microenvironment in spontaneous parturition in humans, strengthening the role of the innate immune system in labor.17,19,21,22,80–83
Leukocyte recruitment appears to be the first step in the conditioning of this microenvironment as term approaches. We have proposed that activated leukocytes extravasate from the local circulation into the choriodecidua in preparation for labor.10,19,84–86 This is accomplished by selective chemotaxis of maternal peripheral leukocytes,19,21,22,86 and is mediated by specific chemokine expression, which results in the infiltration of neutrophils and macrophages.19,23,67,86–89 Once specific leukocyte subsets are recruited into the choriodecidua, they form clusters after expressing selective cell adhesion moelcules (CAMs).8,18,86,90 labor would result by the secretion of at least two waves of activating and effector molecules. Some of these activating molecules include autocrine and paracrine mediators such as pro-inflammatory cytokines, IL-1β (interleukin-1 beta), TNF-α (tumor necrosis factor-alpha), and chemokines such as CXCL8 (chemokine C-X-C motif ligand 8 or IL8) and CXCL10 (or IP-10).19,21,67,91–93 On the other hand, prostaglandins and MMPs (matrix metalloproteinases) act as effector molecules.16,94–102 Together, these molecules elicit local cell responses resulting in the amplification of signaling, the induction of myometrial contractions and extracellular matrix degradation in the cervix and fetal membranes, which promote spontaneous labor and, eventually, delivery.103–105
However, a fundamental question that remains unaddressed is whether the adaptive immune system is involved in physiologic parturition. The issue of whether the onset of labor represent ‘rejection’ of the semi-allograft has remained a speculation for decades.68,106,107
This study was conducted to examine the inflammatory microenvironment in the choriodecidua during spontaneous labor at term with a particular focus on the adaptive immune response. Specifically, we aimed to (i) determine the number of phenotype of the infiltrating leukocytes, (ii) identify key chemokines and receptors, and CAMs participating in the leukocyte recruitment/homing, and (iii) analyze the association of these infiltrating leukocytes with the inflammatory microenvironment found in the choriodecidua during spontaneous labor at term pregnancy.
MATERIALS AND METHODS
PATIENTS AND TISSUES
Fetal membranes (amnion and choriodecidua) were collected during indicated cesarean deliveries from women in the following groups: (i) preterm gestation with indications for preterm delivery (designated as preterm gestation group or PTG, 32.9 ± 2.4 weeks, n = 5); (ii) term gestation not in labor (group TNL), undergoing cesarean delivery for obstetrical indications such as a previous cesarean delivery (38.4 ± 1.1 weeks, n = 7); and (iii) term gestation who underwent spontaneous labor and delivered vaginally without complications (group TL, 39.6 ± 0.31 weeks, n = 6).
Samples were excluded from the study if there was microbiological or clinical evidence of cervicovaginal or intrauterine infection. Inflammation of the chorioamniotic membranes was identified by the presence of a massive polymorphonuclear infiltration and a positive culture for microorganisms. Cultures were performed by rolling a Dacron swab on the surface of the membranes. The swabs were cultured onto blood agar plates under aerobic and anaerobic conditions. Women included in this study belonged to the same ethnic group (Mexican mestizo) and were primiparous. None of these women received oxytocin, antibiotics, or immunosuppressants.
This study was approved by the IRB of the Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes in Mexico City, Mexico. Written informed consistent was obtained from each patient prior to inclusion in the study. The IRB has a Federal Wide Assurance. This study was considered exempt for review by the IRB of Wayne State University.
ISOLATION OF CHORIODECIDUAL LEUKOCYTES
Fetal membranes were washed and immediately placed in sterile saline solution to eliminate blood clots. Choriodecidual leukocyte suspensions were prepared by scraping the choriodecidua using a plastic cell scraper (Corning Incorporated, Life Sciences, Lowell, MA, USA).72 The material was then suspended in 1 mL of 1x PBS (Bio-Rad Laboratories, Hercules, CA, USA) + 0.5% bovine serum albumin + 2 mM ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, St. Louis, MO, USA) and filtered with a MACS pre-separation filter (30 μm) (Miltenyi Biotec, Auburn, CA, USA). Choriodecidual leukocyte suspensions were centrifuged at 300 × g for 10 min and resuspended in 80 μL of 1 x PBS. Finally, 20 μL of anti-CD45 MAb coupled with MACS magnetic beads (Miltenyi Biotec) were added, mixed, and incubated for 20 min at 4 °C. Choriodecidual leukocytes (CD45+ cells) were purified under MS MACS columns and magnetic cell sorting (Miltenyi Biotec). Viability (90–95%) of leukocytes was assessed with the trypan blue exclusion assay.
QUANTIFICATION OF CHORIODECIDUAL LEUKOCYTES
Prior to isolating the choriodecidual leukocytes, fetal membranes from each group of women were spread and measured according to the details described in Fig. S1A. The area of the fetal membranes was calculated following the description of Fig. S1A. Choriodecidual leukocytes were isolated and counted with an automatic cell counter (AC•T 5diff CP Hematology Analyzer; Beckman Coulter, Brea, CA, USA).
PHENOTYPE OF CHORIODECIDUAL LEUKOCYTES
Purified choriodecidual leukocytes were resuspended in 100 μL of 1 x PBS and stained using conjugated monoclonal antibodies (10 μL each) for 15 min on ice, in the dark. The panel of antibodies used in this study is described in Table S1. Choriodecidual leukocytes were then fixed using 500 μL of OptiLyse B (Beckman Coulter), washed, and resuspended in 500 μL of 1 x PBS to be analyzed by flow cytometry (FC-500, Beckman Coulter). The phenotype of leukocytes was analyzed within the CD45+ and CD3+ region, respectively (Fig. S1B).
IMMUNOHISTOCHEMISTRY
Fetal membranes (amnion and choriodecidua) were cut into ~3 cm2 and washed gently in 1 x PBS. Tissues were fixed in 10% neutral-buffered formalin for about 24 hr, rinsed and stored in 70% ethanol. Tissues were then processed for paraffin embedding. Sections (5 μm) were mounted on silane adhesive coated glass slides (Becton Dickinson, Franklin Lakes, NJ, USA) and dried at 37 °C for 12 hr. Sections were blocked with 1 x PBS/1 mg/mL bovine serum albumin/10 mM NaN3 for 30 min prior to incubation with conjugated monoclonal antibodies at recommended concentrations for 1 hr at 37 °C. The phenotype of infiltrated leukocytes was determined using double labeling: CD45-FITC with either CD3-PC5, CD56-PE, CD14-PE-Texas Red or CD19-PC7 (Table S1). Subsets of T cells were localized in these tissues using triple labeling: CD3-FITC, CD4-PC5 and CD8-PE. We also tested whether these cells were naïve- or memory-like T cells using double labeling: CD4-FITC or CD8-PC5 with CD45RA0PE or CD45RO-PE (Table S1).
IL-1β, TNF-α and MMP-9 were also localized using double labeling with monoclonal antibodies at recommended concentrations (Table S1). In addition, we identified the leukocytes that produce MMP-9 using double labeling with the following monoclonal antibodies: MMP-9-FITC and CD45-PE or CD3-PC5 (Table S1).
Sections were finally washed in 1 x PBS containing 0.2% Triton (Sigma-Aldrich) (three buffer changes, 5 min each) and mounted with Vectashield mounting medium (Vector Laboratories, Pet, UK) for visualization using the LSM 510 MetaLaser confocal microscope (Carl Zeis, Herts, UK).
RNA ISOLATION AND cDNA SYNTHESIS
Purified choriodecidual leukocytes were placed in RNAlater (Ambion, Austin, TX, USA) and stored at −70 °C until further processing. Total RNA (ribonucleic acid) was isolated using Trizol reagent (Invitrogen, Grand Island, NY, USA) following the manufacturer’s protocol. Total RNA was quantified by spectrophotometry, and RNA integrity was verified by non-denaturing agarose gel electrophoresis. cDNA (complementary deoxyribonucleic acid) was synthesized with the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Mannheim, Germany), using random hexamer primers. Revers transcription reaction was carried out in Mastercycler Gradient equipment (Eppendorf, Hamburg, Germany) at 25 °C for 10 min, 55 °C for 30 min, and 85 °C for 5 min. cDNA was stored at −20 °C and was used the next day.
REAL-TIME PCR
Quantitative real-time PCR (polymerase chain reaction) was performed in a Light Cycler 2.0 instrument using Light Cycler TaqMan Master kit and TaqMan Probes following the manufacturer’s protocol (Roche Applied Science). Specific primers for mRNA (messenger RNA) sequences of different genes were designed using the ProbeFinder software accessible at www.universalprobelibrary.com (Table S2). ACTB (beta-actin) was used as a reference gene. All primers were designed to have intron spanning sequences, to avoid false positive signals from possible residual genomic DNA. Five hundred nanograms of sample cDNA were added to each reaction. Real-time PCR conditions were as follows: one cycle at 95 °C for 10 min and 45 cycles of denaturation (95 °C, 10 s), annealing (60 °C, 30 s), and extension (72 °C, 1 s). Relative quantification of each molecule was calculated with the Light Cycler Software 4 (Roche Applied Science).
STATISTICAL ANALYSIS
A Shapiro-Wilk test was performed to determine whether the data were normally distributed. ANOVA and post hoc tests were used when this was the case. Kruskal-Wallis tests were used, followed by Mann-Whitney U-tests, when the data were not normally distributed. Statistical analysis was performed using SPSS (IBM Corp, SPSS Inc, Chicago, Il, USA), version 18.0. A P-value of ≤ 0.05 was considered statistically significant.
