Abstract
Structural neuroimaging studies have demonstrated that all regions of the cortex are not affected equally by aging, with frontal regions appearing especially susceptible to atrophy. The “last in, first out” hypothesis posits that aging is, in a sense, the inverse of development: late-maturing regions of the brain are preferentially vulnerable to age-related loss of structural integrity. We tested this hypothesis by analyzing age-related changes in regional cortical thickness via three methods: (1) an exploratory linear regression of cortical thickness and age across the entire cortical mantle (2) an analysis of age-related differences in the thickness of zones of cortex defined by functional/cytoarchitectural affiliation (including primary sensory/motor, unimodal association, heteromodal association, and paralimbic zones), and (3) an analysis of age-related differences in the thickness of regions of cortex defined by surface area expansion in the period between birth and early adulthood. Subjects were grouped as young (aged 18–29, n = 138), middle-aged (aged 30–59, n = 80), young-old (aged 60–79, n = 60), and old–old (aged 80+, n = 38). Thinning of the cortex between young and middle-aged adults was greatest in heteromodal association cortex and regions of high postnatal surface area expansion. In contrast, thinning in old–old age was greatest in primary sensory/motor cortices and regions of low postnatal surface area expansion. In sum, these results lead us to propose a sequential “developmental-sensory” model of aging, in which developmental factors influence cortical vulnerability relatively early in the aging process, whereas later—in more advanced stages of aging—factors specific to primary sensory and motor cortices confer vulnerability. This model offers explicitly testable hypotheses and suggests the possibility that normal aging may potentially allow for multiple opportunities for intervention to promote the structural integrity of the cerebral cortex.
Keywords: Magnetic resonance imaging, Morphometry, Cortex, Aging
Introduction
To better understand why many domains of cognitive and sensorimotor function decline in normal aging, investigators have measured a variety of elements of the structure and function of a multitude of brain regions. In part because of the recent development of new measurement tools, increasing attention has been devoted to the investigation of age-related changes in the morphometry of the human cerebral cortex in vivo. Cortical volume and/or thickness have been measured using various techniques, including manual tracings of regions of interest (Jernigan et al. 1991; Sullivan et al. 1995; Raz et al. 1997; Jernigan et al. 2001; Raz et al. 2004; Allen et al. 2005; Raz et al. 2005), automated or semi-automated parcellation schemes (Resnick et al. 2000; Tisserand et al. 2002; Walhovd et al.; 2005), voxel-based analyses (Good et al. 2001; Taki et al. 2004; Grieve et al. 2005), and surface-based investigations of grey matter density (Sowell et al. 2003) and cortical thickness (Salat et al. 2004; Fjell et al. 2009). Despite the wealth of studies, numerous questions regarding the effects of aging on the gross structure of the cerebral cortex remain. Two key questions include: (1) What cortical regions of undergo the most prominent loss of structural integrity in the process of aging, and (2) Is there an organizing principle behind the pattern of regions affected? (Table 1).
Table 1.
Summary of selected structural MRI studies on aging
| Paper | Method | Limbic and paralimbic | Frontal, parietal, temporal association | Primary sensory and motor |
|---|---|---|---|---|
| Jernigan et al. 1991 | Manual seg., 8 regions, n = 55 | Anterior cingulate | Widespread volume loss in frontal, temporal, parietal lobes | |
| Sullivan et al. 1995 | Manual seg., n = 72 | Hippocampus | Reduction in extra-hippocampal temporal lobe gray matter volume with age | |
| Insausti et al. 1998 | Manual seg., n = 52 | Temporal pole; entorhinal, perirhinal cortex | ||
| Good et al. 2001 | VBM, n = 465 | Insula and cingulate; hippocampus, entorhinal cortex | Superior parietal cortex | Central sulcus |
| Jernigan et al. 2001 | Manual seg., n = 78 | Hippocampus | Volume loss accelerated in frontal lobes relative to other regions | |
| Tisserand et al. 2002 | Manual seg., semi- automated algorithm, VBM, n = 57 | Anterior cingulate, OFC | Entirety of frontal lobes, lateral and orbitofrontal regions predominantly | Striate cortex |
| Resnick et al. 2003 | Semi-automated seg., 4 year, longitudinal, n = 92 | Cingulate; insula; mesial temporal cortex; OFC | Frontal, parietal lobes > temporal lobe; inferior frontal cortex; inferior parietal cortex | Occipital cortex |
| Raz et al. 2004 | Manual seg., n = 200 | Hippocampus, parahippocampal gyrus, OFC; anterior cingulate | Lateral prefrontal, inferior temporal cortex, fusiform; inferior parietal cortex | Precentral and postcentral gyrus; 1° visual cortex |
| Salat et al. 2004 | Surface-wide analysis, cortical thickness, n = 106 | Cingulate, insula, medial temporal cortex | Prefrontal cortex, supramarginal gyrus | Calcarine cortex, 1° somatosensory and motor cortices |
| Taki et al. 2004 | VBM, n = 769 | Diffuse effects, including prominent effects in superior temporal gyrus, precentral gyrus | ||
| Allen et al. Allen et al. 2005 | Manual seg., n = 87 | Hippocampus, parahippocampal gyrus, temporal pole | Lobar effects frontal > temporal > parietal > occipital | |
| Grieve et al. 2005 | VBM, n = 223 | Insula; hippocampus, cingulate | DLPFC, superior and inferior parietal cortex; inferior and middle temporal gyri | Precentral, postcentral gyrus; calcarine cortex |
| Lemaitre et al. 2005 | VBM, n = 662 | Hippocampus, OFC | Angular gyrus, superior parietal cortex | Primary cortices |
| Raz et al. 2005 | Manual seg., 5 year longitudinal, n = 127 | Hippocampus, OFC; entorhinal cortex | Prefrontal cortex, inferior temporal cortex, fusiform, inferior parietal cortex | Visual cortex |
| Walhovd et al. 2005 | Automated seg., n = 73 | Hippocampus | Cortex (global) | |
| Smith et al. 2007 | VBM, n = 122 | Frontal, parietal, temporal cortex | ||
| Fjell et al. 2009 | Surface-wide analysis, cortical thickness, n = 883 | SFG, MFG, IFG; MTG; precuneus; inferior and superior parietal cortex; temporoparietal junction | Superior temporal gyrus | |
Default font indicates regions of age-associated atrophy; italic font denotes regions of relatively preserved volume or thickness
With regard to the first question, although the effects of aging are diffuse, some cortical regions do appear to consistently lose volume and/or thickness to a greater extent than other regions. Numerous studies have reported prominent loss of volume and/or thickness in the prefrontal cortex, particularly dorsolateral and dorsomedial prefrontal cortex (Jernigan et al. 1991; Raz et al. 1997; Resnick et al. 2000; Jernigan et al. 2001; Tisserand et al. 2002; Resnick et al. 2003; Raz et al. 2004; Salat et al. 2004; Allen et al. 2005; Grieve et al. 2005; Raz et al. 2005; Fjell et al. 2009), with many also implicating lateral parietal and lateral temporal association areas (Jernigan et al. 1991; Sullivan et al. 1995; Raz et al. 1997; Resnick et al. 2000; Good et al. 2001; Resnick et al. 2003; Raz et al. 2004; Salat et al. 2004; Allen et al. 2005; Grieve et al. 2005; Raz et al. 2005; Fjell et al. 2009).
