Abstract
Objective
To determine the pregnancy outcome of asymptomatic patients in the second trimester with a non-measurable cervical length (“0 mm”).
Methods
This retrospective cohort study included 78 patients with singleton pregnancies and a sonographic non-measurable cervix detected at 14–28 weeks’ gestation. Patients with cervical cerclage were excluded.
Results
1) 75.3% of the patients delivered before 32 weeks; 2) the median diagnosis-to-delivery interval was 20.5 days; the delivery rate within 7 and 14 days was 28.2% and 35.6%, respectively; 3) patients with a non-measurable cervix diagnosed <24 weeks had a shorter median diagnosis-to-delivery interval than those diagnosed at 24–28 weeks (17.5 vs. 41 days; p=0.009).
Conclusions
1) Asymptomatic women with a non-measurable cervix in the second trimester have a median diagnosis-to-delivery interval of ~3 weeks; 2) almost 65% of these patients will not deliver within two weeks, yet 75% deliver before 32 weeks; 3) the earlier a non-measurable cervix is identified the shorter the diagnosis-to-delivery interval.
Keywords: intra-amniotic infection, inflammation, interleukin-6, IL-6, short cervix, pregnancy, preterm labor, preterm delivery, prematurity, transvaginal ultrasound, sonography, sludge
INTRODUCTION
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide.1–3 A short cervical length is recognized as a powerful predictor of spontaneous preterm birth,4–23 and transvaginal cervical sonography is the most objective and reliable method to assess cervical length.9;14;15;19;24–27 However, there is no agreement as to what is the definition of a sonographic short cervix. 9;14;19 Iams et al.9 reported that a sonographic cervical length of 25 mm or less at 24 weeks of gestation is associated with a prevalence of 4.3% of spontaneous preterm delivery before 35 weeks of gestation and a positive predictive value of only 17.8%. Subsequently, a cut-off of 15 mm was proposed.14;19 The prevalence of a sonographic cervical length of 15 mm or less ranges from 0.6%19 at 14–24 weeks and 1–1.7%14;28–31 at 20–24 weeks. The rate of preterm delivery in these patients varies according to the gestational age at diagnosis, and in asymptomatic women with a sonographic cervical length of ≤15 mm between 14 to 24 weeks of gestation, the rate of spontaneous preterm delivery at 32 weeks gestation or less is 48%.19
Despite the broad range of criteria for the definition of a sonographic short cervix,9;15;18–20;24;32–34 it is generally accepted that the shorter the sonographic cervical length in the mid-trimester, the higher the risk of spontaneous preterm labor/delivery.9;14;15;19;24 However, data regarding pregnancy outcome of asymptomatic patients with a non-measurable cervical length (usually described as a “0 mm” cervix) are limited and based on a small number of patients.14;35 These patients are considered to be at a very high risk of preterm delivery. Furthermore, therapeutic interventions such as vaginal progesterone,36–38 cervical cerclage,39–42 antibiotics,43 or indomethacin44 are of limited success in women with an extremely short cervix.41;44–48
The aim of this study was to determine the pregnancy outcome of asymptomatic patients with a non-measurable cervical length (“0 mm”) diagnosed in the second trimester of pregnancy (14 to 28 weeks’ gestation) by transvaginal sonography.
MATERIAL AND METHODS
Study population
This retrospective cohort study included pregnant women with a singleton pregnancy followed at our Cervix Clinic between January 2002 and December 2008. Using a computer-based search of our clinical and sonographic databases, consecutive asymptomatic patients between 14 and 28 weeks of gestation with a non-measurable cervical length (“0 mm”), as determined by a documented transvaginal ultrasound (TVUS) examination, were identified. Patients with one or more of the following conditions were excluded: 1) multifetal pregnancy; 2) premature contractions, preterm labor or preterm prelabor rupture of membranes at the time of diagnosis; 3) cervical cerclage (placed before or after the diagnosis of a short cervix); 4) placenta previa; and 5) fetuses with chromosomal and/or congenital anomalies.
Patients were diagnosed with a non-measurable cervical length during TVUS evaluation of the cervix. Digital assessment of the cervix was performed in all patients. The ultrasound findings were recorded and stored in a dedicated database. After the diagnosis of a short cervix, the patients were referred to the Labor and Delivery ward for further evaluation and management. Both the sonographic cervical length and the result of the digital vaginal examination were available to the managing physicians. The standard obstetrical practice in our institution is to offer amniocentesis in order to determine the microbial status of the amniotic cavity to patients with an asymptomatic short cervix, based on previous observations, suggesting an association between a sonographic short cervix and histologic chorioamnionitis49 and intra-amniotic infection.43;46;50
All participating women provided written informed consent prior to inclusion in this study. The use of clinical and ultrasound data for research purposes was approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Definitions and study procedures
Gestational age was determined by the last menstrual period or by ultrasound if the sonographic determination of gestational age was not consistent with the menstrual dating by more than one week in the first trimester and by more than two weeks in the second trimester of pregnancy. Gestational age at diagnosis was defined as the earliest gestation at which a cervical length of “0 mm” was documented by TVUS. Data regarding pregnancy outcomes were obtained from the clinical and research records. Patients lost to follow-up and for whom delivery data was not available were censored from the last available follow-up visit.
Patients with an a priori increased risk for spontaneous preterm delivery included those with a history of at least one of the following conditions: 1) one or more previous spontaneous preterm deliveries (≤35 weeks); 2) one or more late mid-trimester spontaneous miscarriages (≥16 weeks); 3) two or more dilatation & curettage (D&C) procedures; and 4) prior cervical surgery (LEEP or cone biopsy).
To maintain an interpretable temporal relationship between the results of amniocentesis and pregnancy outcome, only the results from amniocentesis performed within 7 days of diagnosis of a non-measurable cervical length were included in the statistical analyses. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms (aerobic / anaerobic bacteria or genital mycoplasmas). Intra-amniotic inflammation was defined as an amniotic fluid interleukin (IL)-6 concentration ≥2.6 ng/mL.51 Amniotic fluid IL-6 concentrations were determined using a specific and sensitive immunoassay (R&D Systems, Minneapolis, MN, USA) after all patients were delivered and were not used in clinical management.