RESULTS
T-CELL PROPORTIONS INCREASE IN THE CHORIODECIDUA DURING SPONTANEOUS LABOR
The leukocyte density was significantly higher in tissues from patients in term gestation than in preterm gestation (P = 0.03); however, there was no significant difference in leukocyte density before or after spontaneous labor at term (Fig. 1a). we then investigated whether the leukocyte subset proportions change between the 3 clinical groups. The proportion of T cells and monocytes were lower in tissues from women in preterm gestation than in term gestation not in labor (P < 0.0001 and 0.023). In contrast, granulocyte proportions were higher in tissues from women in preterm gestation than in term gestation not in labor (P < 0.0001). While the proportion of T cells was higher, the proportion of granulocytes was lower in tissues from women who had undergone spontaneous labor than in those who did not have labor (P = 0.006 and 0.03). Therefore, the proportion of T cells in the choriodecidua increase as a function of gestational age (Fig. 1 b).
Figure 1.
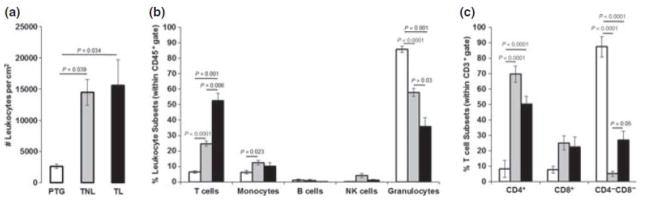
Number and phenotype of choriodecidual leukocytes. PTG, white; TNL, gray; TL, black bars. (a) Number of leukocytes per cm2 of tissue. Leukocyte density increased at term of pregnancy. (b) Leukocyte subsets were analyzed within the CD45+ gate. While T cells increased, granulocytes decreased from PTG to TL. Monocytes were greater in TNL than in PTG. (c) T-cell subsets were analyzed within the CD3+ gate. TNL and TL included a high proportion of CD4+ T cells. CD3+CD4−CD8− DN T cells were higher in TL cells were higher in TL than in TNL. Data shown are means ± SEM, with five to seven tissues per group.
CHORIODECIDUAL LEUKOCYTES INCLUDE CD4+ T CELLS AT TERM PREGNANCY AND ALSO CD4−CD8− T CELLS DURING SPONATENOUS LABOR AT TERM
Next, we investigated the phenotype of T cells in each clinical group. Within T cells, the proportion of CD4+ T cells were higher in tissues from women in term (regardless of whether they had undergone labor) than in preterm gestation (P < 0.0001 each). In contrast, the proportion of double-negative CD3+CD4−CD8− (DN) T cells were higher in tissues from patients in preterm gestation than in term gestation (P < 0.001 each). Importantly, the proportion of DN T cells were higher in the choriodecidua obtained from patients who had undergone spontaneous labor than in those who had not experienced labor (P = 0.05). The proportion of CD8+ T cells did not change significantly among clinical groups (Fig. 1c).
Although we localized T cells in both amniochorion and choriodecidua, they were more abundant in the choriodecidua. In amniochorion, T cells were more abundant in women in term gestation than in preterm gestation. In choriodecidua, T cells were more abundant in women who had undergone spontaneous labor than in women who had not experienced labor (Fig. 2). Monocytes were sporadically detected at term gestation in very low numbers, and B cells and NK cells were undetectable by confocal microscopy (data not shown).
Figure 2.
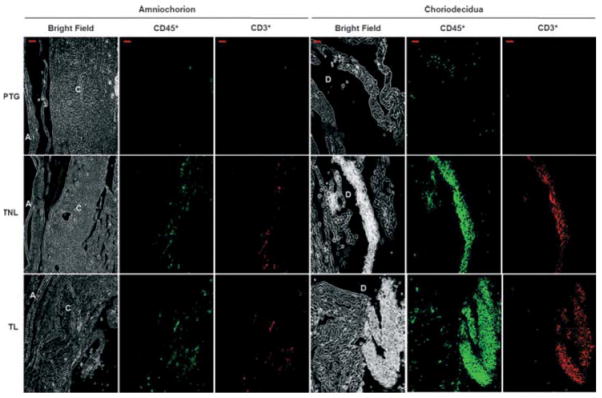
Tissue localization of choriodecidual leukocytes. Photomicrograph of amniochorion (left panel) and choriodecidua (right panel) in each group. A, amnion; C, chorion; D, decidua. Leukocytes (CD45+) were greater in choriodecidua than in amniochorion, and their density seemed to increase from PTG to TL. T cells (CD45+CD3+) and granulocytes (CD45+CD3−CD14−CD56−CD19−) increased from PTG to TL. Bar, 20 μm. Confocal microscopy: magnification x200. Data are representative of three or more independent experiments with five tissues per group.
Next, we localized T-cell subsets in the choriodecidua. CD4+ T cells were higher in tissues from patients in term gestation than in preterm gestation (Fig. 3a), and CD8+ T cells were undetectable or barely detectable (data not shown).
Figure 3.
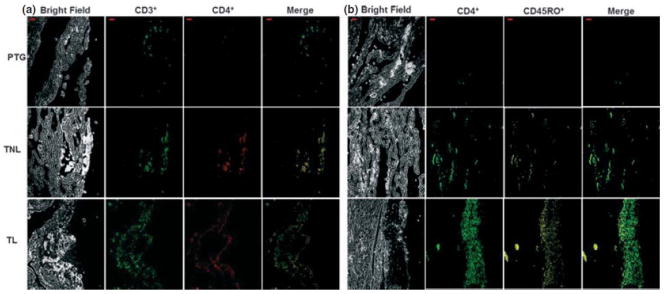
Tissue localization of CD45RO+ memory-like T cells. (a) Photomicrograph of choriodecidua identifying CD4+ T cells in each group. (b) Photomicrograph of choriodecidua identifying CD4+ CD45RO+ memory-like T cells in each group. Merge shows the co-localization of both markers. CD45RO+ memory-like T cells seemed to increase from PTG to TL. Bar, 20 μm. Confocal microscopy: magnification x200. Data are representative of three or more independent experiments with five tissues per group.
CHORIODECIDUAL T CELLS EXPRESS CD4RO+ ‘MEMORY-LIKE T CELLS’ DURING BOTH TERM PREGNANCY AND SPONTANEOUS LABOR AT TERM
Choriodecidual T cells, including mostly CD4+ T cells, expressed CD45RO, a memory-like marker. Although there seems to be more CD4+ memory-like T cells in tissues from women in term gestation with spontaneous labor than in those without labor, the increase observed was not consistent between all histologic samples (Fig. 3b). Such findings were also supported by flow cytometry analysis (Fig. 1c).
In addition, we observed a few CD45RO+ cells that did not express CD4 or CD8 in tissues from patients who had undergone spontaneous labor. These cells were considered to represent memory-like DN T cells (data not shown).
CHORIODECIDUAL LEUKOCYTES EXPRESS SPECIFIC CHEMOKINES/RECEPTORS DURING SPONTANEOUS LABOR AT TERM
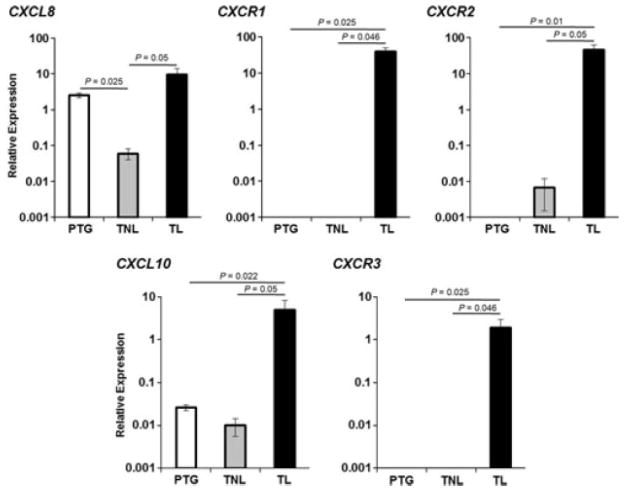
As most of the infiltrated cells were T cells and granulocytes, we determined the mRNA relative expression of chemokines and receptors and related to neutrophil and T-cell recruitment in isolated choriodecidual leukocytes. mRNA expression of the chemokines CXCL8 and CXCL10, and their receptors CXCR1 (chemokine C-X-C motif receptor 1), CXCR2, and CXCR3 was determined. For each chemokine and its receptor(s), the expression was higher in choriodecidua from patients who had experienced labor than in those who had not had labor in term or preterm gestation. However, the expression of these chemokines and their receptors did not change between tissues from women in term gestation without labor and preterm gestation, except CXCL8, which was lower in tissues from women who delivered in term pregnancy without labor than in preterm gestation (P = 0.025) (Fig. 4).
Figure 4.
Chemokine expression. Relative expression of CXCL8 and CXCL10 and its receptors CXCR1-3 in choriodecidual leukocytes from each group. CXCL8 was higher in TL than in TNL, and lower in TNL than in PTG. CXCL10, CXCR1, CXCR2, and CXCR3 were higher in TL than in TNL and PTG. Data are expressed as relative expression using ACTB gene as the reference. Data shown are means ± SEM, with five to seven tissues per group.
CHORIODECIDUAL LEUKOCYTES EXPRESS SPECIFIC CAMs DURING BOTH TERM PREGNANCY AND SPONTANEOUS LABOR AT TERM
Cell adhesion molecules participate in leukocyte infiltration, spreading, and homing.8,18,86,90 We therefore determined the expression of several CAMs in isolated choriodecidual leukocytes. Most of the CAMs were expressed in higher levels in tissues from patients in term gestation than in preterm gestation. Levels of ICAM1 (intercellular adhesion molecule 1), ICAM2, ICAM3, VCAM (vascular cell adhesion molecule), SELP (selctin P), ITGAL (integrin alpha L), and ITGAM (integrin alpha M) were higher in tissues from patients in term gestation who had undergone spontaneous labor than in preterm gestation (P ≤ 0.025). In addition, levels of ICAM1, ICAM2, VCAM, SELP, ITGAL, and ITGAM were greater in tissues from women who had not experienced labor in term gestation than in preterm gestation (P ≤ 0.025). Only levels of ITGAL were higher in tissues from patients who had undergone spontaneous labor than in tissues from women who had not experienced labor in term gestation (P = 0.05) (Fig. 5).