Results pertaining to primary sensory and motor regions of the brain have been more inconsistent across studies. Some but not all studies report volume loss or thinning in somatosensory and motor regions (Good et al. 2001; Raz et al. 2004; Salat et al. 2004; Grieve et al. 2005). Likewise some studies have reported age-related loss of cortical tissue in visual cortices (Resnick et al. 2003; Salat et al. 2004; Raz et al. 2005; Fjell et al. 2009); whereas others have reported preservation of tissue in the same regions (Raz et al. 2004; Grieve et al. 2005). Primary viscerosensory regions of the caudal insular cortex also appear to undergo relatively prominent thinning in normal aging (Fjell et al. 2009).
Similar to primary sensory and motor cortices, results concerning limbic and paralimbic brain regions have been mixed. Multiple studies have reported volume loss in the hippocampus (Jernigan et al. 2001; Raz et al. 2005; Walhovd et al. 2005) and/or other medial temporal regions (Resnick et al. 2003; Raz et al. 2004; Allen et al. 2005), in contrast-to other studies that have reported relative preservation in the hippocampus (Sullivan et al. 1995; Good et al. 2001; Grieve et al. 2005) or entorhinal cortex (Raz et al. 2005). Studies reporting loss of volume or thinning in the cingulate and insula (Good et al. 2001; Resnick et al. 2003) have been balanced by others reporting preservation in the same regions (Jernigan et al. 1991; Raz et al. 2004; Salat et al. 2004), with one study reporting loss of insular volume and preservation of cingulate volume (Grieve et al. 2005). Multiple studies indicate that the orbitofrontal cortex is particularly vulnerable to the effects of aging (Tisserand et al. 2002; Resnick et al. 2003; Raz et al. 2004; Raz et al. 2005), although several investigations have raised the puzzling question of whether ventromedial prefrontal cortex may undergo paradoxical cortical thickening with normal aging (Salat et al. 2004; Fjell et al. 2009).
Given these mixed results, it has been challenging to address the question of whether there are organizing principles underlying the relatively greater selective vulnerability of some regions as opposed to others. Some investigators have approached this question at the level of analysis of selective neuronal or molecular vulnerability (Morrison and Hof 1997), while others have focused on larger-scale topographic factors, our primary interest here. Noting the consistent involvement of the prefrontal cortices in studies of aging, Raz and Rodrigue (Raz and Rodrigue 2006) have considered an anterior-to posterior gradient of volumetric loss. Alternatively, the “last in, first out” hypothesis” of aging posits that such vulnerability may arise because age-related structural changes occur preferentially in regions of the brain that mature relatively late in the life span (Raz 2000; Grieve et al. 2005; Davis et al. 2009; Fjell et al. 2009). This hypothesis has appeal due to the consistency with which association areas appear to lose structural integrity in the aging process, as these regions also tend to mature later than primary sensory/motor regions and paralimbic regions (Huttenlocher 1990; Huttenlocher and Dabholkar 1997; Gogtay et al. 2004; Casey et al. 2005). In challenge to the “last in, first out” hypothesis of aging, advocates of the “common cause” hypothesis have noted that degradation in sensory processing accounts for a large amount of the variance of age-related decline in cognitive performance, suggesting that loss of structural integrity in components of the nervous system that mediate sensory functioning should not be underplayed (Lindenberger and Baltes 1994; Baltes and Lindenberger 1997).
The purpose of the present analysis was to further investigate the topographic patterns of age-related cortical thinning with an emphasis on factors related to functional/cytoarchitectural classification of cortex, as well as trajectories of regional developmental expansion. In addition to an exploratory analysis of age-related thinning across the entire cortical mantle, we conducted two regionally-focused analyses. The first was based upon the functional zones proposed by Mesulam (Mesulam 2000) which built upon the work of pioneering neuroanatomists (Broca 1878; Filimonoff 1947; Bailey and von Bonin 1951; Yakovlev 1959; Pandya and Kuypers 1969; Jones and Powell 1970; Sanides 1970; von Economo and Triarhou 2009): primary sensory/motor, unimodal association cortex, heteromodal association cortex, and paralimbic cortex. The second made use of data from a recent study by Hill et al. (Hill et al. 2010) that reported on regional differences in the expansion of cortical surface area between birth and young adulthood. This study identified a set of cortical regions with a relatively greater postnatal expansion of surface area, including the dorsolateral prefrontal cortex, lateral parietal cortex, lateral temporal cortex, and primary somatosensory and motor cortices. Regions of relatively smaller postnatal expansion of surface area included the visual cortex, auditory cortex, ventromedial temporal cortex, parts of the rostral and orbital prefrontal cortex, and insula.
Our aim was to test the “last in, first out” hypothesis of aging by comparing age effects on cortical thickness in the different cortical functional zones, and by comparing regions of the brain with relatively low postnatal expansion in surface area with those with relatively high expansion. Results suggestive of the “last in, first out” hypothesis would include the finding of relatively late-developing regions such as the heteromodal association cortices exhibiting greater thinning in the aging process than relatively early-developing regions such as paralimbic and primary sensory/motor cortices. Similarly, another finding supportive of the “last in, first out” hypothesis would be greater age-related thinning in regions with a high degree of postnatal surface area expansion than regions with a low degree of postnatal surface area expansion. Alternatively, a high degree of thinning in primary sensory/motor regions and/or regions with a low degree of postnatal expansion would be more consistent with the common cause hypothesis of aging.
Methods
Subjects
Analyses were conducted with images from a subset of subjects from the OASIS dataset (Marcus et al. 2010). This dataset, freely available to the scientific community, comprises 416 right-handed individuals aged 18–96. We restricted our analysis to the subset of subjects without dementia (n = 316, 197 female). At time of enrollment, subjects had no clinical history of mild cognitive impairment, dementia, general neurological or psychiatric illness, or general medical illness with potential impact upon cognitive status. Older adults were evaluated using a structured clinical assessment (including an assessment enabling the use of the Clinical dementia rating (CDR) scale (Morris et al. 1997)), and were included only if their clinical status was that of Normal Cognition (CDR global and sum-of-boxes scores = 0).
Image Acquisition
Three or four high-resolution structural T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) images were acquired on a 1.5T Siemens Vision scanner (Siemens Medical Systems, Erlingan, Germany). MPRAGE parameters were empirically optimized for gray-white contrast (repetition time (TR) 9.7 ms, echo time (TE) 4 ms, flip angle (FA) 10, inversion time (TI) 20 ms, delay time (TD) 200 ms, 256 9 256 (1 9 1 mm2) in-plane resolution, 128 sagittal 1.25 mm slices without gaps, time per acquisition 6.6 min).
Cortical Surface Analysis
FreeSurfer software was used to process and analyze the imaging data (http://surfer.nmr.mgh.harvard.edu, version 4.5). The multiple T1 acquisitions for each subject were motion corrected and averaged to create a single image volume with high contrast-to-noise, and the resulting averaged volume used to segment cerebral white matter (Dale et al. 1999) and subcortical grey matter and ventricular regions (Fischl et al. 2002). Topological defects in the gray/white boundary were corrected (Fischl et al. 2001). Cortical thickness measurements were derived by calculating the distance between the gray/white boundary and the pial surface across the cortical mantle (Fischl and Dale 2000). The accuracy of this method has been previously validated by direct comparisons with manual measures on postmortem brain (Rosas et al. 2002). Test–retest reliability has been confirmed in participants scanned twice on the same scanner and across scanner manufacturers, field strengths, and sequence parameters (Han et al. 2006; Wonderlick et al. 2009).
