Abstract
As an integral member of the filtration barrier in the kidney glomerulus, the podocyte is in a unique geographical position: It is exposed to chemical signals from the urinary space (Bowman’s capsule), it receives and transmits chemical and mechanical signals to/from the glomerular basement membrane upon which it elaborates, and it receives chemical and mechanical signals from the vascular space with which it also communicates. As with every cell, the ability of the podocyte to receive signals from the surrounding environment and to translate them to the intracellular milieu is dependent largely on molecules residing on the cell membrane. These molecules are the first-line soldiers in the ongoing battle to sense the environment, to respond to friendly signals, and to defend against injurious foes. In this review, we take a membrane biologist’s view of the podocyte, examining the many membrane receptors, channels, and other signaling molecules that have been implicated in podocyte biology. Although we attempt to be comprehensive, our goal is not to capture every membrane-mediated pathway but rather to emphasize that this approach may be fruitful in understanding the podocyte and its unique properties.
Keywords: glomerulus, foot process, slit diaphragm, actin cytoskeleton, calcium, angiotensin, synaptopodin, TRPC channels, Rac1, RhoA, integrins, mTOR, autophagy
INTRODUCTION
With every cardiac cycle, the kidney glomerulus filters blood into an ultrafiltrate that will ultimately become urine. Architecturally, the glomerulus or renal corpuscle consists of a glomerular tuft and Bowman’s capsule. The basic unit of the glomerular tuft is a single capillary. The glomerular basement membrane (GBM) provides the primary structural scaffold for the glomerular tuft. Endothelial and smooth muscle–like mesangial cells providing capillary support are located inside the GBM, whereas podocytes are attached to the outer part of the GBM (Figure 1). There are therefore four resident cell types in the glomerulus: endothelial cells, mesangial cells, parietal epithelial cells of Bowman’s capsule, and podocytes (Figure 1a). Podocytes are pericyte-like cells with a complex cellular organization consisting of a cell body, major processes, and foot processes (FPs). Podocyte FPs elaborate into a characteristic interdigitating pattern with FPs of neighboring podocytes, forming in between the filtration slits that are bridged by the glomerular slit diaphragm (SD) (Figure 1b, c). Podocyte FPs and the interposed SD cover the outer part of the GBM and play a major role in establishing the selective permeability of the glomerular filtration barrier, which explains why podocyte injury is typically associated with marked albuminuria. Podocytes are highly differentiated cells with limited capability to undergo cell division in the adult, and the loss of podocytes is a hallmark of progressive kidney disease. The function of podocytes is based largely on the dynamic regulation of their complex cell architecture, in particular the FP structure. Over the past decade, there has been a growing understanding of the role of membrane proteins in transducing extracellular cues into the podocyte intracellular milieu to effect critical architectural changes. Membrane proteins, e.g., ion channels, integrins, and growth factor receptors, are taking center stage in podocyte biology research. Here we provide insight into the physiological and pathophysiological pathways that regulate podocyte structure and function in health and disease.
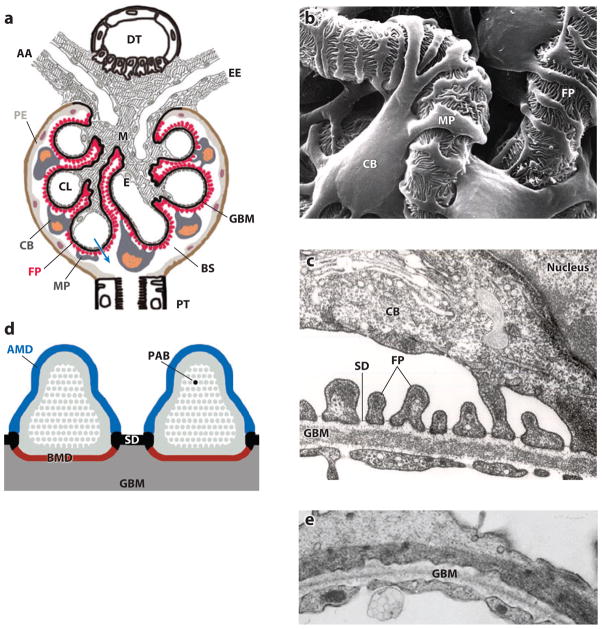
Figure 1.
The function of podocytes is based on their intricate cell architecture. (a) A glomerulus contains a capillary tuft that receives primary structural support from the glomerular basement membrane (GBM). Glomerular endothelial cells (E) lining the capillary lumen (CL) and mesangial cells (M) are located on the blood side of the GBM, whereas podocyte foot processes (FP) cover the outer part of the GBM. Podocyte cell bodies (CB) and major processes (MP) float in Bowman’s space (BS) in primary urine. Along its route from the CL to BS (blue arrow), the plasma ultrafiltrate passes through the fenestrated endothelium, the GBM, and the filtration slits that are covered by the slit diaphragm (SD). AA, afferent arteriole; DT, distal tubule; EE, efferent arteriole; PE, parietal epithelium; PT, proximal tubule. (b) Scanning electron microscopy view from BS highlighting the intricate shape of podocytes. MP link the CB to FP, which interdigitate with FP of neighboring podocytes, thereby forming the filtration slits. (c) Transmission electron microscopy image of the filtration barrier consisting of fenestrated endothelium, GBM, and podocyte FP with the SD covering the filtration slits. (d) Podocyte FP are defined by three membrane domains: the apical membrane domain (AMD) (blue), the basal membrane domain (BMD) (red), and the SD (black). All three domains are connected to the underpinning actin cytoskeleton (gray) and with each other. PAB denotes a single parallel, contractile actin bundle containing α-actinin-4, myosin 9, and synaptopodin. (e) Under conditions of proteinuria, FP lose their normal interdigitating pattern and instead show effacement. A continuous sheet of cytoplasm filled with reorganized actin filaments is clearly visible.