Figure 5.
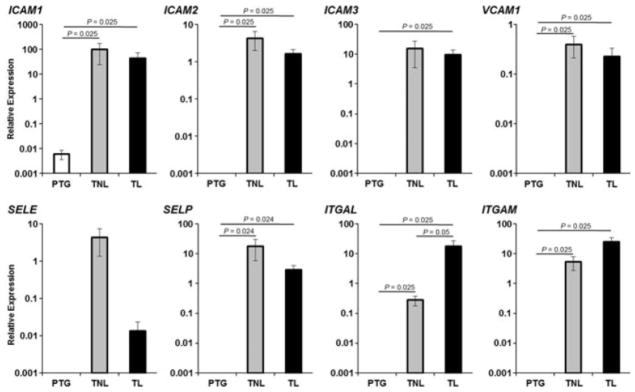
Cell adhesion moelcules expression. Relative expression of ICAM1, ICAM2, ICAM3, VCAM, SELP, SELE, ITGAL and ITGAM were higher in TNL than in PTG. ITGAL was higher in TL than in TNL. Data are expressed as relative expression using ACTB gene as the reference. Data shown are means ± SEM, with five to seven tissues per group.
CHORIODECIDUAL LEUKOCYTES EXPRESS HIGH LEVELS OF TNA-α, IL-1β AND MMP-9 DURING SPONTANEOUS LABOR AT TERM
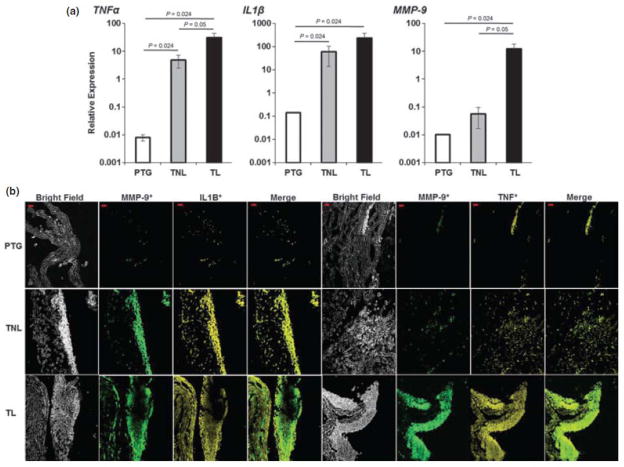
Finally, the expression of TNF-α and IL-1β was determined in choriodecidual leukocytes. mRNA expression of TNF-α, IL-1β and MMP-9 were higher in tissues from women who had not experienced labor in term gestation than in preterm gestation (P = 0.024 each). TNF-α levels were also higher in tissues from patients who had undergone spontaneous labor than in tissues from women who had not experienced labor (P = 0.05) and greater in tissues from women who had not experienced labor in term gestation than in preterm gestation (P = 0.024). IL-1β levels in tissues from women who had not experienced labor in term gestation were higher than in preterm gestation. Although MMP-9 levels did not change significantly between these two clinical groups, the mRNA levels of this enzyme did tend to increase in women at term pregnancy (Fig. 6a). Protein levels of TNF-α, IL-1β, and MMP-9 were also higher in tissues from women who had experienced labor than in those from women who had not experienced labor in term gestation. MMP-9 co-localized with IL-1β and TNF-α (Fig. 6b). MMP-9 was associated with leukocytes, including T cells, and this association was more evident in tissues form women who had experienced labor in term gestation (Fig. 7).
Figure 6.
Labor mediators. (a) Expression of labor mediators. IL-1β, TNF-α, and MMP-9 in choriodecidual leukocytes from each group. All three mediators increased from PTG and TL. Data are expressed as relative expression using ACTB gene as the reference. Data shown are means ± SEM, with five to seven tissues per group. (b) Photomicrograph of choriodecidua from each group. IL-1β, TNF-α, and MMP-9 increased from PTG to TL. Merge shows the co-localization of both markers. Bar, 20 μm. Confocal microscopy: magnification x200. Data are representative of three or more independent experiments with five tissues per group.
Figure 7.
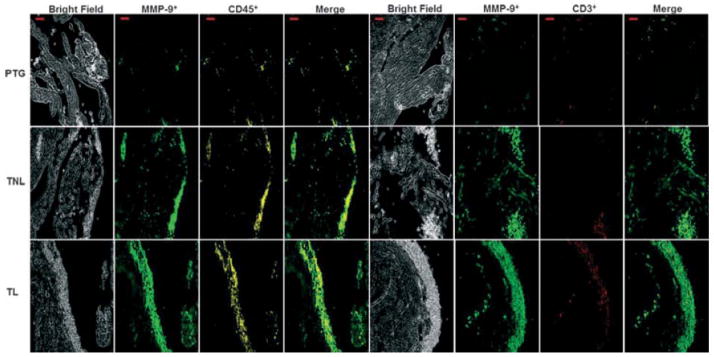
Localization of MMP-9 in choriodecidual leukocytes. Photomicrograph of choriodecidua identifying MMP-9+CD45+ cells or MMP-9+CD3+ T cells in each group. Both MMP-9+ total leukocytes and T cells increased from PTG to TL. Merge shows the co-localization of both markers. Bar, 20 μm. Confocal microscopy: magnification x200. Data are representative of three or more independent experiments with five tissues per group.
DISCUSSION
PRINCIPAL FINDINGS OF THE STUDY
(i) Memory-like CD4+ T cells were present in the choriodecidual tissues of women at term without labor, as well as in women in spontaneous labor at term, indicating that cells involved in the adaptive immune response are present in the maternal-fetal interface at term. (ii) The proportion of ‘memory-like T cells’ is increased in the choriodecidual tissues of women in spontaneous labor. (iii) A new subset ‘memory-like CD4−CD8− double-negative T cells’ are present in the choriodecidual tissues of women in spontaneous labor at term. (iv) Choriodecidual leukocytes, T cells and granulocytes, express chemokines and their receptors (e.g., CXCL8, CXCL10 and CXCR1-5), which we propose participate in the recruitment of these cells during spontaneous labor at term. (v) Choriodecidual leukocytes express specific cell adhesion molecules, which are likely to participate in the homing of these leukocytes into this specific anatomical site as term approaches and when labor occurs, (vi) Choriodecidual T cells and granulocytes isolated from tissues of women in labor express high levels of MMP-9, IL1β, TNF-α, mRNA, as well as protein. Such cytokines have been implicated in the initiation of labor at term (e.g., IL-1) and preterm as they stimulate prostaglandin production and exert other biological functions required for parturition.91,103,108,109 MMP-9 has been implicated in the mechanisms of membrane rupture.14,94–96,98,99,101,102,110 (vii) Collectively, the findings reported herein support a role for the adaptive limb of the immune response in the onset of spontaneous labor at term.
LABOR AS IN INFLAMMATORY PHENOMENON
Unbiased study of the tissues involved in the mechanism of spontaneous labor at term suggests that parturition is an inflammatory response.1–13,15–22,28 Inflammation appears to play a key role in the common pathway of parturition based upon in vivo and in vitro experimentation (uterine contractility, cervical dilatation/repair, rupture of membranes, and detachment of the placenta and membranes.103–105,111–116 Current consensus suggests that the maternal-fetal interface, choriodecidua, is the primary site where maternal leukocytes infiltrate and generate an inflammatory microenvironment during spontaneous labor at term.9,10,15,17–22,35,82,86,117 This process is also important in uterine involution and cervical repair.27,34,130,131
The studies described in this communication were designed to investigate whether the number of leukocytes and discreet subpopulations change as a function of advance in gestational age and spontaneous labor at term. Therefore, we study women who had a preterm gestation, who were not in labor, but require a preterm delivery by cesarean section. Tissues from women not in labor at term were obtained from patients undergoing an elective cesarean delivery before the onset of labor, and choriodecidua was collected from tissues of women who underwent normal parturition at term and received no medications. The key findings of this study are that the number of leukocytes present in the choriodecidual increases with gestational age and that there is a change in the proportion of different leukocytes in the choriodecidual tissues after labor has occurred.
A widely held belief for decades was that granulocytes played a key role in the onset of labor.4,47,80 However, recent evidence in which mice have been depleted of neutrophil during pregnancy indicates that these cells are not necessary for the onset of labor in these species.132 The same appears to be the case for monocytes/macrophages (Gomez-Lopez N, Bijland MT, Olson DM and Robertson SA, unpublished data). The data reported herein suggest that adaptive immune cells, and specifically T cells, play a role in the onset of spontaneous labor at term pregnancy. The relative contribution of the innate and adaptive cells to the process of parturition remains to be defined and it is being investigated by our group.
CHORIODECIDUAL T CELLS: MEMORY-LIKE CD4+ T CELLS DURING TERM PREGNANCY AND SPONTANEOUS LABOR AT TERM
T cells have been localized in the choriodecidua in term pregnancies.35,72,133–139 Choriodecidual T cells at term gestation seem to be activated and have both a regulatory and an effector phenotype.35,134,135,139 Recently, we demonstrated that choriodecidual T cells are recruited into the maternal-fetal interface during spontaneous labor at term.21,82 Here, we demonstrated that the proportion of choriodecidual T cells increases at term pregnancy, and it is maximal during spontaneous labor at term. To our knowledge, this is the first demonstration that the proportion of adaptive immune cells, T cells, increases during spontaneous labor at term pregnancy.