The surface representing the gray-white border was inflated, differences among individuals in the depth of gyri and sulci were normalized, and each subject’s reconstructed brain was then morphed and registered to an average spherical surface representation that optimally aligns sulcal and gyral features across participants. Thickness and surface area measures were then mapped to the inflated surface of each participant’s reconstructed brain (Fischl et al. 1999). This procedure allows visualization of data across the entire cortical surface (i.e., both the gyri and sulci) without interference from cortical folding. The data were smoothed on the surface using an iterative nearest-neighbor averaging procedure. One hundred iterations were applied, which is equivalent to applying a 2-dimensional Gaussian smoothing kernel along the cortical surface with a full-width/half-maximum of 18.4 mm. Data were then resampled for participants into a common spherical coordinate system (Fischl et al. 1999). The procedure provides accurate matching of morphologically homologous cortical locations among participants on the basis of each individual’s anatomy, while minimizing geometric distortion.
Region of Interest Analyses
To test the primary hypotheses of this study, two region-of-interest (ROI) analyses were conducted. For the purposes of these analyses, subjects were classified into one of four age groups: young (18–29 years old, n = 138), middle-aged (30–59 years old, n = 80), young-old (y-old, 60–79 years old, n = 60), and old–old (o-old, 80 + years old, n = 38). Regions were derived from a template of Brodmann areas (BAs) in the Population-Average, Landmark- and Surface-based (PALS) atlas of the human cerebral cortex (Van Essen 2005). Mean thickness of the cortex in each BA in both hemispheres was measured via application of the PALS-BA template to our sample of subjects. The PALS-BA template is part of the newest public release (version 5.0) of FreeSurfer; for the purposes of this analysis, it was mapped from a template of an “average” brain derived from 40 healthy subjects (the fsaverage template) to each individual subject. The insula, not classified in the Brodmann schema, was measured by applying the insula label from the Desikan-Killiany atlas in FreeSurfer (Desikan et al. 2006).
The first ROI analysis was based upon the classification scheme for partitioning the cerebral cortex into functional zones that Mesulam proposed (Mesulam 2000) based on a large body of prior neuroanatomical work (Broca 1878; Filimonoff 1947; Yakovlev 1959; Sanides 1970). In this classification scheme, cortex is classified based upon cytoarchitecture, its involvement in processing within or across one or more domains of sensory or motor functioning, and its connectivity with other regions of the brain. Utilizing this classification scheme, Brodmann areas were classified as primary sensory/motor (idiotypic) cortex, unimodal association (homotypical unimodal) cortex, heteromodal association (homotypical heteromodal) cortex, and paralimbic cortex (Table 2). The insula was included in the paralimbic zone. Linear regression analysis was conducted to examine the relationship between thickness in each functional-cytoarchitectural zone and age (as a continuous independent variable). Additionally, repeated measures analysis of variance (ANOVA) was performed to assess thinning in functional zones in the different age groups in a non-continuous manner. This allowed for the examination of potential effects in discrete age ranges that might not be as readily apparent in a linear regression model.
Table 2 .
Classification of Brodmann areas by postnatal expansion of surface area
| Zone Cortical type |
Low-expansion | Intermediate-expansion | High-expansion |
|---|---|---|---|
| Primary sensory/motor | - | 1, 2, 3, 17, 41, 42, 43 | 4 |
| Unimodal association | 18, 19 | 5, 7, 20, 22, 37, 44 | 6 |
| Heteromodal association | 11, 36 | 8, 10, 31, 39, 40, 36, 47 | 9, 21, 32, 40, 45 |
| Paralimbic | Insula | 23, 24, 25, 26, 27, 28, 29, 30, 35, 38 | - |