From a clinical perspective, the gestational age at delivery is more important than the diagnosis-to-delivery interval per se. For example, an interval to delivery of 6 weeks for a patients diagnosed at 16 weeks is not associated with a better outcome than an interval of only 2 weeks in a patient diagnosed at 26 weeks. To overcome this limitation and to take into account the relative wide range (14–28 weeks) of gestational age at diagnosis included in this study, an “Interval ratio” was calculated for each patient according to the following formula: diagnosis-to-delivery interval (days) / diagnosis-to-37 weeks interval (days). Ratios of ≥1 represent patients who delivered at term (≥37 weeks). The lower the ratio, the shorter the time the patient remained pregnant after diagnosis relative to expected remaining time to term.
Sonographic assessment of the cervix
Transvaginal ultrasound examination was conducted with commercially available two-dimensional (2D) and three-dimensional (3D) ultrasound systems (Acuson Sequoia, Siemens Medical Systems, Mountain View, CA, USA, Voluson 730 Expert™ or Voluson E8, GE Healthcare, Milwaukee, WI, USA) equipped with endovaginal transducers with frequency ranges of 5–7.5 MHz and 5–9 MHz, respectively. All sonographic examinations of the cervical length were performed by Registered Diagnostic Medical Sonographers using a technique previously described,9;24 and reviewed by an experienced physician. Amniotic fluid “sludge” was identified by the presence of dense aggregates of particulate matter in proximity to the internal cervical os, as previously described.52 Two experienced sonographers, blinded to clinical outcome, reviewed the 2D images and 3D volume datasets of the cervix for the presence of amniotic fluid “sludge”, and it was considered to be present only when identified by both examiners.
Statistical analysis
The main outcome variables were the diagnosis-to-delivery interval, the rate of delivery within 7 and 14 days from diagnosis, and the rate of early preterm delivery (<32 weeks of gestation). Patients were further stratified by gestational age at diagnosis (<24 weeks vs. 24–28 weeks). Subjects who had an indicated preterm delivery due to a diagnosis that could not be directly attributed to the initial diagnosis of a short cervix (e.g. preeclampsia, fetal growth restriction, fetal death, etc.) were censored from the statistical analyses at the corresponding gestational age at induction of labor.
Comparisons among groups were performed using Fisher’s exact test for categorical variables, and Mann-Whitney U test for comparisons of continuous variables. Correlation between continuous variables was assessed by Spearman’s rho correlation test. Multivariate logistic regression (backward-stepwise) analyses were performed to determine the relationship between maternal age, gestational age at the time of ultrasound diagnosis, cervical dilation (as continuous variables), nulliparity, an a priori risk for preterm delivery, 17-hydroxyprogesterone caproate prophylactic treatment and the presence of amniotic fluid “sludge” (as categorical variables), and pregnancy outcomes (delivery within 7 days, 14 days and at <32 weeks). A Kaplan-Meier survival analysis was performed to assess the diagnosis-to-delivery interval according to the cervical length and presence or absence of AF “sludge.” A p value of <0.05 was considered statistically significant. The statistical package used was SPSS v.14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
During the study period, 78 asymptomatic patients with a sonographic cervical length of “0 mm” during the second trimester of pregnancy met the inclusion criteria of this study. The earliest gestational age at which an asymptomatic non-measurable cervical length was recorded by TVUS was 17 weeks and 4 days. There was a high correlation between the gestational age at diagnosis and the gestational age at delivery (Spearman’s rho 0.73, p<0.0001).
Demographic and clinical characteristics of the study population are listed in Table 1. Women diagnosed with a non-measurable cervical length between 24 and 28 weeks had a higher median pre-pregnancy BMI than those diagnosed before 24 weeks of gestation (p=0.027). Both the median gestational age at delivery and the median neonatal birthweight were lower in patients diagnosed before 24 weeks than in those diagnosed at 24 – 28 weeks (p<0.001 for both, Table 1). The median cervical dilatation was slightly greater in patients diagnosed before 24 weeks than in those diagnosed earlier in the mid-trimester (p=0.041). Yet, the median diagnosis-to-delivery interval was shorter among the group of patients diagnosed earlier in pregnancy (p=0.009, Table 2). Similarly, the “Interval ratio” (diagnosis-to-delivery interval / diagnosis-to-37 weeks interval) was significantly lower in patients diagnosed before 24 weeks than in those diagnosed between 24 to 28 weeks (p=0.001, Table 2)
Table 1.
Demographic and clinical characteristics of the study population
| Gestational age at diagnosis | ||||
|---|---|---|---|---|
| 14–28 weeks (n=78) |
<24 weeks (n=42) |
24 – 28 weeks (n =36) |
p * | |
| Age (years) | 24 (21.4–29.5) | 25 (22–31.2) | 22.7 (21–27.2) | 0.1 |
| African-American origin | 87.2 (68) | 88.1 (37) | 86.1 (31) | 0.99 |
| Smoking status | 17.9 (14) | 23.8 (10) | 11.1 (4) | 0.2 |
| Pre-pregnancy BMI (kg/m2) | 29.2 (23.9–34.9) | 27.2 (22.3–32) | 32.3 (26.4–40.1) | 0.027 |
| Nulliparity | 50.0 (39) | 47.6 (20) | 52.8 (19) | 0.8 |
| History of D&C | 56.8 (42/74) | 56.4 (22/39) | 57.1 (20/35) | 0.7 |
| History of spontaneous preterm delivery | 33.3 (26) | 35.7 (15) | 30.6 (11) | 0.8 |
| History of cervical surgery | 4.0 (3/75) | 7.5 (3/40) | 0 (0/35) | 0.2 |
| An a priori risk for preterm deliverya | 43.6 (34) | 45.2 (19) | 41.7 (15) | 0.8 |
| 17-OHP caproate treatment | 14.1 (11) | 8.3 (3) | 19 (8) | 0.2 |
| GA at diagnosis (weeks) | 23.6 (21.0–25.5) | 21.6 (20–23) | 25.9 (24.7–26.9) | <0.001 |
| Cervical dilatation at diagnosis (cm) | 1 (0–2.5) | 1 (0–2) | 1.5 (0.5–2.5) | 0.041 |
| GA at delivery (weeks) | 27 (23.9–31.9) | 24.3 (21.2–27.1) | 31.5 (27–36.4) | <0.001 |
| Birthweight (g) | 853 (546–1670) | 670 (375–853) | 1670 (910–2685) | <0.001 |
BMI - body mass index; D&C - dilatation and curettage; 17-OHP caproate - 17 hydroxyprogesterone caproate; GA - Gestational age
Data are presented as % (n) or median (interquartile range)
Between gestational age at diagnosis at <24 weeks and 24–28 weeks
Defined by the presence of history of at least one of the following: 1) ≥2 D&C; 2) spontaneous preterm delivery; and 3) cervical surgery
Table 2.