This study also showed that a high proportion of choriodecidual CD4+ T cells express CD45RO (memory-like T cells) before and after spontaneous labor at term. The fact that these cells express CD45RO suggests that these T cells were generated from early pregnancy or even in a previous pregnancy.140 It is tempting to postulate that memory T cells have T-cell receptors for paternal antigens. The adaptive immune system of the mother could have encountered these antigens in early pregnancy in the context of fetal transfusion of cells into the maternal circulation which occurs physiologically during pregnancy.41,141–148 These cells have a full complement of class I and class II HLA, and there is evidence that mothers have cytotoxic T cells against paternal antigens even during normal pregnancy.134,149–152
A POSSIBLE ROLE FOR CHORIODECIDUAL MEMORY-LIKE CD4+ T CELLS DURING SPONTANTEOUS LABOR AT TERM
Cells have been implicated in the tolerogenic state required during normal pregnancy (to avoid rejection of paternal antigens expressed in fetal tissues, such as white blood cells).49,135,137,138,151,153 A role for effector T cells during labor was suggested approximately thirty years ago.154 Yet, persuasive evidence of their participation in the mechanisms of parturition remains to be proven. We recently reported that T cells infiltrate the site of rupture of the fetal membranes (i.e., choriodecidua), and thus, they may play a role in spontaneous rupture membrane.21,82 Here, we report that choriodecidual memory-like CD4+ T cells express IL-1β, TNF-α and MMP-9 during spontaneous labor at term. These cytokines have been implicated in both term10,17,91,109,155 and preterm labor91,93,108,109,156; however, the traditional thought is that these mediators are produced by cells of the innate immune system. We report for the first time that such mediators are also produced by cells of the adaptive immune system – T cells.
The generation of MMP-9 suggests that T cells may participate in the degradation of extracellular matrix of the fetal membranes and surrounding tissues.4,14,94–96,98,99,101,102,110 T cells producing MMP-9 were recently identified in patients with multiple sclerosis, and they have been implicated in the pathogenesis of this disease.157 Therefore, we propose that memory-like CD4+ T cells producing MMP-9 and pro-inflammatory mediators participate in parturition during the process of spontaneous labor at term pregnancy.
Several studies have also demonstrated that maternal circulating T cells during pregnancy are the result of the expansion of the total number of regulatory T cells or Tregs.140,153,158–162 Although the proportion of circulating regulatory T cells does not change in late gestation, the suppressive activity of these cells decreases during spontaneous labor at term.161 We therefore suggest that the changes in functional properties of T regulatory cells, suppression, play an important role in the initiation of labor.163 The role may not be restricted to spontaneous labor at term but they may also play an important role in preterm labor, particularly associated with maternal anti-fetal rejection.
A NOVEL FINDING: MEMORY-LIKE CD4−CD8−DOUBLE-NEGATIVE T CELLS IN THE CHORIODECIDUAL INTERFACE DURING SPONTANEOUS LABOR AT TERM
Interestingly, 30% of CD4−CD8− DN T cells are in the choriodecidua during spontaneous labor at term. They also seemed to express CD45RO; therefore, they were considered as memory-like T cells. Although DN T cells were previously reported,164 here we demonstrated that their proportion increases during spontaneous labor at term. Human DN T cells are non-conventional T cells that show tropism for mucosa and behave more like innate rather than adaptive immune cells.165 Human DN T cells act as either suppressors or promoters of an immune response. Their suppressor role in the effector function of T cells is mediated by an active cell contact-dependent mechanism. Human DN T cells can also produce pro-inflammatory cytokines and thus enhance the inflammatory response.166,167 We suggest that the choriodecidual memory-like DN T cells may play both roles as they could suppress effector T cells (e.g., CD4+ T cells) and/or help in the production of cytokines during spontaneous labor at term. More studies are needed to clarify their function.
HOW ARE MEMORY-LIKE T CELLS RECRUITED INTO THE CHORIODECIDUA DURING TERM PREGNANCY AND SPONTANEOUS LABOR AT TERM?
T-cell recruitment and homing are mediated by specific chemokines and CAMs. T-cell chemo-attractants include CCL5,168 IL-16,169 CXCL10, CXCL9, and CXCL11.67,170, 171 Recently it has been demonstrated that early pregnancy decidual stromal cells restrict T-cell recruitment into the decidua to regulate the maternal immune response against the fetus.172 Such findings suggest that the decidua has an active role in controlling the migration of maternal T cells into the maternal-fetal interface.172,173 Our observations are in keeping with this hypothesis and suggest that both decidual stromal cells and leukocytes coordinate the migration of several immunological cells, including T cells, into the maternal-fetal interface all throughout pregnancy and during labor. We previously demonstrated that CCL5 (also known as RANTES) is expressed by the human fetal membranes (i.e., choriodecidua) during term pregnancy and that its local concentration does not change with labor.19 However, in vivo observations suggest that RANTES increases in the amniotic fluid with spontaneous labor at term and with intra-amniotic infection/inflammation.174 As CCL5 can recruit memory T cells168 and its local concentrations in the choriodecidua increase during term pregnancy,19 there seems to be a temporal association between the infiltration of memory-like CD4+ T cells and the expression of a chemokine capable of recruiting these cells at term pregnancy. Therefore, we propose that CCL5 participates in the recruitment of memory-like CD4+ T cells during term pregnancy, In addition, our data showed that CXCL10 and its receptor CXCR3 are highly expressed in choriodecidual leukocytes during spontaneous labor at term. This finding is consistent with our previous observation where we found that CXCL10 levels in the choriodecidual tissues are higher during labor than in the absence of labor.19 As both levels of CXCL10 and the proportion of memory-like DN T cells increased during labor, we suggest that CXCL10 may be involved in memory-like DN T-cell recruitment during spontaneous labor at term. CXCL10 has been implicated in the mechanism responsible for maternal anti-fetal rejection and chronic chorioamnionitis, which is the most common lesion associated with spontaneous late preterm labor.67 Therefore, the observations reported herein have implications beyond the control of normal parturition.
Cell adhesion molecules play a critical role in controlling adhesion and the homing of T cells into reproductive tissues.8 It has been described in a murine model that ITGAL and ICAM1 mediate T-cell recruitment during pregnancy.175 Here, we found that in human ITGAL and it ligands, ICAMs 1-3 are over-expressed in choriodecidual leukocytes during term pregnancy and spontaneous labor at term. Because these CAMs have been related to the recruitment of human effector memory CD4+ T cells,176 we suggest that they may also be responsible for the infiltration and accumulation of memory-like T cells into the choriodecidua during term pregnancy and spontaneous labor at term.
WHAT IS THE ROLE OF GRANULOCYTES IN THE CHORIODECIDUA DURING SPONTANEOUS LABOR AT TERM?
Granulocytes are present in reproductive tissues during pregnancy.4,9,10,13,177 The results of the present study indicate that granulocytes are present in the choriodecidua during late gestation. We found that although leukocytes are scarce in the choriodecidua before term pregnancy, most of them are granulocytes (~80%). These cells, and in particular neutrophils, have been sparingly found in the choriodecidua before the onset of labor.10 Even though granulocytes were present approximately in 60% of leukocytes in the choriodecidua at term, they represented only 35% of leukocytes in tissues obtained in women who experienced labor. Our data and that of others, who have reported that decidual neutrophils in term pregnancies provide a rich source of extracellular matrix proteases4,16,178–180 and cytokines,12,33,74,80,92,181 suggest that granulocytes may play a role before and during spontaneous labor at term (before labor for the preparative stages for parturition). As the depletion of neutrophils in the mouse does not affect the timing or course of labor132 and that other leukocytes and stromal cells produce these and other related immunological mediators,83,182–188 it would seem that granulocytes are not absolutely required for labor in mice. We favor a model in which there is cooperation of the different cell types to achieve normal parturition at term.
WHAT FACTORS ARE IMPLCIATED IN THE RECRUITMENT OF GRANULOCYTES INTO THE CHORIODECIDUA DURING TERM PREGNANCY?
Neutrophil recruitment and homing are mediated by specific chemokines and CAMs. CXCL8 is highly expressed in the amniochorion and choriodecidua in spontaneous labor.12,19,21,22,74,80,92,181 Coincidently, our results showed that CXCL8 and it receptors, CXCR1 ANC CXCR2, are greatly expressed in choriodecidual leukocytes during spontaneous labor at term pregnancy. Previous studies have associated neutrophil influx into decidua with CXCL8 levels.12,19,21,22,74,80,92,181 Although we did not observe an increase in the proportion of granulocytes in the choriodecidua during spontaneous labor at term, we did observe that choriodecidual granulocytes express high levels of CXCL8 and its receptors, CXCR1 and CXCR2. This suggests that infiltration of granulocytes into the choriodecidua expresses CXCL8 as a labor mediator to promote labor at term, rather than to recruit granulocytes. An alternative could be that the choriodecidual leukocytes express high levels of CXCL8 to recruit more granulocytes, which will be required to repair surrounding tissue (e.g., cervix) during the postpartum period.131,132 CAMs like selectin E, VCAM-1, and ICAM-1 have been linked to neutrophil recruitment into reproductive tissues.13,110 We put forward evidence supporting this hypothesis as our data showed that VCAM and ICAM1 are highly expressed at term pregnancy, where granulocytes are abundant in the choriodecidua. Taken together, these data allow us to suggest that the synchronized action of CXCL8 produced by fetal membranes and choriodecidual leukocyte expression of CXCL8 receptors and CAMs (e.g., VCAM and ICAM-1) results in the recruitment and homing of granulocytes into the choriodecidua during term pregnancy and spontaneous labor.