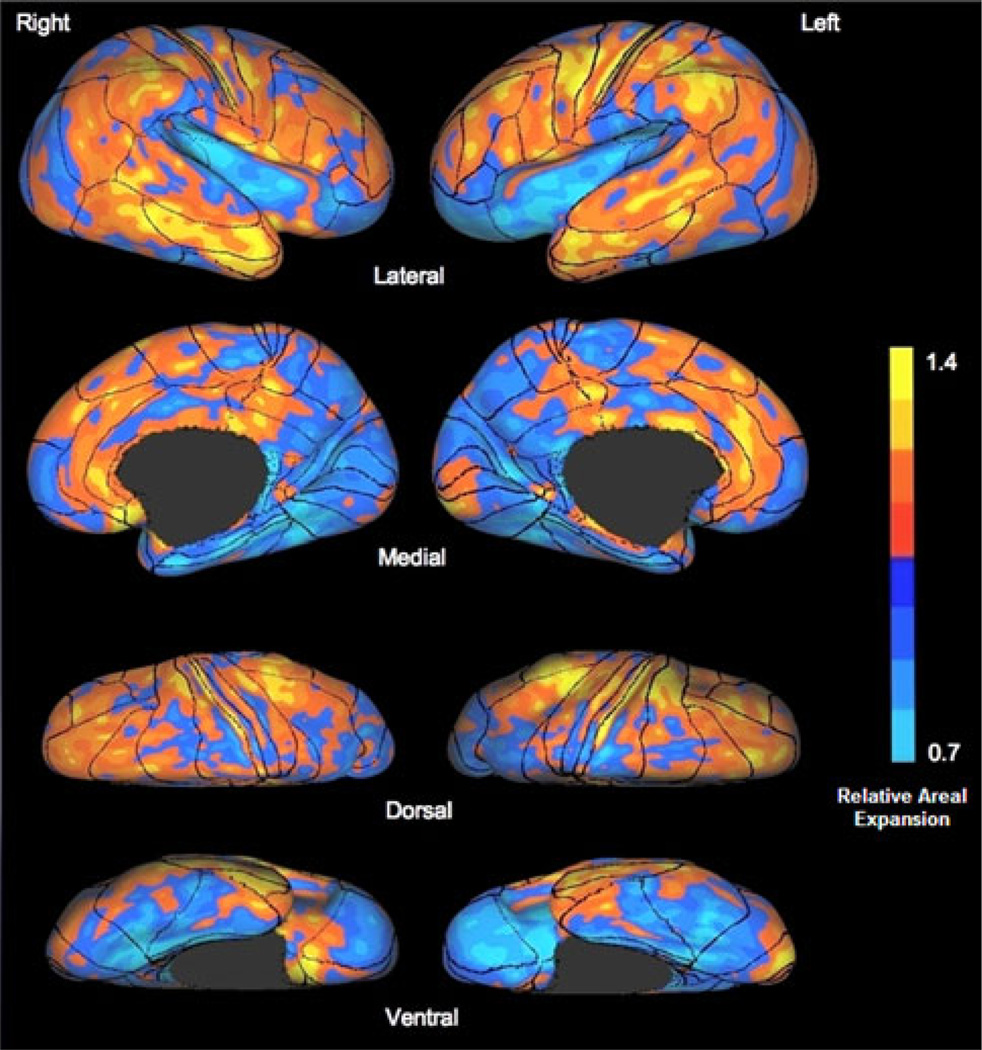
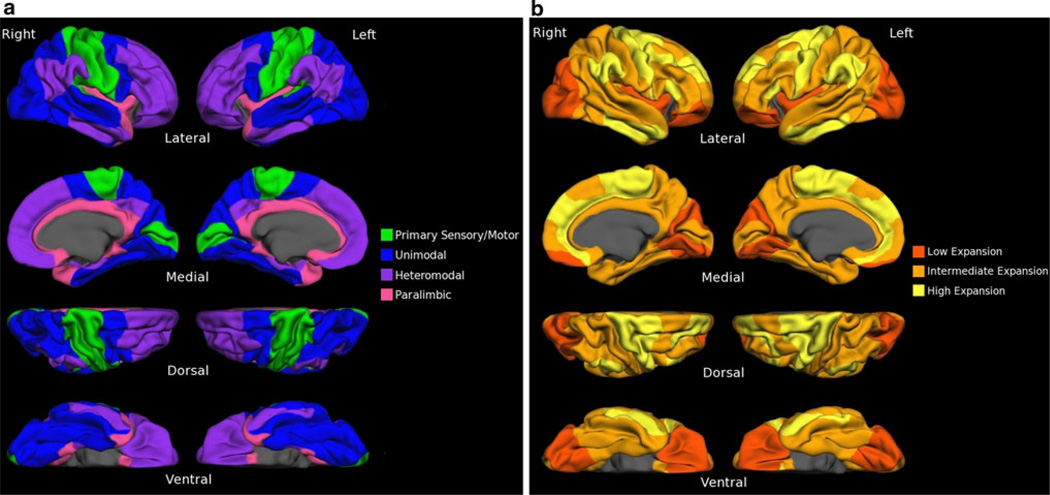
For purposes of the second ROI analysis, the PALS-BA template was overlaid upon the postnatal cortical expansion maps derived by Hill et al. (Hill et al. 2010) (Fig. 1). From this figure, BAs were qualitatively classified as high-expanding, intermediate-expanding, or low-expanding regions based upon both the fraction of the BA expanding and the magnitude of expansion (Table 2). BAs determined to be intermediate-expanding were excluded from further analysis. Mean cortical thickness values were derived for the set of BAs classified as low-expanding and those classified as high-expanding. Linear regression was conducted to investigate the relationship between age and thickness of regions (low-expanding, high-expanding). As in the analysis of functional zones described above, the effect of aging on regional thickness of the age groups as discrete entities was investigated via repeated measures ANOVA. Figure 2 depicts the BA ROIs and the two types of classification used in the present analyses of these ROIs.
Fig. 1.
Postnatal cortical surface expansion with Brodmann area (BA) overlay. This figure, adapted from Fig. 1 in (Hill et al. 2010), depicts relative and absolute areal expansion of the cerebral cortex after birth through young adulthood on a standard mesh average inflated surface. BAs are derived from a template in the Population-Average, Landmark- and Surface-based atlas of the human cerebral cortex (Van Essen 2005)
Fig. 2.
Classification of Brodmann areas (BAs). BAs are visualized on the fsaverage cortical surface template, a surface generated as the topographic average of 40 subjects. BAs are classified into a functional zones based on cytoarchitecture/functional affiliation, and b as low-expansion, intermediate-expansion, or high-expansion regions based upon the work of (Hill et al. 2010)
In addition to these primary ROI-based analyses, three exploratory whole-cortex analyses were conducted to illustrate the large-scale patterns in the data. Linear regression models were constructed utilizing age as the independent variable and cortical thickness at each point across the cortical mantle the dependent variable. These regression analyses were run in the entire sample, and also in the young/middle-aged subgroup and in the young-old/old–old subgroup to illustrate the patterns identified in the ROI analyses above. The resulting cortical surface statistical maps were displayed at arbitrary statistical thresholds to emphasize the differences between groups.
Results
Cortical Surface Analysis
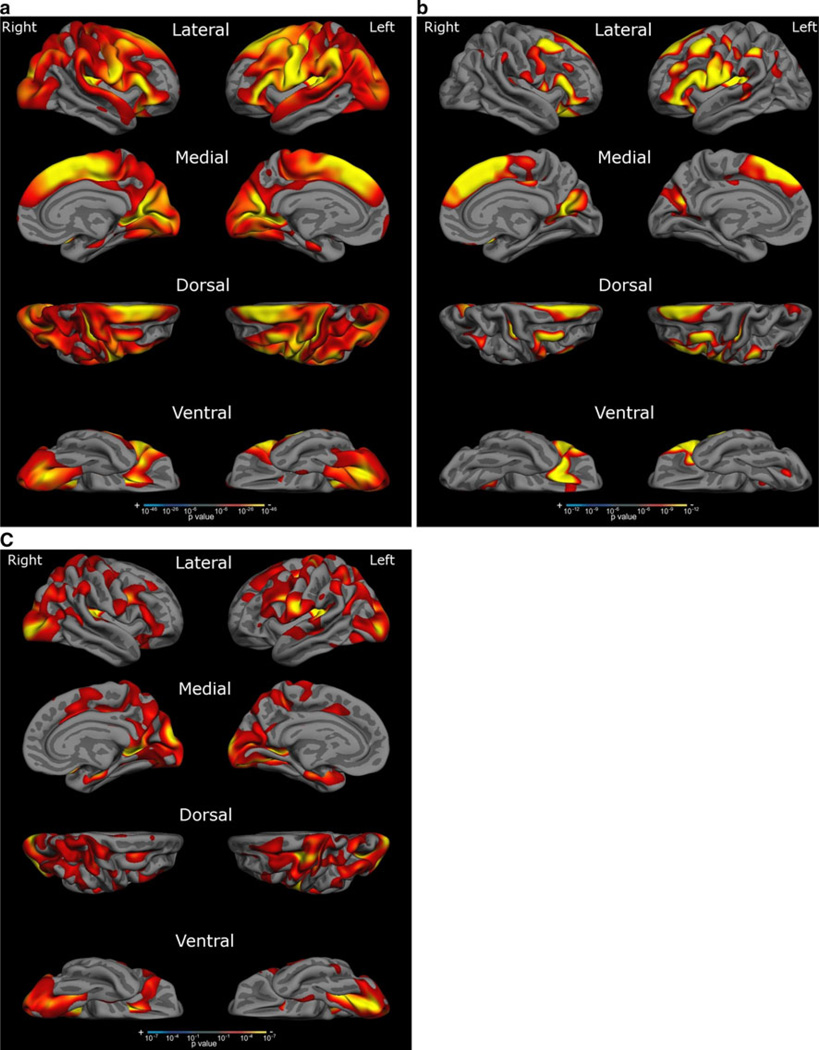
Figure 3a displays regression maps for cortical thickness and age in the entire sample of subjects. The lateral view reveals widespread areas of thinning, with the most prominent thinning occurring in the superior, middle and inferior frontal gyri (including opercularis, orbitalis, and triangularis), inferior frontal sulcus, anterior ramus of the lateral sulcus, precentral gyrus and sulcus, central sulcus, subcentral gyrus, supramarginal gyrus, angular gyrus, intraparietal sulcus, superior temporal gyrus, middle occipital gyrus and sulcus, transverse occipital sulcus, superior occipital gyrus, occipital pole, and caudal insula. The medial view reveals thinning in the superior frontal gyrus, paracentral lobule and sulcus, cuneus, lingual gyrus, and superior occipital gyrus. The dorsal view reveals thinning in the superior frontal gyrus, middle frontal gyrus, precentral gyrus, supramarginal gyrus, angular gyrus, cuneus, middle occipital gyrus, superior occipital gyrus, and occipital pole. The ventral view reveals thinning in the orbital and triangular parts of the inferior frontal gyrus, the lingual gyrus, and the inferior occipital gyrus and sulcus. Regions relatively preserved in the above images include the anterior prefrontal cortex, middle and inferior temporal gyri, superior parietal lobule, precuneus, rostral insula, and medial temporal cortex.
