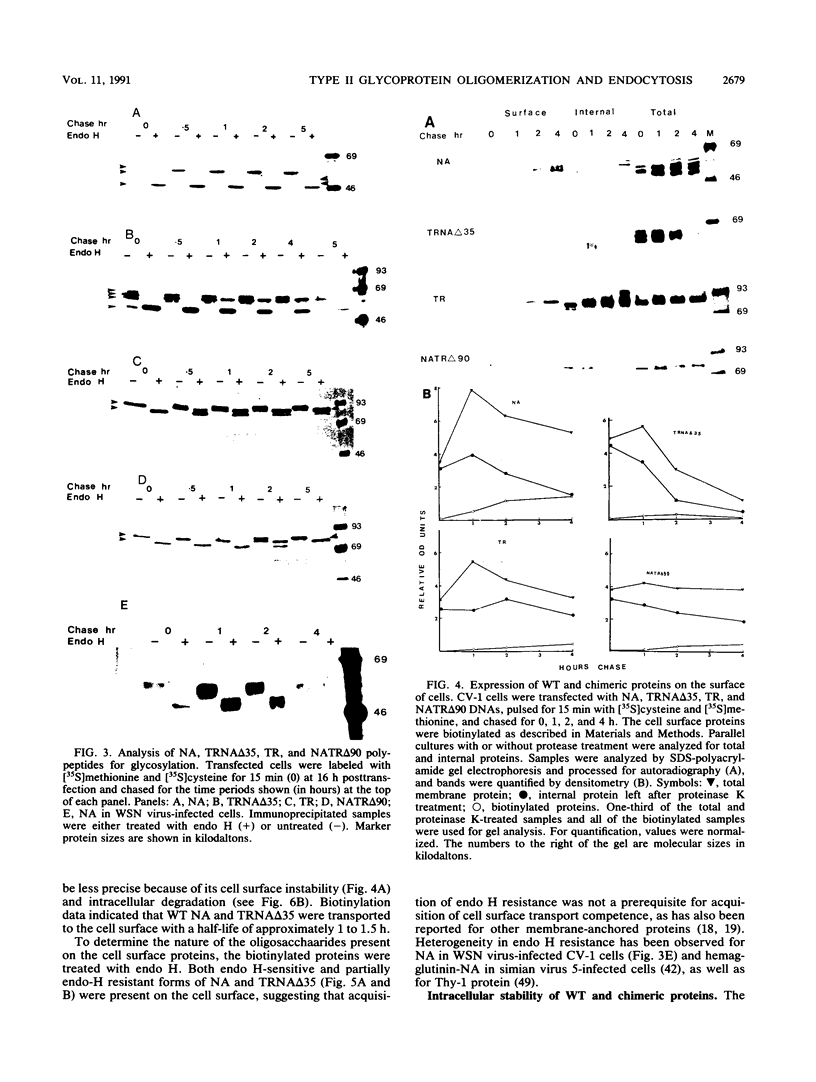
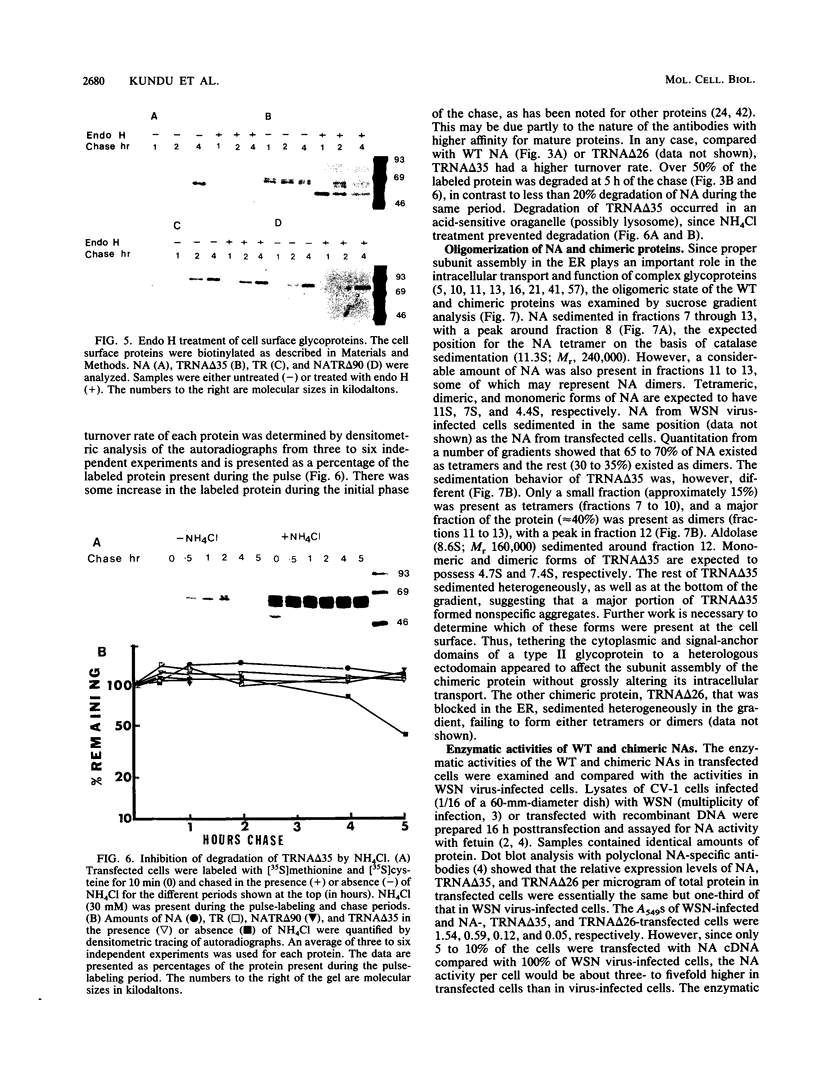
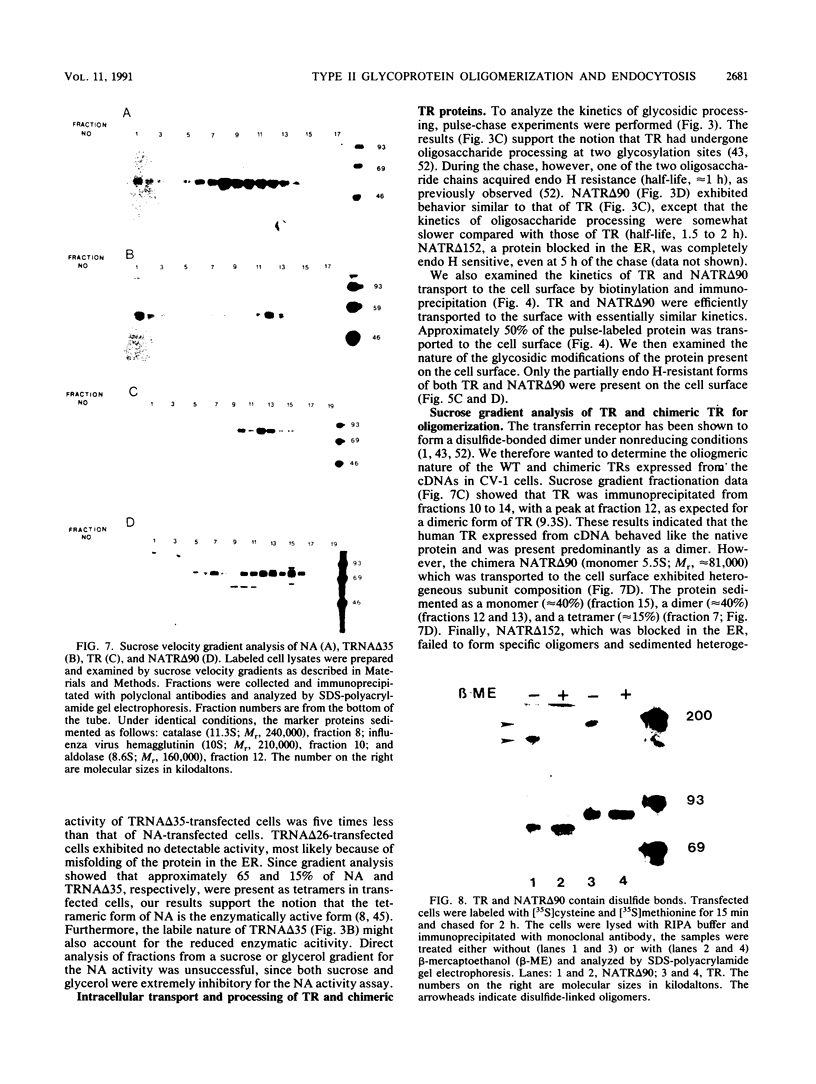
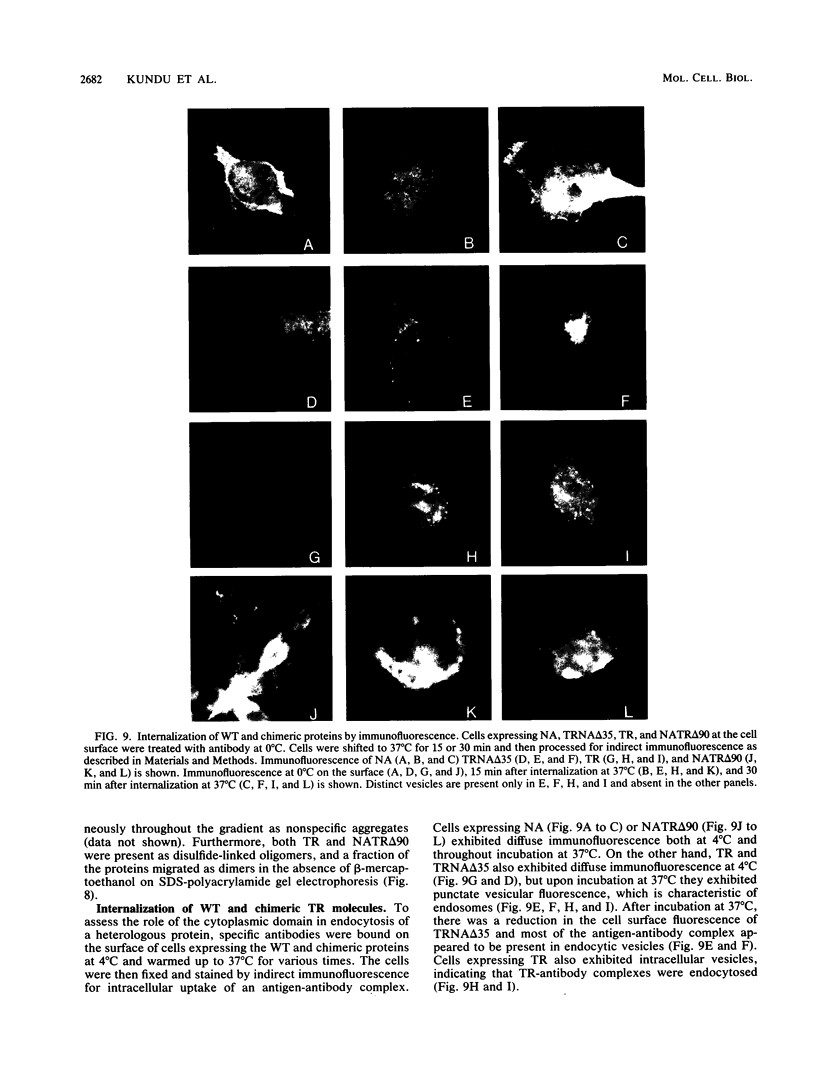
Abstract
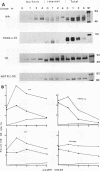
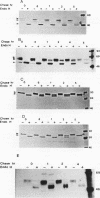
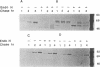
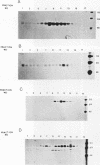
We investigated the role of cytoplasmic and anchor domains of type II glycoproteins in intracellular transport, oligomerization, and endocytosis by expressing the wild-type and chimeric genes in mammalian cells. Chimeric genes were constructed by exchanging the DNA segments that encode the cytoplasmic and anchor domains between the human influenza virus (A/WSN/33) neuraminidase (NA) and transferrin receptor (TR). The chimeric proteins in which domains were exchanged precisely were productively targeted to the cell surface. However, the proteins appeared to assemble differently in the intracellular compartment. For example, while TR existed predominantly as a dimer, NATR delta 90, containing the cytoplasmic and signal-anchor domains of NA and the ectodomain of TR, was present as a tetramer, a dimer, and a monomer. Similarly, the influenza virus NA existed predominantly as a tetramer but TRNA delta 35, in which the cytoplasmic and signal-anchor domains of TR were joined to the ectodomain of NA, existed predominantly as a dimer, suggesting that the cytoplasmic and anchor domains of type II glycoproteins affect the subunit assembly of heterologous ectodomains. In addition, we analyzed the role of the cytoplasmic domain in endocytosis. NA and NATR delta 90 did not undergo endocytosis, whereas both TR and TRNA delta 35 were internalized efficiently, demonstrating that the NH2 cytoplasmic domain of TR was capable of internalizing a heterologous ectodomain (NA) from the cell surface.