STRENGTHS AND LIMITATIONS
This is the first study identifying adaptive immune cells, memory-like CD4+, and double-negative T cells, which are able to produce MMP-9 in the choriodecidua during spontaneous labor at term pregnancy. We recognized the following limitation: (i) the identification of memory T cells is very complex and uses the expression of several markers including CD45RO, CD45RA, CCR7, CCR4, CCR5, CD62L, CD27, CD28, CXCR5, CXCR3 and CRTH2.189 In our study, we only analyzed the expression of CD45RO and CD45RA; therefore, we referred to these cells as ‘memory-like T cells’ and further analysis of their phenotype is required; (ii) additional studies are necessary to clarify the role of choriodecidual memory-like CD4+ T cells during term pregnancy and spontaneous labor at term; (iii) the function of double-negative T cells in the choriodecidua during spontaneous labor at term remains to be elucidated; (iv) choriodecidual granulocytes include neutrophils, basophils and eosinophils; therefore, future studies are needed to establish the proportion of each granulocyte and their possible role during term pregnancy and spontaneous labor at term. The tissues obtained from women with preterm gestations were derived from patients who had complications such as congenital anomalies. It is possible that these patients do not represent the state of the decidual in normal pregnancy. However, it is extremely difficult in humans to obtain preterm tissues in normal women; therefore, such tissues are the best representation that can be obtained at this time of the cellular composition of the choriodecidual in preterm pregnancy.
CONCLUSION
Human labor involves the establishment of an inflammatory microenvironment in the choriodecidua that includes adaptive immune cells, such as two newly identified T-cell subsets, ‘memory-like CD4+ T cells’ and ‘memory-like double-negative T cells’. This microenvironment also includes granulocytes, macrophages and an extensive signaling network composed of chemokines, cytokines and cell adhesion molecules. We propose that cells and mediators create a specific microenvironment in the maternal-fetal interface that plays an important role in spontaneous labor at term pregnancy (Fig. 8). Further research is needed to investigate the function of these T cells during term and preterm labor. An improved understanding of the mechanisms of term and preterm labor will assist in the prevention of prematurity, the most important challenge to modern obstetrics.
Figure 8.
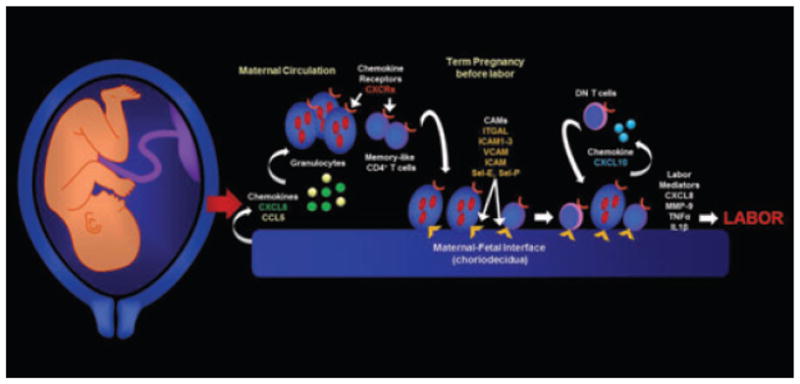
Conceptual framework. Choriodecidual tissues express chemokines to recruit maternal circulating leukocytes at term pregnancy. Before the onset of labor, choriodecidual memory-like CD4+ T cells and other leukocytes such as granulocytes express cell adhesion molecules to remain into this anatomical space. Infiltrating leukocytes release CXCL10 to recruit double-negative (DN) T cells. Together they release labor mediators (e.g., CXCL8, IL-1β, TNF-α and MMP-9) to participate in labor at the end of gestation.
Supplementary Material
Figure S1. (A) Representative diagram of spread out fetal membranes. Area was calculated by measuring the dotted lines. (B) Representative dot-plots showing the dual parameters used for the flow cytometric analysis. Monocytes (CD45+CD14+), NK cells (CD45+CD56+), B cells (CD45+CD19+) and T cells (CD45+CD3+) were analyzed within the CD45+ gate. T cell subsets were analyzed within the CD3+ gate, P4 CD3+CD8+, P5 CD3+CD4+, P6 CD3+CD4−CD8−.
Table S1. Panel of antibodies used in this study.
Table S2. Primers and probes used for performing real time PCR.
Acknowledgments
We gratefully acknowledge Dr. Jorge Beltran-Montoya and Dr. Guadalupe Estrada-Gutierrz from the Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes, for their contribution to the execution of this study. We also thank Andrew Lobb, Board of Scientific Counselors, from the Department of Obstetrics and Gynecology, Wayne State University, for editorial assistance.
GRANT SUPPORT
F.V-O was supported by CONACyT-SALUD 736 and 69353. N.G-L was sponsored by the Molly Towell Perinatal Research Foundation. This work was supported, in part, by the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH/DHHS (RR).
References
- 1.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 2.Liggins G. Cervical ripening as an inflammatory reaction. In: Ellwood D, Anderson A, editors. The Cervix in Pregnancy and Labor: Clinical and Biochemical Investigations. Edinburgh: Churchill Livingstone; 1981. pp. 1–9. [Google Scholar]
- 3.Uldbjerg N, Ulmsten U, Ekman G. The ripening of the human uterine cervix in terms of connective tissue biochemistry. Clin Obstet Gynecol. 1983;26:14–26. doi: 10.1097/00003081-198303000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Osmers R, Rath W, Adelmann-Grill BC, Fittkow C, Kuloczik M, Szeverenyi M, Tschesche H, Kuhn W. Origin of cervical collagenase during parturition. Am J Obstet Gynecol. 1992;166:1455–1460. doi: 10.1016/0002-9378(92)91619-l. [DOI] [PubMed] [Google Scholar]
- 5.Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Sennstrom MK, Brauner A, Lu Y, Granstrom LM, Malmstrom AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur J Obstet Gynecol Reprod Biol. 1997;74:89–92. doi: 10.1016/s0301-2115(97)02757-7. [DOI] [PubMed] [Google Scholar]
- 7.Luo L, Ibaragi T, Maeda M, Nozawa M, Kasahara T, Sakai M, Sasaki Y, Tanebe K, Saito S. Interleukin-8 levels and granulocyte counts in cervical mucus during pregnancy. Am J Reprod Immunol. 2000;43:78–84. doi: 10.1111/j.8755-8920.2000.430203.x. [DOI] [PubMed] [Google Scholar]
- 8.Ledingham MA, Thomson AJ, Jordan F, Young A, Crawford M, Norman JE. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet Gynecol. 2001;97:235–242. doi: 10.1016/s0029-7844(00)01126-1. [DOI] [PubMed] [Google Scholar]
- 9.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–449. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 10.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 11.Stjernholm-Vladic Y, Stygar D, Mansson C, Masironi B, Akerberg S, Wang H, Ekman-Ordeberg G, Sahlin L. Factors involved in the inflammatory events of cervical ripening in humans. Reprod Biol Endocrinol. 2004;2:74. doi: 10.1186/1477-7827-2-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 13.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 14.Roh CR, Oh WJ, Yoon BK, Lee JH. Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: a cytokine-mediated process in uterine smooth muscle cells. Mol Hum Reprod. 2000;6:96–102. doi: 10.1093/molehr/6.1.96. [DOI] [PubMed] [Google Scholar]
- 15.Keski-Nisula L, Aalto ML, Katila ML, Kirkinen P. Intrauterine inflammation at term: a histopathologic study. Hum Pathol. 2000;31:841–846. doi: 10.1053/hupa.2000.8449. [DOI] [PubMed] [Google Scholar]
- 16.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon BH. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 17.Elliott CL, Loudon JA, Brown N, Slater DM, Bennett PR, Sullivan MH. IL-1beta and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am J Reprod Immunol. 2001;46:260–267. doi: 10.1034/j.1600-0897.2001.d01-11.x. [DOI] [PubMed] [Google Scholar]
- 18.Osman I, Crawford M, Jordan F, Young A, Norman J, Thomson A. Expression and localization of cell adhesion molecules in human fetal membranes during parturition. J Reprod Immunol. 2004;63:11–21. doi: 10.1016/j.jri.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009;80:122–131. doi: 10.1016/j.jri.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Mittal P, Romero R, Mazaki-Tovi S, Tromp G, Tarca AL, Kim YM, Chaiworapongsa T, Kusanovic JP, Erez O, Than NG, Hassan SS. Fetal membranes as an interface between inflammation and metabolism: increased aquaporin 9 expression in the presence of spontaneous labor at term and chorioamnionitis. J Matern Fetal Neonatal Med. 2009;22:1167–1175. doi: 10.3109/14767050903019692. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines- Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205:235, e215–224. doi: 10.1016/j.ajog.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204:364, e369–316. doi: 10.1016/j.ajog.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod. 2002;8:399–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 24.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394, e391–324. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J., Jr The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–786. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Hassan SS, Romero R, Tarca AL, Draghici S, Pineles B, Bugrim A, Khalek N, Camacho N, Mittal P, Yoon BH, Espinoza J, Kim CJ, Sorokin Y, Malone J., Jr Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2007;197:250, e251–257. doi: 10.1016/j.ajog.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmons BC, Mahendroo M. Processes regulating cervical ripening differ from cervical dilation and postpartum repair: insights from gene expression studies. Reprod Sci. 2007;14:53–62. doi: 10.1177/1933719107309587. [DOI] [PubMed] [Google Scholar]