Fig. 3.
Areas of age-related cortical thinning. This figure depicts a linear regression of cortical thickness and age across the entire cortical mantle, showing areas of relatively more prominent thinning in red-yellow. a depicts thinning across all age groups, b in the subgroups of young and middle-aged subjects, and c in the subgroup of young-old and old–old subjects. Areas of prominent thinning in the entire sample include the inferior, middle and superior frontal gyri, precentral gyrus, inferior parietal lobule, superior temporal gyrus, and occipital cortex. Thinning in the sample of young and middle-aged subjects was marked in multiple frontal and parietal regions of high postnatal cortical surface expansion. Thinning in the sample of young-old and old–old subjects was notable in a more distributed set of regions, including but not limited to widespread portions of the occipital cortex. This map corresponds more closely to regions of low postnatal cortical surface expansion (see Fig. 2)
Figure 3b, c display regression maps for the subsets of young and middle-aged subjects (Fig. 3b) and young-old and old–old subjects (Fig. 3c). The regression map in young and middle-aged subjects reveals correlation of thickness with age in multiple frontal locations, as well as some parietal regions, largely in areas of high postnatal expansion. The regression map in young-old and old–old subjects reveals a more distributed pattern with prominent involvement of the lateral and medial occipital cortex, including multiple areas of low postnatal expansion.
Region of Interest Analyses
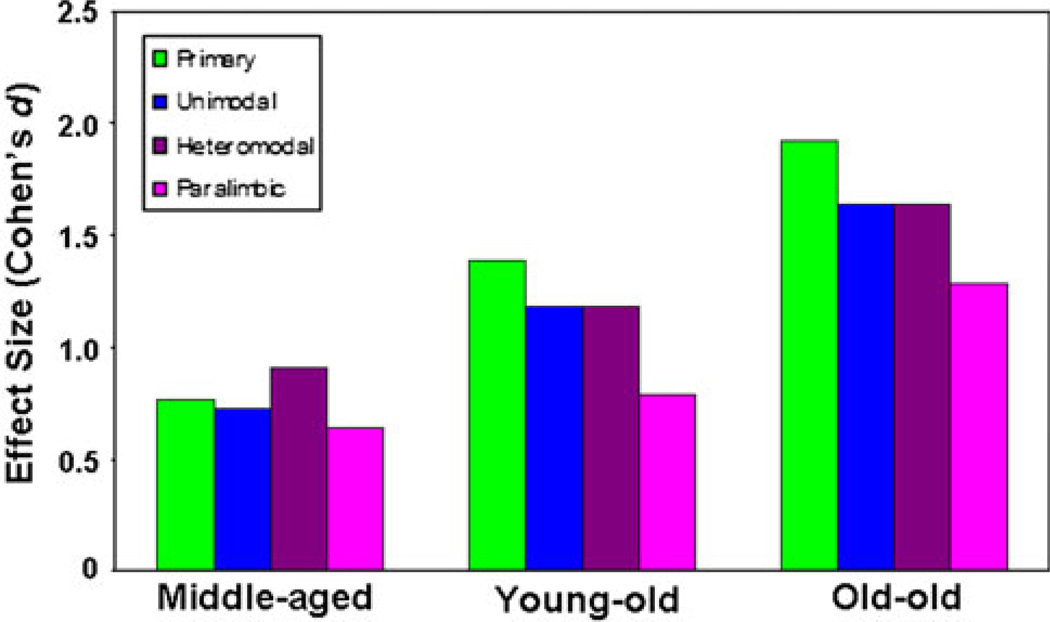
The first ROI analysis examined effects of age on the thickness of the four major cortical functional-cytoarchitectural zones: primary sensory/motor cortex, unimodal association cortex, heteromodal cortex, and paralimbic cortex (Table 3, Fig. 4). In this ANOVA there was a main effect of age group on thickness across all types of cortex (F(3.312) = 90.0, P < 10−20, with decreases in thickness between the young (mean thickness = 2.37 ± 0.007 mm), middle-aged (mean thickness = 2.28 ± 0.009 mm), y-old (mean thickness = 2.23 ± 0.01 mm), and o-old subjects (mean thickness = 2.17 ± 0.013 mm). Likewise, there was a main effect of cortical zone on cortical thickness across age groups (F(3.936) = 1400.5, P < 10−10), with paralimbic cortex (2.39 ± 0.007 mm) thicker than heteromodal association cortex (2.36 ± 0.005 mm), heteromodal association cortex thicker than unimodal association cortex (2.22 ± 0.005 mm), and unimodal association cortex thicker than primary sensory/motor cortex (2.07 ± 0.006 mm). This relationship held across all age groups, with the exception of a comparable thickness between heteromodal and paralimbic cortex in the o-old group (P = 0.14). A functional zone by age group interaction was present (F(9.936) = 5.61, P < 10−4), reflecting relatively greater thinning in the primary sensory/motor and unimodal association zones from both middle-age to y-old age and y-old age to o-old age than was observed in the heteromodal association and paralimbic zones (Table 3). In contrast, the effect size (Cohen’s d) was greatest for thinning in the heteromodal association zone from the young age group to the middle age group (Fig. 4). The relatively greater thinning in primary and unimodal zones from middle-age through o-old age was also reflected in somewhat stronger correlation coefficients across the entire age span for these zones, the strongest occurring in the primary sensory/motor cortices (r = 0.73, P < 10−20), followed by the unimodal cortices (r = 0.64, P < 10−20) and heteromodal cortices (r = 0.62, P < 10−20). The correlation was relatively weaker, albeit still strong, in the paralimbic (r = 0.50, P < 10−20) zone.
Table 3.
Quantitative metrics of age-related thinning by region
| Zone | Mean thickness and SD (mm) |
Effect size (Cohen’s d) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortical type | Young | Middle-aged | Y-old | O-old | MA versus Y | Y-old versus Y | O-old versus Y | ||||
| Primary sensory/motor | 2.20 | 0.08 | 2.10* | 0.09 | 2.02* | 0.10 | 1.95* | 0.10 | 0.77 | 1.38 | 1.92 |
| Unimodal association | 2.32 | 0.08 | 2.24* | 0.08 | 2.19* | 0.09 | 2.14† | 0.09 | 0.73 | 1.18 | 1.64 |
| Heteromodal association | 2.46 | 0.09 | 2.36* | 0.08 | 2.33‡ | 0.09 | 2.28† | 0.09 | 0.91 | 1.18 | 1.64 |
| Paralimbic | 2.48 | 0.12 | 2.39* | 0.11 | 2.37 | 0.13 | 2.30† | 0.11 | 0.64 | 0.79 | 1.29 |
| Low-expansion | 2.38 | 0.10 | 2.30* | 0.09 | 2.23* | 0.10 | 2.14* | 0.09 | 0.67 | 1.25 | 2.00 |
| High-expansion | 2.50 | 0.09 | 2.39* | 0.09 | 2.35‡ | 0.09 | 2.30 | 0.10 | 0.92 | 1.25 | 1.67 |
Y young (18–29 years), MA middle-aged (30–59 years), Y-old young-old (60–79 years), O-old old–old (80+ years)
P < 0.001;
P < 0.01;
P < 0.05 for change in thickness relative to previous age group
Fig. 4.