Diagnosis-to-delivery interval and the rate of spontaneous preterm delivery
| Gestational age at diagnosis | ||||
|---|---|---|---|---|
| 14–28 (n=78) |
< 24 weeks (n=42) |
24 – 28 weeks (n =36) |
p * | |
| Diagnosis-to-delivery interval (days) | 20.5 (5.7–57) | 17.5 (4–38.5) | 41 (13–75.5) | 0.009 |
| “Interval ratio”† | 0.23 (0.06–0.57) | 0.16 (0.04–0.37) | 0.49 (0.16–0.9) | 0.001 |
| Delivery within 7 days | 28.2 (22) | 38.1 (16) | 16.7 (6) | 0.04 |
| Delivery within 14 days | 35.6 (28) | 45.2 (19) | 25 (9) | 0.1 |
| Gestational age at delivery | ||||
| <24 weeks | NA | 45.2 (19) | NA | NA |
| <28 weeks | 53.8 (42) | 76.2 (32) | 27.8 (10) | <0.001 |
| <32 weeks | 75.3 (58/77) | 92.9 (39) | 54.3 (19/35) | <0.001 |
| <34 weeks | 81.3 (61/75) | 95.1 (39/41) | 64.7 (22/34.) | 0.001 |
| <37 weeks | 89.3 (67/75) | 97.6 (40/41) | 79.4 (27/34) | 0.02 |
Data are presented as median (interquartile range) or % (n)
“Interval ratio” – Diagnosis-to-delivery interval / diagnosis-to-37weeks interval
Between gestational age at diagnosis at <24 weeks and 24–28 weeks
NA – not available
The rate of delivery within 7 days and at <32 weeks: the association with gestational age at diagnosis
Table 2 displays the rate of preterm delivery within 7 and 14 days from diagnosis, as well as the rate of delivery at <24, <28, <32, <34 and <37 weeks of gestation. Patients diagnosed to have a non-measurable cervical length before 24 weeks had a higher rate of preterm delivery within 7 days from diagnosis (38.1% vs. 16.7%, p=0.04) and at <32 weeks (92.9% vs. 54.3%, p<0.001) than those diagnosed at 24 to 28 weeks of gestation.
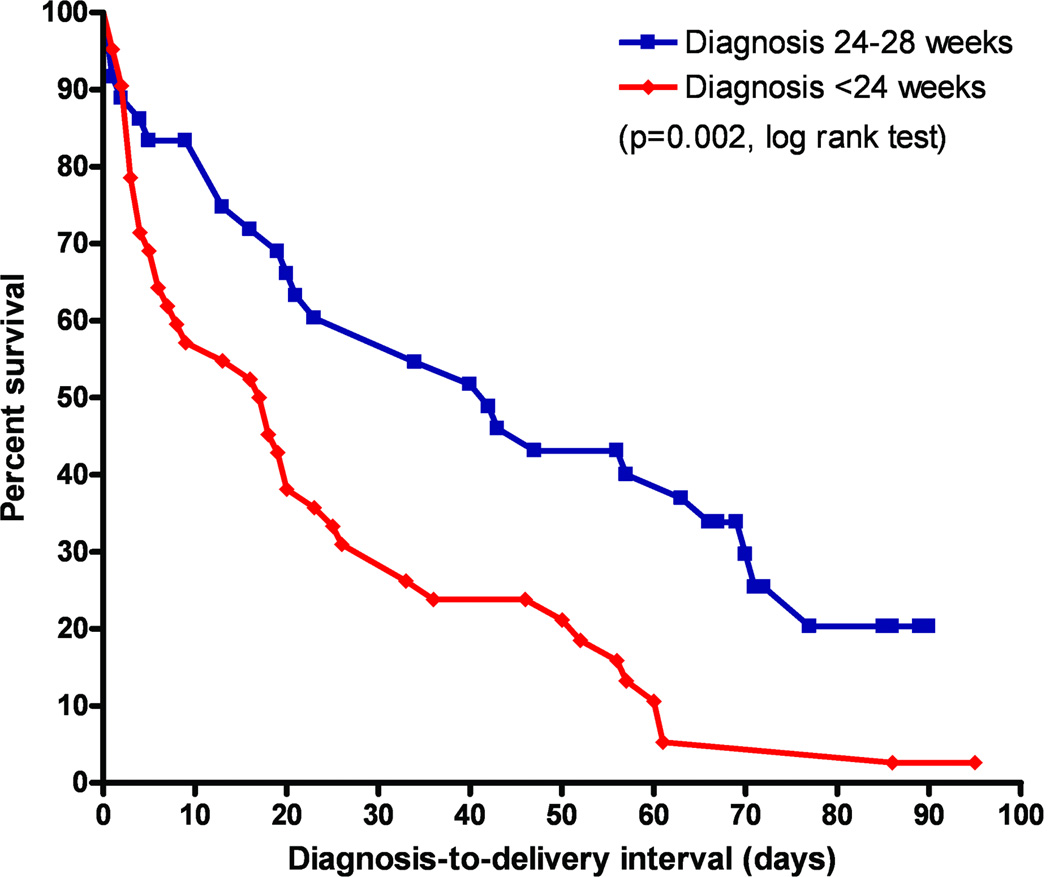
Kaplan-Meier survival curves of asymptomatic patients diagnosed with a non-measurable cervical length at <24 weeks and those diagnosed between 24 and 28 weeks of gestation are displayed in Figure 1. One patient was lost to follow-up and was censored at 32 weeks (the last available follow-up visit). All undelivered patients were censored at term (37 completed weeks of gestation). The survival curves of patients diagnosed at <24 weeks and of those diagnosed at 24–28 weeks differed significantly (p=0.002, log rank test,).