- 28.Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200:104, e101–111. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 29.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Vaisbuch E, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Yeo L, Draghici S, Kim JS, Uldbjerg N, Kim CJ. The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process. J Matern Fetal Neonatal Med. 2009;22:1183–1193. doi: 10.3109/14767050903353216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan SS, Romero R, Pineles B, Tarca AL, Montenegro D, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Espinoza J, Nhan-Chang CL, Draghici S, Kim CJ. MicroRNA expression profiling of the human uterine cervix after term labor and delivery. Am J Obstet Gynecol. 2010;202:80, e81–88. doi: 10.1016/j.ajog.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang CL, Chaiworapongsa T, Lye S, Kusanovic JP, Lipovich L, Mazaki-Tovi S, Hassan SS, Mesiano S, Kim CJ. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38:617–643. doi: 10.1515/JPM.2010.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nhan-Chang CL, Romero R, Tarca AL, Mittal P, Kusanovic JP, Erez O, Mazaki-Tovi S, Chaiworapongsa T, Hotra J, Than NG, Kim JS, Hassan SS, Kim CJ. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol. 2010;202:462, e461–441. doi: 10.1016/j.ajog.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakamoto Y, Moran P, Searle RF, Bulmer JN, Robson SC. Interleukin-8 is involved in cervical dilatation but not in prelabour cervical ripening. Clin Exp Immunol. 2004;138:151–157. doi: 10.1111/j.1365-2249.2004.02584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005;66:161–173. doi: 10.1016/j.jri.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Sindram-Trujillo A, Scherjon S, Kanhai H, Roelen D, Claas F. Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol. 2003;49:261–268. doi: 10.1034/j.1600-0897.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 36.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 37.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 38.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25:375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 39.Riley JK. Trophoblast immune receptors in maternal-fetal tolerance. Immunol Invest. 2008;37:395–426. doi: 10.1080/08820130802206066. [DOI] [PubMed] [Google Scholar]
- 40.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63:624–636. doi: 10.1111/j.1600-0897.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 41.Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R. Tolerance mechanisms in pregnancy: a reappraisal of the role of class I paternal MHC antigens. Am J Reprod Immunol. 2010;63:93–103. doi: 10.1111/j.1600-0897.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 42.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178:3345–3351. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- 45.von Rango U. Fetal tolerance in human pregnancy–a crucial balance between acceptance and limitation of trophoblast invasion. Immunol Lett. 2008;115:21–32. doi: 10.1016/j.imlet.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Abrahams VM. Thirty years of reproductive immunology: an introduction. Am J Reprod Immunol. 2010;63:411–412. doi: 10.1111/j.1600-0897.2010.00849.x. [DOI] [PubMed] [Google Scholar]
- 47.Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol. 2010;63:445–459. doi: 10.1111/j.1600-0897.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 48.Zenclussen ML, Thuere C, Ahmad N, Wafula PO, Fest S, Teles A, Leber A, Casalis PA, Bechmann I, Priller J, Volk HD, Zenclussen AC. The persistence of paternal antigens in the maternal body is involved in regulatory T-cell expansion and fetal-maternal tolerance in murine pregnancy. Am J Reprod Immunol. 2010;63:200–208. doi: 10.1111/j.1600-0897.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 49.Dimova T, Nagaeva O, Stenqvist AC, Hedlund M, Kjellberg L, Strand M, Dehlin E, Mincheva-Nilsson L. Maternal Foxp3 expressing CD4 + CD25 + and CD4 + CD25− regulatory T-cell populations are enriched in human early normal pregnancy decidua: a phenotypic study of paired decidual and peripheral blood samples. Am J Reprod Immunol. 2011;66 (Suppl 1):44–56. doi: 10.1111/j.1600-0897.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 50.Ernerudh J, Berg G, Mjosberg J. Regulatory T helper cells in pregnancy and their roles in systemic versus local immune tolerance. Am J Reprod Immunol. 2011;66 (Suppl 1):31–43. doi: 10.1111/j.1600-0897.2011.01049.x. [DOI] [PubMed] [Google Scholar]
- 51.Fraccaroli L, Grasso E, Zeitler E, Lombardi E, Gogorza S, Etchepareborda JJ, Nagle C, Cortelezzi M, Perez Leiros C, Ramhorst R. Modulation of maternal LIF producers T cells by trophoblast and paternal antigens. Am J Reprod Immunol. 2011;65:133–145. doi: 10.1111/j.1600-0897.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 52.Jin LP, Fan DX, Li DJ. Regulation of costimulatory signal in maternal-fetal immune tolerance. Am J Reprod Immunol. 2011;66:76–83. doi: 10.1111/j.1600-0897.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, Chaiworapongsa T, Hassan SS, Kim CJ. Maternal HLA panelreactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–526. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nevers T, Kalkunte S, Sharma S. Uterine Regulatory T cells, IL-10 and hypertension. Am J Reprod Immunol. 2011;66 (Suppl 1):88–92. doi: 10.1111/j.1600-0897.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramhorst R, Fraccaroli L, Aldo P, Alvero AB, Cardenas I, Leiros CP, Mor G. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol. 2012;67:17–27. doi: 10.1111/j.1600-0897.2011.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spencer PS, Hakam SM, Laissue PP, Jabeen A, Jain P, Hayrabedyan S, Todorova K, Blanch A, McElhinney JM, Muhandiram N, Alkhatib S, Dealtry GB, Miranda-Sayago JM, Fernandez N. Key cellular components and interactive histocompatibility molecules regulating tolerance to the fetal allograft. Am J Reprod Immunol. 2012;68:95–99. doi: 10.1111/j.1600-0897.2012.01138.x. [DOI] [PubMed] [Google Scholar]
- 57.Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vasarhelyi B, Rigo J., Jr Molvarec A: the frequency of peripheral blood CD4 + CD25high FoxP3 + and CD4 + CD25− FoxP3 + regulatory T cells in normal pregnancy and pre-eclampsia. Am J Reprod Immunol. 2012;68:175–180. doi: 10.1111/j.1600-0897.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- 58.Kerr MG. Immunological rejection as a cause of abortion. J Reprod Fertil Suppl. 1968;3 (Suppl 3):49–55. [PubMed] [Google Scholar]
- 59.Redman CW. Immune factors and recurrent abortion: a review. Am J Reprod Immunol. 1983;4:179–181. doi: 10.1111/j.1600-0897.1983.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 60.Kilpatrick DC. Immune mechanisms and pre-eclampsia. Lancet. 1987;2:1460–1461. doi: 10.1016/s0140-6736(87)91156-1. [DOI] [PubMed] [Google Scholar]
- 61.Scott JR, Rote NS, Branch DW. Immunologic aspects of recurrent abortion and fetal death. Obstet Gynecol. 1987;70:645–656. [PubMed] [Google Scholar]
- 62.Labarrere CA. Allogeneic recognition and rejection reactions in the placenta. Am J Reprod Immunol. 1989;21:94–99. doi: 10.1111/j.1600-0897.1989.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 63.Aksel S. Immunologic aspects of reproductive diseases. JAMA. 1992;268:2930–2934. [PubMed] [Google Scholar]
- 64.Labarrere CA, Faulk WP. Microvascular perturbations in human allografts: analogies in preeclamptic placentae. Am J Reprod Immunol. 1992;27:109–116. doi: 10.1111/j.1600-0897.1992.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 65.Chaouat G, Ledee-bataille N, Zourbas S, Dubanchet S, Sandra O, Martal J, Ostojojic S, Frydman R. Implantation: can immunological parameters of implantation failure be of interest for pre-eclampsia? J Reprod Immunol. 2003;59:205–217. doi: 10.1016/s0165-0378(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 66.Chaouat G, Ledee-Bataille N, Zourbas S, Ostojic S, Dubanchet S, Martal J, Frydman R. Cytokines, implantation and early abortion: re-examining the Th1/Th2 paradigm leads to question the single pathway, single therapy concept. Am J Reprod Immunol. 2003;50:177–186. doi: 10.1034/j.1600-0897.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 67.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, Kim JS. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, Kusanovic JP, Chaiworapongsa T, Hassan SS, Yoon BH, Kim CJ. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS ONE. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matthiesen L, Kalkunte S, Sharma S. Multiple pregnancy failures: an immunological paradigm. Am J Reprod Immunol. 2012;67:334–340. doi: 10.1111/j.1600-0897.2012.01121.x. [DOI] [PubMed] [Google Scholar]
- 70.Lessin DL, Hunt JS, King CR, Wood GW. Antigen expression by cells near the maternal-fetal interface. Am J Reprod Immunol Microbiol. 1988;16:1–7. doi: 10.1111/j.1600-0897.1988.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 71.Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods. 1990;132:181–189. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- 72.Vargas ML, Santos JL, Ruiz C, Montes MJ, Aleman P, Garcia-Tortosa C, Garcia-Olivares E. Comparison of the proportions of leukocytes in early and term human decidua. Am J Reprod Immunol. 1993;29:135–140. doi: 10.1111/j.1600-0897.1993.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 73.Castrechini NM, Murthi P, Qin S, Kusuma GD, Wilton L, Abumaree M, Gronthos S, Zannettino A, Gude NM, Brennecke SP, Kalionis B. Decidua Parietalis-Derived Mesenchymal Stromal Cells Reside in a Vascular Niche Within the Choriodecidua. Reprod Sci. 2012;19:1302–1314. doi: 10.1177/1933719112450334. [DOI] [PubMed] [Google Scholar]
- 74.Dudley DJ, Trautman MS, Mitchell MD. Inflammatory mediators regulate interleukin-8 production by cultured gestational tissues: evidence for a cytokine network at the chorio-decidual interface. J Clin Endocrinol Metab. 1993;76:404–410. doi: 10.1210/jcem.76.2.8432783. [DOI] [PubMed] [Google Scholar]
- 75.Narahara H, Johnston JM. Effects of endotoxins and cytokines on the secretion of platelet-activating factor-acetylhydrolase by human decidual macrophages. Am J Obstet Gynecol. 1993;169:531–537. doi: 10.1016/0002-9378(93)90614-o. [DOI] [PubMed] [Google Scholar]
- 76.Narahara H, Nishioka Y, Johnston JM. Secretion of plateletactivating factor acetylhydrolase by human decidual macrophages. J Clin Endocrinol Metab. 1993;77:1258–1262. doi: 10.1210/jcem.77.5.7521345. [DOI] [PubMed] [Google Scholar]
- 77.Kelly RW. Inflammatory mediators and parturition. Rev Reprod. 1996;1:89–96. doi: 10.1530/ror.0.0010089. [DOI] [PubMed] [Google Scholar]
- 78.Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, Jones RL. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- 79.Kim SY, Romero R, Tarca AL, Bhatti G, Kim CJ, Lee J, Elsey A, Than NG, Chaiworapongsa T, Hassan SS, Kang GH, Kim JS. Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. Am J Reprod Immunol. 2012;68:8–27. doi: 10.1111/j.1600-0897.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelly RW, Leask R, Calder AA. Choriodecidual production of interleukin-8 and mechanism of parturition. Lancet. 1992;339:776–777. doi: 10.1016/0140-6736(92)91896-g. [DOI] [PubMed] [Google Scholar]