Thinning in functional zones between young, middle-aged, young-old, and old–old age groups. This figure depicts effect sizes (Cohen’s d) for age-related thinning in each age group relative to the young adult age group. Slightly greater thinning was observed in heteromodal association cortex in between young and middle age, whereas thinning later in the process of aging was most prominent in primary sensory/motor cortex
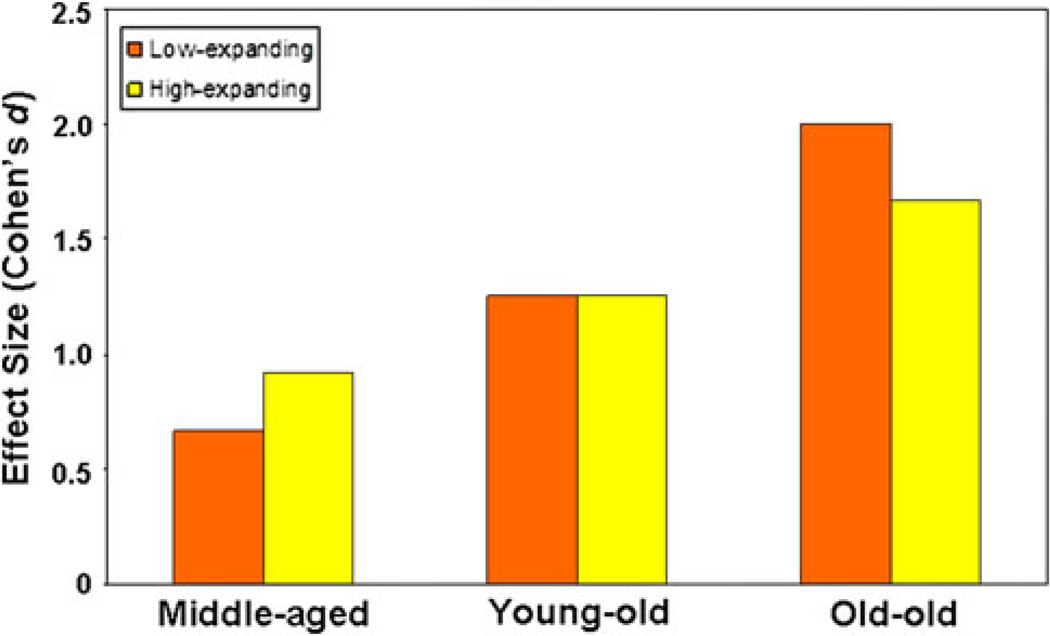
In the analysis of thickness of low-expanding and high-expanding cortical regions, there was a main effect of cortical region on thickness (F(1,312) = 488.4, P < 0.0001), with the high-expanding regions having a greater mean cortical thickness (2.39 ± 0.006 mm) than the low-expanding regions (2.26 ± 0.006 mm). Again there was a main effect of age group on mean cortical thickness across regions (F(3.312) = 91.0, P < 0.0001). An age group by region interaction (F(3.312) = 5.37, P = 0.001) was driven by greater thinning in the high-expanding regions than the low-expanding regions in the middle-aged group compared to the young group (F(1.216) = 4.36, P < 0.05) and greater thinning in the low-expanding regions than the high-expanding regions in the o-old age group compared to the young group (F(1.174) = 6.63, P = 0.01). There was no difference in thinning between the low-expanding region and the high-expanding regions in the group of y-old subjects compared to young subjects (F(1.196) = 0.16, P = 0.69). Figure 5 displays effect sizes for thickness in low-expanding cortex and high-expanding cortex in the middle-aged, y-old, and o-old groups compared to the young group. Linear regression again confirmed strong correlations between age and cortical thickness in both low-expanding and high-expanding regions, with a slightly stronger correlation between age and thickness in the low-expanding regions (r = 0.69, P < 10−20) than the high-expanding regions (r = 0.64, P < 10−20).
Fig. 5.
Evidence for a sequential “developmental-sensory” hypothesis of cortical aging. This figure depicts effect sizes (Cohen’s d) for age-related thinning in postnatal low-expansion versus high-expansion regions in middle-aged, young-old, and old–old adults compared to young adults. In middle-age greater thinning occurs in highexpansion regions, whereas, in old–old age greater thinning occurs in low-expansion regions
Discussion
Across the cortical surface, thinning was observed in many of the regions consistently implicated in prior studies of aging and cortical morphometry. A majority of studies have reported loss of volume and/or thinning in frontal and parietal association cortex (Jernigan et al. 1991; Good et al. 2001; Jernigan et al. 2001; Resnick et al. 2003; Raz et al. 2004; Salat et al. 2004; Taki et al. 2004; Allen et al. 2005; Grieve et al. 2005; Lemaitre et al. 2005; Raz et al. 2005; Walhovd et al. 2005; Fjell et al. 2009). Our analysis similarly revealed prominent thinning in the superior, middle, and inferior frontal gyri (dorsolateral and ventrolateral prefrontal cortex), as well as the angular and supramarginal gyri (inferior parietal lobule). Recent studies using resting state and task-related functional connectivity MRI have demonstrated that the dorsolateral prefrontal cortex and inferior parietal lobule contain nodes in frontoparietal networks important for attention, executive function, and cognitive control (Corbetta and Shulman 2002; Koechlin et al. 2003; Dosenbach et al. 2008; Vincent et al. 2008; Spreng et al. 2010). Age-related thinning in cortical regions subserving attention and executive function is in keeping with the “frontal lobe” hypothesis of aging, borne out of numerous psychological experiments demonstrating agerelated decrements in performance on tasks of complex attention and executive functioning (Braver et al. 2001; DiGirolamo et al. 2001; Andres et al. 2006; Kemps and Newson 2006; West and Schwarb 2006).
One goal of our analysis was to determine whether this age-related thinning in frontal and parietal association areas reflects a pattern of greater thinning in heteromodal association cortex compared to the other cortical zones. Figure 3a reveals that prominent thinning was not confined to heteromodal areas, but also occurred in primary somatosensory, motor, auditory, and visual areas, as well as the caudal insula, considered to be a primary viscerosensory region (Craig 2002). Thinning was similarly present in many unimodal association areas contiguous with primary regions, including premotor regions in the frontal lobe, auditory association areas in the superior temporal gyrus, and visual association areas in the occipital lobe. These results are consistent with prior studies reporting agerelated effects in primary sensory/motor and modality-specific association areas of cortex (Good et al. 2001; Tisserand et al. 2002; Raz et al. 2004; Salat et al. 2004; Lemaitre et al. 2005; Raz et al. 2005; Fjell et al. 2009).
In comparison to the primary sensory/motor and association zones, cortical thickness in the paralimbic zone was relatively spared in the course of aging. Previous studies have reported morphometric sparing of paralimbic regions including the anterior cingulate, insula, medial temporal cortex, and orbitofrontal cortex (Jernigan et al. 1991; Good et al. 2001; Raz et al. 2004; Salat et al. 2004; Grieve et al. 2005; Raz et al. 2005). Cytoarchitecturally, this zone represents a transition between relatively primitive three-layered archicortex of limbic regions and the more differentiated six layered structure of association cortex (Mesulam 2000). Phylogenetically, it represents a zone of structural consistency and similarity across mammalian species (MacLean 1990). As with other phylogenetically-preserved regions, paralimbic cortex follows a relatively simple developmental trajectory and is considered mature relatively early in the life span (Shaw et al. 2008; Hill et al. 2010). Its preservation thus lends support to the “last in, first out” hypothesis of aging (Grieve et al. 2005).