Figure 1. Survival curves according to gestational age at diagnosis.
A Kaplan-Meier survival analysis of the diagnosis-to-delivery interval (days) according to the gestational age at diagnosis (<24 weeks and 24–28 weeks) in asymptomatic women with a non-measurable cervical length in the second trimester of pregnancy. All patients were censored at 37 completed weeks of gestation. The survival curves differed significantly between the two groups (p=0.002, log rank test).
The association between an a priori risk for preterm delivery, a non-measurable cervical length, and preterm birth
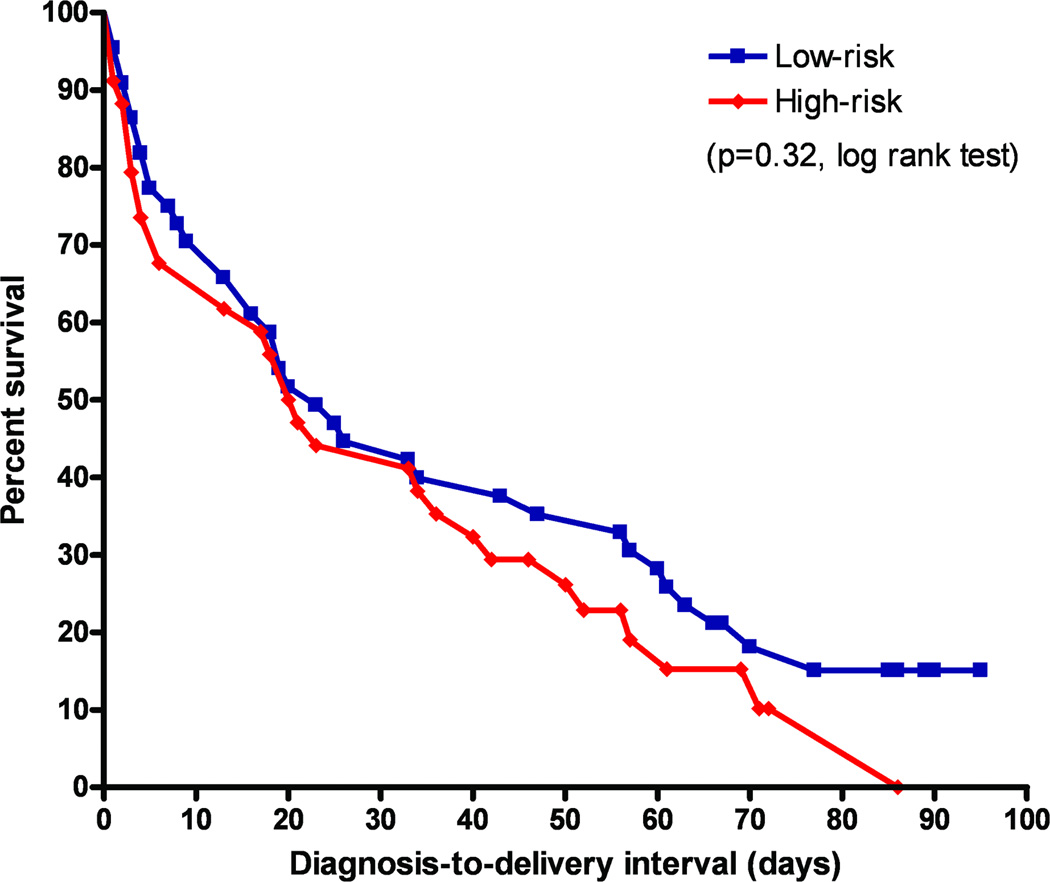
Nineteen patients (45.2%) in the group diagnosed at <24 weeks and 15 patients (41.7%) in the group diagnosed between 24 and 28 weeks had an a priori increased risk for a spontaneous preterm delivery (p=0.8). There was no significant difference between the Kaplan-Meier survival curves of patients with or without an a priori increased risk for a preterm delivery (p=0.32, log rank test; Figure 2).
Figure 2. Survival curves according to an a priori risk for preterm delivery.
A Kaplan-Meier survival analysis of the diagnosis-to-delivery interval (days) according to the presence of an a priori increased risk for spontaneous preterm delivery (HRP) among asymptomatic women with a non-measurable cervical length in the second trimester of pregnancy. An a priori risk for preterm delivery was defined by history of at least one of the following: 1) ≥2 D&C; 2) spontaneous preterm delivery; and 3) cervical surgery. All patients were censored at 37 completed weeks of gestation. The survival curves of patients with and without such a history did not differ significantly (p=0.32, log rank test).
Amniotic fluid “sludge”
Amniotic fluid “sludge” was observed by TVUS in 59% (46/78) of the patients. The rate of amniotic fluid “sludge” was higher among patients diagnosed before 24 weeks than among those diagnosed later in the mid-trimester (76.2% vs. 38.9%, p=0.001). Among patients diagnosed before 24 weeks, those with amniotic fluid “sludge” had a shorter median diagnosis-to-delivery interval (11 days, IQR 3.2–25.7 vs. 39.5 days, IQR 14.5–67.2, respectively; p=0.026) and a lower “Interval ratio” (0.09, IQR 0.03–0.23 vs. 0.37, IQR 0.15–0.63, p=0.016) than those without “sludge”. In addition, among patients diagnosed before 24 weeks, the rate of preterm delivery at <32 weeks was higher in patients with “sludge” than in those without this sonographic finding [100% (32/32) vs. 70% (7/10), p=0.01], but not among those diagnosed at 24 to 28 weeks [53.8% (7/13) vs. 54.5% (12/22), p=0.99]. In contrast, among patients diagnosed between 24 to 28 weeks, the median diagnosis-to-delivery interval (34 days, IQR 12–72.2 vs. 45 days, IQR 13–77.7, respectively; p=0.7) as well as the “Interval ratio” (0.48, IQR 0.14–0.9 vs. 0.55, IQR 0.18–0.93, respectively; p=0.8) were not significantly different between patients with or without amniotic fluid “sludge.”