- 81.Vega-Sanchez R, Flores A, Castillo M, Gomez N, Vadillo-Ortega F. Expression, Tissular Traffic and Activation of MMP-9 in Human Fetal Membranes during Labor. Reprod Sci. 2008;15:164. [Google Scholar]
- 82.Gomez-Lopez N, Hernandez-Santiago S, Lobb AP, Olson DM, Vadillo-Ortega F. Normal and Premature Rupture of Fetal Membranes at Term Delivery Differ in Regional Chemotactic Activity and Related Chemokine/Cytokine Production. Reprod Sci. 2012 doi: 10.1177/1933719112452473. In press. [DOI] [PubMed] [Google Scholar]
- 83.Horton JS, Yamamoto SY, Bryant-Greenwood GD. Relaxin augments the inflammatory IL6 response in the choriodecidua. Placenta. 2012;33:399–407. doi: 10.1016/j.placenta.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luppi P, Irwin TE, Simhan H, Deloia JA. CD11b Expression on circulating leukocytes increases in preparation for parturition. Am J Reprod Immunol. 2004;52:323–329. doi: 10.1111/j.1600-0897.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- 85.Yuan M, Jordan F, McInnes IB, Harnett MM, Norman JE. Leukocytes are primed in peripheral blood for activation during term and preterm labour. Mol Hum Reprod. 2009;15:713–724. doi: 10.1093/molehr/gap054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88:625–633. doi: 10.1189/jlb.1209796. [DOI] [PubMed] [Google Scholar]
- 87.Cohen J, Ghezzi F, Romero R, Ghidini A, Mazor M, Tolosa JE, Goncalves LF, Gomez R. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol. 1996;35:23–29. doi: 10.1111/j.1600-0897.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 88.Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23:239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- 89.Gomez-Lopez N, Laresgoiti-Servitje E, Olson DM, Estrada-Gutierrez G, Vadillo-Ortega F. The role of chemokines in term and premature rupture of the fetal membranes: a review. Biol Reprod. 2010;82:809–814. doi: 10.1095/biolreprod.109.080432. [DOI] [PubMed] [Google Scholar]
- 90.Kruse A, Martens N, Fernekorn U, Hallmann R, Butcher EC. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol Reprod. 2002;66:333–345. doi: 10.1095/biolreprod66.2.333. [DOI] [PubMed] [Google Scholar]
- 91.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP, Mitchell MD. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–238. [PubMed] [Google Scholar]
- 92.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 93.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 94.Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, Lei H, Muschel RJ, Stetler-Stevenson WG, Strauss JF., 3rd 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol. 1995;146:148–156. [PMC free article] [PubMed] [Google Scholar]
- 95.Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998;179:1248–1253. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 96.Athayde N, Romero R, Gomez R, Maymon E, Pacora P, Mazor M, Yoon BH, Fortunato S, Menon R, Ghezzi F, Edwin SS. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med. 1999;8:213–219. doi: 10.1002/(SICI)1520-6661(199909/10)8:5<213::AID-MFM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 97.Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–920. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 98.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000;183:887–894. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 99.Edwin SS, Romero R, Rathnasabapathy CM, Athaydel N, Armant DR, Subramanian MG. Protein kinase C stimulates release of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by human decidual cells. J Matern Fetal Neonatal Med. 2002;12:231–236. doi: 10.1080/jmf.12.4.231.236. [DOI] [PubMed] [Google Scholar]
- 100.Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, Strauss JF., 3rd A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277:6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- 101.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, Mazor M, Maymon E, Berry S. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–1130. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 102.Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. 2002;87:1353–1361. doi: 10.1210/jcem.87.3.8320. [DOI] [PubMed] [Google Scholar]
- 103.Mitchell MD, Romero RJ, Edwin SS, Trautman MS. Prostaglandins and parturition. Reprod Fertil Dev. 1995;7:623–632. doi: 10.1071/rd9950623. [DOI] [PubMed] [Google Scholar]
- 104.Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol. 2003;1:122. doi: 10.1186/1477-7827-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gleicher N. Does the immune system induce labor? Lessons from preterm deliveries in women with autoimmune diseases. Clin Rev Allergy Immunol. 2010;39:194–206. doi: 10.1007/s12016-009-8180-8. [DOI] [PubMed] [Google Scholar]
- 108.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 109.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 110.Rath W, Winkler M, Kemp B. The importance of extracellular matrix in the induction of preterm delivery. J Perinat Med. 1998;26:437–441. doi: 10.1515/jpme.1998.26.6.437. [DOI] [PubMed] [Google Scholar]
- 111.Skinner SJ, Liggins GC. Glycosaminoglycans and collagen in human amnion from pregnancies with and without premature rupture of the membranes. J Dev Physiol. 1981;3:111–121. [PubMed] [Google Scholar]
- 112.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 113.Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 114.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14:237–241. doi: 10.1093/humrep/14.1.237. [DOI] [PubMed] [Google Scholar]
- 115.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24(Suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 116.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 117.Hayano C, Koi H, Ogawa K, Nagata K, Matsumoto Y, Nakamura M, Aso T. Accumulation of CD16 + cells with secretion of Ksp37 in decidua at the end of pregnancy. Am J Reprod Immunol. 2002;48:57–62. doi: 10.1034/j.1600-0897.2002.01100.x. [DOI] [PubMed] [Google Scholar]
- 118.Johansson M, Bromfield JJ, Jasper MJ, Robertson SA. Semen activates the female immune response during early pregnancy in mice. Immunology. 2004;112:290–300. doi: 10.1111/j.1365-2567.2004.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- 120.Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007;18:287–298. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 121.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4 + CD25 + T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy – the contribution of seminal fluid. J Reprod Immunol. 2009;83:109–116. doi: 10.1016/j.jri.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Robertson SA. Immune regulation of conception and embryo implantation-all about quality control? J Reprod Immunol. 2010;85:51–57. doi: 10.1016/j.jri.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 124.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3 + regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3 + cell pool and CCL19-mediated recruitment. Biol Reprod. 2011;85:397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 125.Robertson SA, Chin PY, Glynn DJ, Thompson JG. Peri-conceptual cytokines–setting the trajectory for embryo implantation, pregnancy and beyond. Am J Reprod Immunol. 2011;66 (Suppl 1):2–10. doi: 10.1111/j.1600-0897.2011.01039.x. [DOI] [PubMed] [Google Scholar]
- 126.Vujaklija DV, Gulic T, Sucic S, Nagata K, Ogawa K, Laskarin G, Saito S, Haller H, Rukavina D. First trimester pregnancy decidual natural killer cells contain and spontaneously release high quantities of granulysin. Am J Reprod Immunol. 2011;66:363–372. doi: 10.1111/j.1600-0897.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 127.Bondarenko GI, Durning M, Golos TG. Immunomorphological changes in the rhesus monkey endometrium and decidua during the menstrual cycle and early pregnancy. Am J Reprod Immunol. 2012;68:309–321. doi: 10.1111/j.1600-0897.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95:1–14. doi: 10.1016/j.jri.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 129.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 130.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182:2700–2707. doi: 10.4049/jimmunol.0803138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab. 2010;21:353–361. doi: 10.1016/j.tem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006;74:236–245. doi: 10.1095/biolreprod.105.044891. [DOI] [PubMed] [Google Scholar]
- 133.Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, Kanhai HH, Claas FH, Scherjon SA. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl A):S47–S53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 134.Tilburgs T, Scherjon SA, Roelen DL, Claas FH. Decidual CD8 + CD28− T cells express CD103 but not perforin. Hum Immunol. 2009;70:96–100. doi: 10.1016/j.humimm.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 135.Tilburgs T, Scherjon SA, van der Mast BJ, Haasnoot GW, Versteeg VDV-MM, Roelen DL, van Rood JJ, Claas FH. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009;82:148–157. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 136.Tilburgs T, van der Mast BJ, Nagtzaam NM, Roelen DL, Scherjon SA, Claas FH. Expression of NK cell receptors on decidual T cells in human pregnancy. J Reprod Immunol. 2009;80:22–32. doi: 10.1016/j.jri.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 137.Tilburgs T, Claas FH, Scherjon SA Elsevier Trophoblast Research Award Lecture. unique properties of decidual T cells and their role in immune regulation during human pregnancy. Placenta. 2010;31(Suppl):S82–S86. doi: 10.1016/j.placenta.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 138.Tilburgs T, Scherjon SA, Claas FH. Major histocompatibility complex (MHC)-mediated immune regulation of decidual leukocytes at the fetal-maternal interface. J Reprod Immunol. 2010;85:58–62. doi: 10.1016/j.jri.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 139.Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM, Prins F, van Lith JM, van der Mast BJ, Roelen DL, Scherjon SA, Claas FH. Human decidual tissue contains differentiated CD8 + effector-memory T cells with unique properties. J Immunol. 2010;185:4470–4477. doi: 10.4049/jimmunol.0903597. [DOI] [PubMed] [Google Scholar]