The analysis of cortical surface area expansion by Hill et al. afforded a more direct way to examine effects of aging in regions of the brain that continue expanding during the first two decades of life compared with those relatively mature (from that perspective) at birth. As with the relationship between age and thickness in cortical zones, the relationship between age and thickness in low-expanding and high-expanding regions of cortex was complex. Many areas with significant postnatal expansion of surface area thinned considerably with aging, including the superior, middle, and inferior frontal gyri, precentral and postcentral gyri, inferior parietal lobule, and superior temporal gyrus. Regions classified as postnatally low-expanding areas that correspondingly did not thin prominently with aging included the medial temporal cortex and the rostral insula. In contrast, the occipital cortex was a region of relatively low postnatal expansion in surface area in which significant thinning occurred with aging, and the middle temporal gyrus was a region of high postnatal expansion in surface area in which very little thing occurred with aging.
The timing of age-related thinning in cortical zones and expansion regions was notable and may help to disambiguate the implications of results with respect to the “last in, first out” hypothesis of aging. As a group, regions of high postnatal expansion in surface area thinned more prominently in middle age than those with low postnatal expansion in surface area (Figs. 3b and 5). As this stage represents the earliest period of time in the process of aging, these results support the notion that late developing regions are indeed the first to undergo structural compromise. Within the age range of 60–79, thinning appears equivalent in low-expansion and high-expansion regions, whereas individuals aged 80 or older demonstrated more thinning in the regions of the brain with less postnatal expansion in surface area (Figs. 3c and 5). The most significant thinning in this “old–old” age range with respect to cortical zones occurred in the primary and unimodal zones, lending a possible anatomical correlate to the observation that declines in cognition track with declines in sensation in this age range (Lindenberger and Baltes 1994). Of note, correlations between visual and auditory performance and cognition reported by Lindenberger and Baltes occurred in a group of individuals aged 70–103, consistent with the “common cause” hypothesis as being applicable later in the course of aging. As the OASIS dataset does not contain information on visual, auditory, or somatosensory acuity, any link between motor and/or sensory functioning and cortical thickness based on our data is purely speculative. Further research is required to study the relationship between peripheral sensory/motor functions and thickness of corresponding regions of cortex.
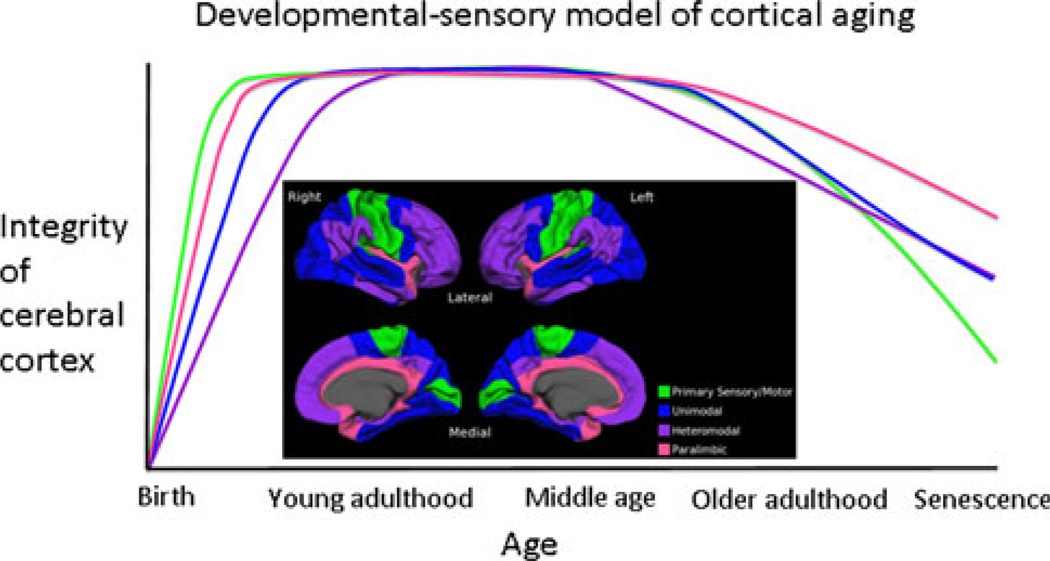
On the basis of our observations, a sequential “developmental-sensory” hypothesis of aging, incorporating “last in, first out” early in the course of aging and “common cause” late in the course appears plausible (Fig. 6). Such a model could arise because processes that underlie structural changes in the cortex differ early versus late in the process of aging or because different types of cortex have varying susceptibility to any given combination of factors in any given period of time. At this time it is unclear how or why the level of differentiation of the cerebral cortex would confer a varying degree of vulnerability to agerelated insults or injuries. Answers to these questions will first require a more thorough understanding of the histopathological changes that give rise to cortical thinning and loss of volume in normal aging.
Fig. 6.
Proposed schematic for a “developmental-sensory” hypothesis of cortical aging. The model proposes that primary sensory/motor and paralimbic regions reach mature levels of thickness before unimodal and heteromodal association cortices. Early aging is marked by thinning in heteromodal regions, whereas, late aging is marked by thinning in primary sensory/motor regions
Leaving aside common age-related neuropathologies, these changes are poorly understood at present. Postmortem studies have revealed a diversity of microscopic structural changes in the course of aging, including neuronal shrinkage (Terry et al. 1987), reduction in synaptic density (Morrison and Hof 1997), reduced complexity of dendritic spines (Scheibel et al. 1975; Jacobs et al. 1997), loss of presynaptic terminals (Masliah et al. 1993), and altered microvasculature (Riddle et al. 2003). While some studies have suggested loss of neurons (Brody 1955; Coleman and Flood 1987), others have reported preservation of neuron number and density (Morrison and Hof 1997; Freeman et al. 2008), thus suggesting that reduced neuronal complexity (or, loss of neuropil) may factor more prominently in age-related cortical thinning. Cognitively normal older individuals also harbor some degree of accumulation of disease-related neuropathological abnormalities including amyloid plaques and neurofibrillary tangles (Dayan 1970; Crystal et al. 1988; Price et al. 1991; Morris et al. 1996; Bennett 2006; Price et al. 2009) and cerebrovascular changes (Chaves et al. 2004; Warsch and Wright 2010). The contribution of these changes to thinning of the cortex in healthy older adults is unclear, especially given the spatially distinct profiles of thinning in aging (Salat et al. 2004; Fjell et al. 2009) and Alzheimer disease (Dickerson et al. 2009). Thinning of the cortex may also be secondary to changes in other locations of the brain, including subcortical white matter, where studies have revealed extensive loss of myelinated fibers in the aging process (Pakkenberg and Gundersen 1997; Marner et al. 2003). Pertinent to the “last in, first out” hypothesis of aging, investigators have reported age-related myelin pallor restricted to cortico-cortical tracts with relatively long postnatal cycles of myelination (Kemper 1994). This finding was corroborated by a recent diffusion tensor tractography study demonstrating that the sequence of reduction in structural integrity of white matter tracts in aging inverts the sequence of myelination during development (Davis et al. 2009).