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul Jabbar M., Nayak D. P. Signal processing, glycosylation, and secretion of mutant hemagglutinins of a human influenza virus by Saccharomyces cerevisiae. Mol Cell Biol. 1987 Apr;7(4):1476–1485. doi: 10.1128/mcb.7.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E., Gironès N., Davis R. J. Intermolecular disulfide bonds are not required for the expression of the dimeric state and functional activity of the transferrin receptor. EMBO J. 1989 Aug;8(8):2231–2240. doi: 10.1002/j.1460-2075.1989.tb08347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos T. J., Davis A. R., Nayak D. P. NH2-terminal hydrophobic region of influenza virus neuraminidase provides the signal function in translocation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2327–2331. doi: 10.1073/pnas.81.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos T. J., Nayak D. P. Identification of defects in the neuraminidase gene of four temperature-sensitive mutants of A/WSN/33 influenza virus. Virology. 1986 Oct 15;154(1):85–96. doi: 10.1016/0042-6822(86)90432-0. [DOI] [PubMed] [Google Scholar]
- Boulay F., Doms R. W., Webster R. G., Helenius A. Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J Cell Biol. 1988 Mar;106(3):629–639. doi: 10.1083/jcb.106.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Ward C. W. Structure and diversity of influenza virus neuraminidase. Curr Top Microbiol Immunol. 1985;114:177–255. doi: 10.1007/978-3-642-70227-3_5. [DOI] [PubMed] [Google Scholar]
- Compton T., Ivanov I. E., Gottlieb T., Rindler M., Adesnik M., Sabatini D. D. A sorting signal for the basolateral delivery of the vesicular stomatitis virus (VSV) G protein lies in its luminal domain: analysis of the targeting of VSV G-influenza hemagglutinin chimeras. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4112–4116. doi: 10.1073/pnas.86.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Bos T. J., Nayak D. P. Active influenza virus neuraminidase is expressed in monkey cells from cDNA cloned in simian virus 40 vectors. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3976–3980. doi: 10.1073/pnas.80.13.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Cell surface expression of membrane-anchored v-sis gene products: glycosylation is not required for cell surface transport. J Cell Biol. 1986 Dec;103(6 Pt 1):2311–2322. doi: 10.1083/jcb.103.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert S. W., Lamb R. A. Cell surface expression of glycosylated, nonglycosylated, and truncated forms of a cytoplasmic protein pyruvate kinase. J Cell Biol. 1988 Sep;107(3):865–876. doi: 10.1083/jcb.107.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiti A. L., Nayak D. P. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J Virol. 1982 Feb;41(2):730–734. doi: 10.1128/jvi.41.2.730-734.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Rothenberger S., Kühn L. C. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988 Aug 12;54(4):485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Jabbar M. A., Nayak D. P. Intracellular interaction of human immunodeficiency virus type 1 (ARV-2) envelope glycoprotein gp160 with CD4 blocks the movement and maturation of CD4 to the plasma membrane. J Virol. 1990 Dec;64(12):6297–6304. doi: 10.1128/jvi.64.12.6297-6304.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S. Q., Spencer T., Miller K., Hopkins C., Trowbridge I. S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990 Feb;110(2):283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Ktistakis N. T., Thomas D., Roth M. G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990 Oct;111(4):1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarovits J., Roth M. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell. 1988 Jun 3;53(5):743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Lobigs M., Zhao H. X., Garoff H. Function of Semliki Forest virus E3 peptide in virus assembly: replacement of E3 with an artificial signal peptide abolishes spike heterodimerization and surface expression of E1. J Virol. 1990 Sep;64(9):4346–4355. doi: 10.1128/jvi.64.9.4346-4355.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McQueen N. L., Nayak D. P., Jones L. V., Compans R. W. Chimeric influenza virus hemagglutinin containing either the NH2 terminus or the COOH terminus of G protein of vesicular stomatitis virus is defective in transport to the cell surface. Proc Natl Acad Sci U S A. 1984 Jan;81(2):395–399. doi: 10.1073/pnas.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen N. L., Nayak D. P., Stephens E. B., Compans R. W. Basolateral expression of a chimeric protein in which the transmembrane and cytoplasmic domains of vesicular stomatitis virus G protein have been replaced by those of the influenza virus hemagglutinin. J Biol Chem. 1987 Nov 25;262(33):16233–16240. [PubMed] [Google Scholar]
- McQueen N., Nayak D. P., Stephens E. B., Compans R. W. Polarized expression of a chimeric protein in which the transmembrane and cytoplasmic domains of the influenza virus hemagglutinin have been replaced by those of the vesicular stomatitis virus G protein. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9318–9322. doi: 10.1073/pnas.83.24.9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S. T., Nayak D. P. Function of two discrete regions is required for nuclear localization of polymerase basic protein 1 of A/WSN/33 influenza virus (H1 N1). Mol Cell Biol. 1990 Aug;10(8):4139–4145. doi: 10.1128/mcb.10.8.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Jabbar M. A. Structural domains and organizational conformation involved in the sorting and transport of influenza virus transmembrane proteins. Annu Rev Microbiol. 1989;43:465–501. doi: 10.1146/annurev.mi.43.100189.002341. [DOI] [PubMed] [Google Scholar]