- 140.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 142.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in preeclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 143.Poon LL, Leung TN, Lau TK, Lo YM. Presence of fetal RNA in maternal plasma. Clin Chem. 2000;46:1832–1834. [PubMed] [Google Scholar]
- 144.Hahn S, Holzgreve W. Fetal cells and cell-free fetal DNA in maternal blood: new insights into pre-eclampsia. Hum Reprod Update. 2002;8:501–508. doi: 10.1093/humupd/8.6.501. [DOI] [PubMed] [Google Scholar]
- 145.Ng EK, Tsui NB, Lau TK, Leung TN, Chiu RW, Panesar NS, Lit LC, Chan KW, Lo YM. mRNA of placental origin is readily detectable in maternal plasma. Proc Natl Acad Sci U S A. 2003;100:4748–4753. doi: 10.1073/pnas.0637450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Menezo Y, Elder K, Viville S. Soluble HLA-G release by the human embryo: an interesting artefact? Reprod Biomed Online. 2006;13:763–764. doi: 10.1016/s1472-6483(10)61021-8. [DOI] [PubMed] [Google Scholar]
- 147.Illanes S, Parra M, Serra R, Pino K, Figueroa-Diesel H, Romero C, Arraztoa JA, Michea L, Soothill PW. Increased free fetal DNA levels in early pregnancy plasma of women who subsequently develop preeclampsia and intrauterine growth restriction. Prenat Diagn. 2009;29:1118–1122. doi: 10.1002/pd.2372. [DOI] [PubMed] [Google Scholar]
- 148.Scharfe-Nugent A, Corr SC, Carpenter SB, Keogh L, Doyle B, Martin C, Fitzgerald KA, Daly S, O’Leary JJ, O’Neill LA. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188:5706–5712. doi: 10.4049/jimmunol.1103454. [DOI] [PubMed] [Google Scholar]
- 149.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 150.de Groot CJ, van der Mast BJ, Visser W, De Kuiper P, Weimar W, Van Besouw NM. Preeclampsia is associated with increased cytotoxic T-cell capacity to paternal antigens. Am J Obstet Gynecol. 2010;203:496, e491–496. doi: 10.1016/j.ajog.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 151.Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. Fetalspecific CD8 + cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol. 2012;189:1072–1080. doi: 10.4049/jimmunol.1200544. [DOI] [PubMed] [Google Scholar]
- 152.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, Lee J, Sundell IB, Mittal P, Kusanovic JP, Hassan SS, Kim JS. Peripheral CD300a+CD8 + T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol. 2012;67:184–197. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 154.Pearson RD. Immunogenicity, parturition and the prostaglandins. Med Hypotheses. 1979;5:1297–1303. doi: 10.1016/0306-9877(79)90097-5. [DOI] [PubMed] [Google Scholar]
- 155.Sato TA, Gupta DK, Keelan JA, Marvin KW, Mitchell MD. Expression of interleukin-1beta mRNA in murine uterine and gestational tissues: relationship with gestational age. Am J Reprod Immunol. 2001;46:413–419. doi: 10.1034/j.1600-0897.2001.d01-33.x. [DOI] [PubMed] [Google Scholar]
- 156.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 157.Sato W, Tomita A, Ichikawa D, Lin Y, Kishida H, Miyake S, Ogawa M, Okamoto T, Murata M, Kuroiwa Y, Aranami T, Yamamura T. CCR2 + CCR5 + T Cells Producing Matrix Metalloproteinase-9 and Osteopontin in the Pathogenesis of Multiple Sclerosis. J Immunol. 2012;189:5057–5065. doi: 10.4049/jimmunol.1202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25 + CD4 + regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao JX, Zeng YY, Liu Y. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol. 2007;75:71–81. doi: 10.1016/j.jri.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 160.Xiong H, Zhou C, Qi G. Proportional changes of CD4 + CD25 + Foxp3 + regulatory T cells in maternal peripheral blood during pregnancy and labor at term and preterm. Clin Invest Med. 2010;33:E422. doi: 10.25011/cim.v33i6.14594. [DOI] [PubMed] [Google Scholar]
- 161.Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90:935–944. doi: 10.1038/icb.2012.33. [DOI] [PubMed] [Google Scholar]
- 162.Steinborn A, Schmitt E, Kisielewicz A, Rechenberg S, Seissler N, Mahnke K, Schaier M, Zeier M, Sohn C. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol. 2012;167:84–98. doi: 10.1111/j.1365-2249.2011.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gomez-Lopez N, Laresgoiti-Servitje E. T regulatory cells: regulating both term and preterm labor? Immunol Cell Biol. 2012;90:919–920. doi: 10.1038/icb.2012.48. [DOI] [PubMed] [Google Scholar]
- 164.Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol. 2004;62:125–137. doi: 10.1016/j.jri.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 165.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 166.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 167.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 168.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 169.Cruikshank WW, Greenstein JL, Theodore AC, Center DM. Lymphocyte chemoattractant factor induces CD4-dependent intracytoplasmic signaling in lymphocytes. J Immunol. 1991;146:2928–2934. [PubMed] [Google Scholar]
- 170.Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H. Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab. 2004;89:2470–2476. doi: 10.1210/jc.2003-031293. [DOI] [PubMed] [Google Scholar]
- 171.Hirota Y, Osuga Y, Koga K, Yoshino O, Hirata T, Morimoto C, Harada M, Takemura Y, Nose E, Yano T, Tsutsumi O, Taketani Y. The expression and possible roles of chemokine CXCL11 and its receptor CXCR3 in the human endometrium. J Immunol. 2006;177:8813–8821. doi: 10.4049/jimmunol.177.12.8813. [DOI] [PubMed] [Google Scholar]
- 172.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Silasi M, Mor G. Decidual stromal cells as regulators of T-cell access to the maternal-fetal interface. Am J Reprod Immunol. 2012;68:279–281. doi: 10.1111/aji.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, Yoon BH. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181:989–994. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 175.Blois S, Tometten M, Kandil J, Hagen E, Klapp BF, Margni RA, Arck PC. Intercellular adhesion molecule-1/LFA-1 cross talk is a proximate mediator capable of disrupting immune integration and tolerance mechanism at the feto-maternal interface in murine pregnancies. J Immunol. 2005;174:1820–1829. doi: 10.4049/jimmunol.174.4.1820. [DOI] [PubMed] [Google Scholar]
- 176.Manes TD, Pober JS. Identification of endothelial cell junctional proteins and lymphocyte receptors involved in transendothelial migration of human effector memory CD4 + T cells. J Immunol. 2011;186:1763–1768. doi: 10.4049/jimmunol.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dawson DW. Eosinophils and pregnancy. J Obstet Gynaecol Br Emp. 1953;60:727–731. doi: 10.1111/j.1471-0528.1953.tb07268.x. [DOI] [PubMed] [Google Scholar]
- 178.Leppi J. Role of neutrophils in dilation during parturition. Am J Obstet Gynecol. 1981;141:354–355. doi: 10.1016/s0002-9378(16)32648-5. [DOI] [PubMed] [Google Scholar]
- 179.Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, Uldbjerg N. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–246. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 180.Lockwood CJ, Toti P, Arcuri F, Paidas M, Buchwalder L, Krikun G, Schatz F. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol. 2005;167:1443–1449. doi: 10.1016/S0002-9440(10)61230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kelly RW, Illingworth P, Baldie G, Leask R, Brouwer S, Calder AA. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod. 1994;9:253–258. doi: 10.1093/oxfordjournals.humrep.a138491. [DOI] [PubMed] [Google Scholar]
- 182.Canavan TP, Simhan HN. Innate immune function of the human decidual cell at the maternal-fetal interface. J Reprod Immunol. 2007;74:46–52. doi: 10.1016/j.jri.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 183.Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG. 2007;114:1326–1334. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- 184.Pavlov O, Pavlova O, Ailamazyan E, Selkov S. Characterization of cytokine production by human term placenta macrophages in vitro. Am J Reprod Immunol. 2008;60:556–567. doi: 10.1111/j.1600-0897.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 185.Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci. 2010;17:209–218. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- 186.Horton JS, Yamamoto SY, Bryant-Greenwood GD. Relaxin modulates proinflammatory cytokine secretion from human decidual macrophages. Biol Reprod. 2011;85:788–797. doi: 10.1095/biolreprod.110.089201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schatz F, Kayisli UA, Vatandaslar E, Ocak N, Guller S, Abrahams VM, Krikun G, Lockwood CJ. Toll-like receptor 4 expression in decidual cells and interstitial trophoblasts across human pregnancy. Am J Reprod Immunol. 2012;68:146–153. doi: 10.1111/j.1600-0897.2012.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zaga-Clavellina V, Martha RV, Flores-Espinosa P. In vitro secretion profile of pro-inflammatory cytokines IL-1beta, TNF-alpha, IL-6, and of human beta-defensins (HBD)-1, HBD-2, and HBD-3 from human chorioamniotic membranes after selective stimulation with Gardnerella vaginalis. Am J Reprod Immunol. 2012;67:34–43. doi: 10.1111/j.1600-0897.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- 189.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A) Representative diagram of spread out fetal membranes. Area was calculated by measuring the dotted lines. (B) Representative dot-plots showing the dual parameters used for the flow cytometric analysis. Monocytes (CD45+CD14+), NK cells (CD45+CD56+), B cells (CD45+CD19+) and T cells (CD45+CD3+) were analyzed within the CD45+ gate. T cell subsets were analyzed within the CD3+ gate, P4 CD3+CD8+, P5 CD3+CD4+, P6 CD3+CD4−CD8−.
Table S1. Panel of antibodies used in this study.
Table S2. Primers and probes used for performing real time PCR.