Intra-amniotic infection and/or inflammation
Forty-six patients (60.5% of the study population) had undergone an amniocentesis within 7 days of diagnosis. Amniotic fluid samples for retrospective analysis of IL-6 concentration were available for 41 patients (89%). Table 3 presents the rate of intra-amniotic infection and/or inflammation among these patients. Overall, the rate of intra-amniotic infection among asymptomatic patients with a non-measurable cervical length in the second trimester of pregnancy was 10.9% (5/46). While the rate of intra-amniotic inflammation (IL-6 ≥2.6 ng/mL) in these patients was 63.4% (26/41) and among patients who had a negative amniotic fluid culture the rate was 58.3% (21/36). There were no significant differences in the rate of intra-amniotic infection and/or inflammation between women diagnosed <24 weeks and those diagnosed at 24 to 28 weeks (Table 3). Of note, there was no correlation between amniotic fluid IL-6 concentrations and gestational age at amniocentesis (Spearman’s rho −0.11, p=0.47). However, there was a negative correlation between IL-6 concentration and diagnosis-to-delivery interval (Spearman’s rho −0.54, p<0.001). Furthermore, the rate of intra-amniotic inflammation among patients who delivered within 7 days was higher than that of those who had a diagnosis-to-delivery interval >7 days [83.3% (15/18) vs. 47.8% (11/23), p=0.02)].
Table 3.
Rate of intra-amniotic infection and/or intra-amniotic inflammation
| Gestational age at diagnosis | ||||
|---|---|---|---|---|
| 14–28 weeks (n=78) |
<24 weeks (n=42) |
24 – 28 weeks (n =36) |
p * | |
| Amniocentesis within 7 days | 60.5 (46) | 73.8 (31) | 41.7 (15) | 0.006 |
| Intra-amniotic infection | 10.9 (5/46) | 9.7 (3/31) | 13.3 (2/15) | NS |
| Amniotic fluid white blood cell count ≥50 cells/mm3 | 17.4 (8/46) | 19.4 (6/31) | 13.3 (2/15) | NS |
| Intra-amniotic inflammation** | 63.4 (26/41) | 63.0 (17/27) | 64.3 (9/14) | NS |
| Intra-amniotic infection and/or inflammation | 65.9 (27/41) | 66.7 (18/27) | 64.3 (9/14) | NS |
Data are presented as % (n)
Between gestational age at diagnosis at <24 weeks and 24–28 weeks
Defined as IL-6 concentration ≥2.6 ng/mL
Among patients diagnosed before 24 weeks, the rate of intra-amniotic infection/inflammation was higher in women with amniotic fluid “sludge” than in those without “sludge” [77.3%, (17/22) vs. 20% (1/5), p=0.03]. In contrast, among patients diagnosed at 24 to 28 weeks, there was no difference in the rate of intra-amniotic infection/inflammation between those with and without amniotic fluid “sludge” ([50%, (3/6) vs. 75% (6/8), p=0.58].
Logistic regression analyses
Multivariable logistic regression (backward-stepwise) analyses of maternal age, gestational age at diagnosis, cervical dilation (as continuous variables), an a priori risk for preterm delivery, nulliparity, the presence of amniotic fluid “sludge” and progesterone treatment (as categorical variables) as explanatory variables for the pregnancy outcome revealed that only gestational age at diagnosis (OR 0.71, 95% CI 0.56–0.9, p=0.004) and cervical dilatation (OR 1.78, 95% CI 1.18–2.69, p=0.006) were independently associated with a preterm delivery within 7 days. In other words, the risk to deliver within 7 days of diagnosis of a non-measurable cervical length decreased by 29% for every 1 week increase in gestational age at diagnosis and increased by 78% for every 1 cm of cervical dilatation. In addition, only gestational age at diagnosis (OR 0.57, 95% CI 0.4–0.83, p=0.003) and the presence of amniotic fluid “sludge” (OR 4.25, 95% CI 1.02–17.75, p=0.047) were independently associated with a preterm delivery at <32 weeks of gestation.
COMMENT
Principal findings of this study
1) Asymptomatic women in the second trimester of pregnancy with a sonographic cervical length of “0 mm” had a median diagnosis-to-delivery interval of almost 3 weeks: one-third of the patients delivered within two weeks and 75% delivered before 32 weeks’ gestation; 2) patients with a non-measurable cervical length diagnosed before 24 weeks had a shorter diagnosis-to-delivery interval than those diagnosed between 24 to 28 weeks, regardless of the presence or absence of an a priori risk for preterm delivery; and 3) approximately two-thirds of patients with a non-measurable cervical length have intra-amniotic infection/inflammation.