- Ng D. T., Randall R. E., Lamb R. A. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J Cell Biol. 1989 Dec;109(6 Pt 2):3273–3289. doi: 10.1083/jcb.109.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A. Conversion of a class II integral membrane protein into a soluble and efficiently secreted protein: multiple intracellular and extracellular oligomeric and conformational forms. J Cell Biol. 1990 Apr;110(4):999–1011. doi: 10.1083/jcb.110.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C., Braun M., Weber B., Wendland M., Schmidt B., Pohlmann R., Waheed A., von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990 Nov;9(11):3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Puddington L., Machamer C. E., Rose J. K. Cytoplasmic domains of cellular and viral integral membrane proteins substitute for the cytoplasmic domain of the vesicular stomatitis virus glycoprotein in transport to the plasma membrane. J Cell Biol. 1986 Jun;102(6):2147–2157. doi: 10.1083/jcb.102.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Doyle C., Sambrook J., Gething M. J. Heterologous transmembrane and cytoplasmic domains direct functional chimeric influenza virus hemagglutinins into the endocytic pathway. J Cell Biol. 1986 Apr;102(4):1271–1283. doi: 10.1083/jcb.102.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Sivasubramanian N., Nayak D. P. Mutational analysis of the signal-anchor domain of influenza virus neuraminidase. Proc Natl Acad Sci U S A. 1987 Jan;84(1):1–5. doi: 10.1073/pnas.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990 Sep;111(3):955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Yellen A., Bächi T. Monoclonal antibodies localize events in the folding, assembly, and intracellular transport of the influenza virus hemagglutinin glycoprotein. Cell. 1988 Mar 25;52(6):843–852. doi: 10.1016/0092-8674(88)90426-6. [DOI] [PubMed] [Google Scholar]