Potential weaknesses of this study include those related to the sample of subjects and the methods employed. The cross-sectional nature of the sample introduces the possibility that sources of variability between subjects other than age may influence results. Longitudinal datasets would be extremely valuable for these sorts of investigations. In addition, as with any sample of “healthy” older individuals not constrained by absence of biomarker evidence of disease, there is a high likelihood that the sample contains individuals with preclinical neurodegenerative or other as-yet silent underlying disease. As for the methods, this type of cortical thickness analysis relies upon automated segmentation of brain tissue into grey matter and white matter and the localization of the grey-white and pial boundaries, which could be influenced by image contrast and MR tissue parameters, both of which are known to exhibit regionally varying change with aging (Salat et al. 2009). Systematic age-related effects on these variables could influence measures of cortical thickness, although the use of multiple scans for each individual, motion correction, and smoothing procedures were employed to minimize these potential effects (Jernigan et al. 2001; Davatzikos and Bryan 2002; Salat et al. 2004; Han et al. 2006; Fjell et al. 2009).
Conclusion
Consistent with previous studies, the present study revealed that age-related thinning of the cerebral cortex is prominent across a range of structurally and functionally heterogeneous regions including heteromodal regions such as the prefrontal and inferior parietal cortices and modality-specific regions in the frontal, temporal, parietal and occipital lobes. Early in the course of aging, thinning was greatest in the heteromodal association zone and regions of high postnatal surface area expansion. This trend was reversed late in the course of aging, when rates of thinning in modality-specific zones and regions of low postnatal surface area expansion eclipsed rates of thinning in transmodal zones and regions of high postnatal surface area expansion. A sequential “developmental-sensory” hypothesis of aging could account for these findings, and serve as context for further investigation into mechanisms of cortical thinning active at different time points in the course of aging.
Acknowledgment
This study was supported by grants from the NIA R01-AG29411, R21-AG29840, P50-AG005134, and NCRR P41-RR14075, U24-RR021382, Harvard NeuroDiscovery Center, and the Alzheimer’s Association.
Footnotes
This is one of several papers published together in Brain Topography on the “Special Issue: Brain Imaging across the Lifespan”.
Contributor Information
Scott M. McGinnis, Frontotemporal Dementia Unit, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA Departments of Neurology, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Massachusetts Alzheimer’s Disease Research Center, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Division of Cognitive and Behavioral Neurology, Department of Neurology, Brigham & Women’s Hospital, 221 Longwood Avenue, Boston, MA 02115, USA, smmcginnis@partners.org.
Michael Brickhouse, Frontotemporal Dementia Unit, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Departments of Psychiatry, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Belen Pascual, Frontotemporal Dementia Unit, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Bradford C. Dickerson, Frontotemporal Dementia Unit, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA Departments of Neurology, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Massachusetts Alzheimer’s Disease Research Center, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Departments of Psychiatry, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
References
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. Discussion 1279-1282. [DOI] [PubMed] [Google Scholar]
- Andres P, Parmentier FB, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: a test of the frontal hypothesis of aging. Neuropsychologia. 2006;44:2564–2568. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bailey P, von Bonin G. The isocortex of man. Urbana: University of Illinois Press; 1951. [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20:S63–S68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Janowsky JS, Taylor SF, Yesavage JA, Mumenthaler MS, Jagust WJ, Reed BR. Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. J Exp Psychol Gen. 2001;130:746–763. [PubMed] [Google Scholar]
- Broca P. Anatomie comparée des circonvolutions cérébrales: le grand lobe limbique dans la série des mammifêres. Revue Anthropologique. 1878;1:384–498. [Google Scholar]
- Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955;102:511–516. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chaves PH, Kuller LH, O’Leary DH, Manolio TA, Newman AB. Subclinical cardiovascular disease in older adults: insights from the Cardiovascular Health Study. Am J Geriatr Cardiol. 2004;13:137–151. doi: 10.1111/j.1076-7460.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging. 1987;8:521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Bryan RN. Morphometric analysis of cortical sulci using parametric ribbons: a study of the central sulcus. J Comput Assist Tomogr. 2002;26:298–307. doi: 10.1097/00004728-200203000-00024. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan AD. Quantitative histological studies on the aged human brain. II. Senile plaques and neurofibrillary tangles in senile dementia (with an appendix on their occurrence in cases of carcinoma) Acta Neuropathol. 1970;16:95–102. doi: 10.1007/BF00687664. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E. General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimonoff IN. A rational subdivision of the cerebral cortex. Arch Neurol Psychiatry. 1947;58:296–311. doi: 10.1001/archneurpsyc.1947.02300320047002. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, Hedley-Whyte ET, Locascio JJ, Lipsitz LA, Hyman BT. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:1205–1212. doi: 10.1097/NEN.0b013e31818fc72f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci USA. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: localization of age-related changes. Biol Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuropathological changes during aging and dementia. In: Albert MLKJ, editor. Clinical neurology of aging. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Kemps E, Newson R. Comparison of adult age differences in verbal and visuo-spatial memory: the importance of ‘pure’, parallel and validated measures. J Clin Exp Neuropsychol. 2006;28:341–356. doi: 10.1080/13803390490918228. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26:900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: role in paleocerebral functions. New York: Plenum Press; 1990. [DOI] [PubMed] [Google Scholar]
- Marcus DS, Fotenos AF, Csernansky JG, Morris JC, Buckner RL. Open access series of imaging studies: longitudinal MRI data in nondemented and demented older adults. J Cogn Neurosci. 2010;22:2677–2684. doi: 10.1162/jocn.2009.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Principles of behavioral and cognitive neurology. New York: Oxford University Press; 2000. [Google Scholar]
- Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Thal LJ, Woodbury P. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s disease cooperative study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. aging of the brain and its impact on cognitive performance: integration of structural and functional findings. London: Erlbaum; 2000. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Lee SY, van der Kouwe AJ, Greve DN, Fischl B, Rosas HD. Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. Neuroimage. 2009;48:21–28. doi: 10.1016/j.neuroimage.2009.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: Noback CR, Montagna W, editors. The primate brain. New York: Appleton-Century-Crofts; 1970. pp. 137–208. [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxelbased morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, Sato K, Ono S, Kinomura S, Nakagawa M, Sugiura M, Watanabe J, Kawashima R, Fukuda H. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging. 2004;25:455–463. doi: 10.1016/j.neurobiolaging.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surfacebased (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Economo C, Triarhou LC. Cellular structure of the human cerebral cortex. Basel: Karger Publishers; 2009. [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275-1268. [DOI] [PubMed] [Google Scholar]
- Warsch JR, Wright CB. The aging mind: vascular health in normal cognitive aging. J Am Geriatr Soc. 2010;58(Suppl 2):S319–S324. doi: 10.1111/j.1532-5415.2010.02983.x. [DOI] [PubMed] [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal function on the neural correlates of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20:468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- Wonderlick JS, Ziegler DA, Hosseini-Varnamkhasti P, Locascio JJ, Bakkour A, van der Kouwe A, Triantafyllou C, Corkin S, Dickerson BC. Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage. 2009;44:1324–1333. doi: 10.1016/j.neuroimage.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI. Pathoarchitectonic studies of cerebral malformations. III. Arrhinencephalies (holotelencephalies) J Neuropathol Exp Neurol. 1959;18:22–55. doi: 10.1097/00005072-195901000-00003. [DOI] [PubMed] [Google Scholar]