“Non-measurable cervical length” – Clinical significance of the most extreme short cervix
The present study was explicitly designed to address the question of the clinical significance and natural history of the “non-measurable cervical length” (0 mm) in asymptomatic patients in the second trimester of pregnancy. Though these patients are considered to be at a very high risk for impending preterm delivery; the current data concerning the natural history of such patients is scarce. Indeed, the expected rate of preterm delivery of these patients is extrapolated from data obtained from patients with a short cervix (defined as <15 or <25 mm) or from observations which included a small number of patients with a “0 mm” cervical length.14;35
Our findings are in agreement with the study by Berghella et al35 in which the authors reported that the gestational age at which cervical length is measured significantly affects the calculation of risk of spontaneous preterm birth. Using a logistic regression model to calculate the risk of preterm delivery <32 weeks, the authors reported a risk of 60–70% for patients diagnosed with a “0 mm” cervix before 24 weeks of gestation. A slightly lower risk (54–59%) was calculated for women with a “0 mm” cervix diagnosed between 24 and 28 weeks of gestation. The present study demonstrates a higher rate of preterm delivery at <32 weeks, with a more striking difference in this rate between patients diagnosed <24 weeks of gestation (93%) and those diagnosed between 24 and 28 weeks (51.5%). Moreover, our study includes the largest sample size of asymptomatic patients with a non-measurable cervical length. This allowed us to report that the rate of preterm delivery within 14 days was relatively low, and almost two-thirds of the patients were still pregnant two weeks after ultrasound diagnosis. Moreover, women diagnosed with a non-measurable cervical length at <24 weeks of gestation had a significantly higher rate (38.1%) of preterm delivery within 7 days of ultrasound than patients diagnosed between 24 and 28 weeks (16.7%), despite a similar rate of intra-amniotic infection/inflammation in these two groups of patients. These data are beneficial in counseling patients with a non-measurable cervical length regarding possible management interventions (i.e. cervical cerclage, although we cannot argue on its beneficial effect from this study) or treatments (i.e. betamethasone).
Amniotic fluid “sludge” in asymptomatic non-measurable cervical length
Amniotic fluid ‘sludge,’ defined as particulate matter seen in proximity of the internal cervical os during a transvaginal sonographic examination of the cervix, occurs in 1% of uncomplicated term pregnancies.52 Our group demonstrated that among patients with preterm labor, amniotic fluid “sludge” is a risk factor for microbial invasion of the amniotic cavity, histological chorioamnionitis, and impending spontaneous preterm delivery.52 Moreover, among asymptomatic patients at high risk for preterm birth (based upon the presence of history of spontaneous preterm delivery, previous mid-trimester loss, a cervical length <25 mm, mullerian duct anomalies, or a history of cone biopsy), amniotic fluid “sludge” was reported to be an independent risk factor for spontaneous preterm delivery, preterm PROM, microbial invasion of the amniotic cavity, and histological chorioamnionitis.53
In the present study, the rate of amniotic fluid “sludge” among asymptomatic women with a non-measurable cervical length in the mid-trimester was 59%. Importantly, the rate of amniotic fluid “sludge” was significantly higher in women diagnosed at <24 weeks that in those diagnosed at 24 to 28 weeks. Consistent with the aforementioned studies, among asymptomatic patients with a non-measurable cervical length, amniotic fluid “sludge” was independently associated with a preterm delivery at <32 weeks. Of note, amniotic fluid “sludge” was significantly associated with a shorter diagnosis-to-delivery interval and a higher rate of preterm delivery at <32 weeks only among patients diagnosed before 24 weeks of gestation. Consistent with this finding, amniotic fluid “sludge” was associated with a higher rate of intra-amniotic infection/ inflammation only in patients diagnosed before 24 weeks.
An asymptomatic sonographic non-measurable short cervix - a clinical manifestation of intra-amniotic infection/inflammation
The present study does not allow us to determine whether a non-measurable cervix is the result of microbial invasion of the amniotic cavity or a predisposing factor. Nevertheless, in our study, a non-measurable cervical length was associated with a rate of intra-amniotic infection of 11%. This finding is in accordance with a previous report by our group in which 9% of asymptomatic women in the mid-trimester with a cervical length <25 mm and without cervical dilatation have a microbiologically-proven intra-amniotic infection.43
Intra-amniotic inflammation was present in almost two-thirds of patients with an asymptomatic non-measurable cervical length who had an amniocentesis within one week of ultrasound diagnosis. The rate of intra-amniotic inflammation in these patients is remarkably higher than that reported in patients with a cervical length ≤15 mm (23%),54 preterm labor (23%),51 or with preterm PROM (30%),55 and only slightly lower than that of patients with acute cervical insufficiency (79%).56 Consistent with previous reports of patients with a short cervix,54;57;58 intra-amniotic inflammation, even in the absence of proven MIAC (data not shown), was a risk factor for preterm delivery within 7 days of the diagnosis of a non-measurable cervical length. Furthermore, amniotic fluid concentrations of IL-6 significantly correlated with diagnosis-to-delivery interval.
An a priori risk for preterm delivery is not contributory to the risk assessment of asymptomatic patients with a non-measurable cervical length
A history of a spontaneous preterm birth,59–61 a cervical surgery,62 or termination of pregnancy27;63 have all been previously demonstrated to be associated with an increased risk of preterm delivery. In our study, however, an a priori risk for a recurrent spontaneous preterm birth was not contributory to the risk assessment of asymptomatic patients with a non-measurable cervical length. Indeed, the survival curves of “low-risk” and “high-risk” patients for preterm delivery were similar. It is possible that among patients with an extremely short cervix, the presence of an a priori risk factor for preterm delivery does not confer an additional risk.
Individualized risk assessment has been advocated in the prediction of preterm parturition. As a single variable, sonographic cervical length is currently the most powerful predictor of preterm birth. The results of the present study indicate that even in the presence of a non-measurable cervical length the diagnosis-to-delivery interval varies considerably. Paradoxically, in this subset of patients with an extremely high rate of preterm delivery before 32 weeks (75%), we can not use the most reliable tool available to further refine risk assessment. Potential variables that may be beneficial in this context include the patient’s history, gestational age at diagnosis, the rate of cervical shortening over time, cervical dilatation, the presence of intra-amniotic infection and/or inflammation, the presence of amniotic fluid “sludge” and the fetal fibronectin test. In the present study, we were able to demonstrate that gestational age at diagnosis, cervical dilatation, and amniotic fluid “sludge,” were independently associated with one or more of the pregnancy outcomes. Further studies are needed in order to determine the relevance and relative importance of each of the abovementioned putative variables.
In conclusion, our findings demonstrate that asymptomatic women in the second trimester of pregnancy with a non-measurable sonographic cervical length have a median diagnosis-to-delivery interval that is largely dependent on the gestational age at diagnosis. Patients diagnosed before 24 weeks of gestation have a significantly shorter diagnosis-to-delivery interval than those diagnosed after 24 weeks. However, since most patients with a “0 mm” cervix in the mid-trimester will not deliver within two weeks, further studies are warranted in order to develop a customized integrated risk assessment to predict pregnancy outcome. Until that time, the findings of the present study can be helpful to physicians in the counseling and clinical management of patients with a non-measurable cervical length diagnosed in the second trimester of pregnancy.
Acknowledgment
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. The authors wish to acknowledge the contributions of the Registered Diagnostic Medical Sonographers staff and the Research Nurses of the Perinatology Research Branch:
Footnotes
Presented at the 56th Annual Meeting of the Society for Gynecological Investigation, March 18–21, 2009 Glasgow, Scotland
Reference List
- 1.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N.Engl.J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 2.Steer P. The epidemiology of preterm labour. BJOG. 2005;112(Suppl 1):1–3. 1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2005. Natl.Vital Stat.Rep. 2006;55:1–18. [PubMed] [Google Scholar]
- 4.Kushnir O, Vigil DA, Izquierdo L, Schiff M, Curet LB. Vaginal ultrasonographic assessment of cervical length changes during normal pregnancy. Am J Obstet Gynecol. 1990;162:991–993. doi: 10.1016/0002-9378(90)91302-s. [DOI] [PubMed] [Google Scholar]
- 5.Andersen HF. Transvaginal and transabdominal ultrasonography of the uterine cervix during pregnancy. J Clin Ultrasound. 1991;19:77–83. doi: 10.1002/jcu.1870190204. [DOI] [PubMed] [Google Scholar]
- 6.Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound Obstet Gynecol. 1992;2:402–409. doi: 10.1046/j.1469-0705.1992.02060402.x. [DOI] [PubMed] [Google Scholar]
- 7.Iams JD, Paraskos J, Landon MB, Teteris JN, Johnson FF. Cervical sonography in preterm labor. Obstet Gynecol. 1994;84:40–46. [PubMed] [Google Scholar]
- 8.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–1103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 9.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A et al. The length of the cervix and the risk of spontaneous premature delivery. N.Engl.J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa I, Tanaka K, Takahashi K, Tanaka T, Aoki K, Torii Y et al. Transvaginal ultrasonographic cervical assessment for the prediction of preterm delivery. J Matern Fetal Med. 1996;5:305–309. doi: 10.1002/(SICI)1520-6661(199611/12)5:6<305::AID-MFM2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Berghella V, Kuhlman K, Weiner S, Texeira L, Wapner RJ. Cervical funneling: sonographic criteria predictive of preterm delivery. Ultrasound Obstet Gynecol. 1997;10:161–166. doi: 10.1046/j.1469-0705.1997.10030161.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad AH, Copper RL et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network. Am J Public Health. 1998;88:233–238. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman ER, Mellon C, Vintzileos AM, Ananth CV, Walters C, Gipson K. Longitudinal assessment of endocervical canal length between 15 and 24 weeks' gestation in women at risk for pregnancy loss or preterm birth. Obstet Gynecol. 1998;92:31–37. doi: 10.1016/s0029-7844(98)00120-3. [DOI] [PubMed] [Google Scholar]
- 14.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 15.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks' gestation and the risk of preterm delivery. Obstet.Gynecol. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 16.Watson WJ, Stevens D, Welter S, Day D. Observations on the sonographic measurement of cervical length and the risk of premature birth. J Matern Fetal Med. 1999;8:17–19. doi: 10.1002/(SICI)1520-6661(199901/02)8:1<17::AID-MFM4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Hibbard JU, Tart M, Moawad AH. Cervical length at 16–22 weeks' gestation and risk for preterm delivery. Obstet Gynecol. 2000;96:972–978. doi: 10.1016/s0029-7844(00)01074-7. [DOI] [PubMed] [Google Scholar]
- 18.Andrews WW, Copper R, Hauth JC, Goldenberg RL, Neely C, Dubard M. Second-trimester cervical ultrasound: associations with increased risk for recurrent early spontaneous delivery. Obstet Gynecol. 2000;95:222–226. doi: 10.1016/s0029-7844(99)00483-4. [DOI] [PubMed] [Google Scholar]
- 19.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 20.Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, III et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;%19;286:1340–1348. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 21.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18:200–203. doi: 10.1046/j.1469-0705.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 22.Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193:1170–1174. doi: 10.1016/j.ajog.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 23.Matijevic R, Grgic O, Vasilj O. Is sonographic assessment of cervical length better than digital examination in screening for preterm delivery in a low-risk population? Acta Obstet Gynecol Scand. 2006;85:1342–1347. doi: 10.1080/00016340600935722. [DOI] [PubMed] [Google Scholar]
- 24.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet.Gynecol. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 25.Tekesin I, Eberhart LH, Schaefer V, Wallwiener D, Schmidt S. Evaluation and validation of a new risk score (CLEOPATRA score) to predict the probability of premature delivery for patients with threatened preterm labor. Ultrasound Obstet.Gynecol. 2005;26:699–706. doi: 10.1002/uog.2633. [DOI] [PubMed] [Google Scholar]
- 26.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–367. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 27.Visintine J, Berghella V, Henning D, Baxter J. Cervical length for prediction of preterm birth in women with multiple prior induced abortions. Ultrasound Obstet Gynecol. 2008;31:198–200. doi: 10.1002/uog.5193. [DOI] [PubMed] [Google Scholar]
- 28.Heath VC, Southall TR, Souka AP, Novakov A, Nicolaides KH. Cervical length at 23 weeks of gestation: relation to demographic characteristics and previous obstetric history. Ultrasound Obstet Gynecol. 1998;12:304–311. doi: 10.1046/j.1469-0705.1998.12050304.x. [DOI] [PubMed] [Google Scholar]
- 29.To MS, Alfirevic Z, Heath VC, Cicero S, Cacho AM, Williamson PR et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–1853. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 30.Palma-Dias RS, Fonseca MM, Stein NR, Schmidt AP, Magalhaes JA. Relation of cervical length at 22–24 weeks of gestation to demographic characteristics and obstetric history. Braz.J Med.Biol Res. 2004;37:737–744. doi: 10.1590/s0100-879x2004000500016. [DOI] [PubMed] [Google Scholar]
- 31.Celik E, To M, Gajewska K, Smith GC, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol. 2008;31:549–554. doi: 10.1002/uog.5333. [DOI] [PubMed] [Google Scholar]
- 32.Tongsong T, Kamprapanth P, Srisomboon J, Wanapirak C, Piyamongkol W, Sirichotiyakul S. Single transvaginal sonographic measurement of cervical length early in the third trimester as a predictor of preterm delivery. Obstet Gynecol. 1995;86:184–187. doi: 10.1016/0029-7844(95)00152-h. [DOI] [PubMed] [Google Scholar]
- 33.Berghella V, Tolosa JE, Kuhlman K, Weiner S, Bolognese RJ, Wapner RJ. Cervical ultrasonography compared with manual examination as a predictor of preterm delivery. Am J Obstet Gynecol. 1997;177:723–730. doi: 10.1016/s0002-9378(97)70259-x. [DOI] [PubMed] [Google Scholar]
- 34.To MS, Skentou C, Cicero S, Liao AW, Nicolaides KH. Cervical length at 23 weeks in triplets: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2000;16:515–518. doi: 10.1046/j.1469-0705.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 35.Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110:311–317. doi: 10.1097/01.AOG.0000270112.05025.1d. [DOI] [PubMed] [Google Scholar]
- 36.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N.Engl.J.Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 37.DeFranco EA, O'Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S et al. Vaginal progesterone decreases the risk of early preterm birth and improves neonatal outcome in women with a short cervix. Ultrasound Obstet Gynecol. 2007 doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 38.Dodd JM, Flenady VJ, Cincotta R, Crowther CA. Progesterone for the prevention of preterm birth: a systematic review. Obstet Gynecol. 2008;112:127–134. doi: 10.1097/AOG.0b013e31817d0262. [DOI] [PubMed] [Google Scholar]
- 39.Heath VC, Souka AP, Erasmus I, Gibb DM, Nicolaides KH. Cervical length at 23 weeks of gestation: the value of Shirodkar suture for the short cervix. Ultrasound Obstet Gynecol. 1998;12:318–322. doi: 10.1046/j.1469-0705.1998.12050318.x. [DOI] [PubMed] [Google Scholar]
- 40.Althuisius SM. The short and funneling cervix: when to use cerclage? Curr.Opin.Obstet Gynecol. 2005;17:574–578. doi: 10.1097/01.gco.0000188727.53622.f9. [DOI] [PubMed] [Google Scholar]
- 41.Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106:181–189. doi: 10.1097/01.AOG.0000168435.17200.53. [DOI] [PubMed] [Google Scholar]
- 42.Owen J. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened mid-trimester cervical length. Am J Obstet Gynecol 199(6A (Supp)), S3. 2009;201:375, e1–e8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat.Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berghella V, Rust OA, Althuisius SM. Short cervix on ultrasound: does indomethacin prevent preterm birth? Am J Obstet Gynecol. 2006;195:809–813. doi: 10.1016/j.ajog.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Hassan SS, Romero R, Maymon E, Berry SM, Blackwell SC, Treadwell MC et al. Does cervical cerclage prevent preterm delivery in patients with a short cervix? Am J Obstet Gynecol. 2001;184:1325–1329. doi: 10.1067/mob.2001.115119. [DOI] [PubMed] [Google Scholar]
- 46.Rust OA, Atlas RO, Reed J, van GJ, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098–1105. doi: 10.1067/mob.2001.118163. [DOI] [PubMed] [Google Scholar]
- 47.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311–1317. doi: 10.1016/j.ajog.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 48.Fox NS, Chervenak FA. Cervical cerclage: a review of the evidence. Obstet Gynecol Surv. 2008;63:58–65. doi: 10.1097/OGX.0b013e31815eb368. [DOI] [PubMed] [Google Scholar]
- 49.Guzman ER, Shen-Schwarz S, Benito C, Vintzileos AM, Lake M, Lai YL. The relationship between placental histology and cervical ultrasonography in women at risk for pregnancy loss and spontaneous preterm birth. Am J Obstet Gynecol. 1999;181:793–797. doi: 10.1016/s0002-9378(99)70303-0. [DOI] [PubMed] [Google Scholar]
- 50.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652–655. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 51.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 52.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM et al. The prevalence and clinical significance of amniotic fluid 'sludge' in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–352. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 53.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E et al. Clinical significance of the presence of amniotic fluid 'sludge' in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, Yeo L, Mittal P, Yoon BH, Romero R. Patients with an asymptomatic short cervix (<=15mm) have a high rate of subclinical intra-amniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010 doi: 10.1016/j.ajog.2010.02.007. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 56.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633–638. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 57.Keeler SM, Kiefer DG, Rust OA, Vintzileos A, Atlas RO, Bornstein E, et al. Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome? Am J Obstet Gynecol. 2009;201:276, e1–e6. doi: 10.1016/j.ajog.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 58.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:374–375. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 59.Mercer BM, Goldenberg RL, Moawad AH, Meis PJ, Iams JD, Das AF et al. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1999;181:1216–1221. doi: 10.1016/s0002-9378(99)70111-0. [DOI] [PubMed] [Google Scholar]
- 60.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195:643–650. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 61.Goldenberg RL, Andrews WW, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth Project: placental histology in recurrent spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2006;195:792–796. doi: 10.1016/j.ajog.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 62.Berghella V, Pereira L, Gariepy A, Simonazzi G. Prior cone biopsy: prediction of preterm birth by cervical ultrasound. Am J Obstet Gynecol. 2004;191:1393–1397. doi: 10.1016/j.ajog.2004.06.087. [DOI] [PubMed] [Google Scholar]
- 63.Shah PS, Zao J. Induced termination of pregnancy and low birthweight and preterm birth: a systematic review and meta-analyses. BJOG. 2009;116:1425–1442. doi: 10.1111/j.1471-0528.2009.02278.x. [DOI] [PubMed] [Google Scholar]