PODOCYTE ARCHITECTURE: THE CENTRAL ROLE OF FOOT PROCESSES
Mature podocytes consist of three morphologically distinct segments: a cell body, major processes, and FPs (1). From the cell body, microtubule-rich major processes split into FPs (Figure 1b) containing an actin-based cytoskeleton (Figure 1c, d). Podocyte FPs elaborate into a highly branched interdigitating network with FPs of neighboring podocytes. The SD (Figure 1c) is a multiprotein complex similar to an adherens junction. The SD bridges the filtration slits between opposing podocyte FPs (1), thereby establishing the final barrier to urinary protein loss (2). FPs are characterized by a podosome-like cortical network of short, branched actin filaments and by the presence of highly ordered, parallel contractile actin filament bundles (Figure 1d) (3), which are thought to modulate the permeability of the filtration barrier through changes in FP morphology. The function of podocytes is based largely on their complex architecture, in particular on the maintenance of highly ordered, parallel, contractile actin filament bundles in FPs (3). In fact, similar to (pseudo)unipolar sensory neurons, the podocyte cell body and primary processes may simply play a trophic, supporting role to the critically important FPs. FPs are functionally defined by three membrane domains: the apical membrane domain, the SD, and the basal membrane domain associated with the GBM (Figure 1d). All three domains are physically and functionally linked to the FP actin cytoskeleton, thus lending a central role to actin for both podocyte function and dysfunction. Interference with any of the three FP domains changes the actin cytoskeleton from parallel, contractile bundles (3) into a dense network. The results are (a) FP effacement reflected by the simplification of the FP structure and loss of the normal interdigitating pattern (Figure 1e) and (b) proteinuria (4). FP effacement requires the active reorganization of actin filaments (5, 6), a process regulated at the molecular level by a multitude of signaling events involving, but not limited to, integrin activation, G protein–coupled receptor (GPCR) and growth factor receptor activity, and calcium (Ca2+) influx pathways as upstream modulators of the actin cytoskeleton. In vivo studies have shown that FP effacement is always temporally related to the emergence of proteinuria (4, 7, 8) and thus suggest a causative link between these two events, which is a widely held belief in our field. However, the molecular steps leading to FP effacement are so hierarchical and precise that they lend credence to what remains a tantalizing hypothesis: FP effacement may be the podocyte’s desperate attempt to adapt to toxic cues to prevent proteinuria (W. Kriz, personal communication).
PODOCYTE ORIGINS: PODOCYTE MATURATION IS ASSOCIATED WITH A PHYSIOLOGICAL EPITHELIAL-TO-MESENCHYMAL TRANSITION
During renal development, epithelial precursors give rise to mature podocytes, which are mesenchyme-like cells. Glomerular development proceeds in four stages: the renal vesicle stage, the S-shaped body stage, the capillary loop stage, and the maturing-glomeruli stage (9, 10). The transition from the S-shaped body stage to the capillary loop stage is critical for podocyte differentiation (1). During the early developmental stages, simple undifferentiated epithelial cells with apically located tight junctions form the immature podocyte precursor cell population (11). At this stage, the expression of podocalyxin (12) and of the tight junction protein ZO-1 (13) commences, and expression of the podocyte-specific transcription factor Wilm’s tumor protein 1 (WT-1) is highest (14). As these immature podocytes enter the capillary loop stage, they lose their mitotic activity and begin to establish their characteristic complex cell architecture, including the appearance of FPs and the reorganization of cell-cell junctions into SDs (11), which are essentially modified adherens junctions (15). At this stage, ZO-1 migrates from its apical location down to the level of the slit membrane, where it is observed in a punctate pattern along the filtration slits (13). A similar apical-to-basal redistribution pattern occurs in the case of the atypical protein kinase C (aPKC), an important cell polarity protein (16–18). The phenotypic conversion occurring during the S-shaped body stage is accompanied by the expression of synaptopodin (19) and by the reappearance of vimentin (20), a phenotypic marker of mesenchymal cells.
The canonical developmental pathway leading to the formation of podocyte FPs is governed by the coordinated activities of numerous membrane or membrane-associated proteins or the transcription factors that regulate the expression of such membrane proteins. Mouse genetic studies have shown a central role for α3 integrin in the formation of mature FPs (21), a phenotype mirrored by the podocyte-specific deletion of β1 integrin (22, 23). The deletion of the nonclassical protocadherin FAT1 revealed the importance of this molecule for proper FP formation (24). Disruption of the cytoskeletal adaptor proteins Nck1/2, which are linked to the critical SD protein nephrin (25), resulted in complete failure to develop FPs (26), thereby phenocopying α3 integrin knockout mice. This result is not surprising, as Nck proteins are critically involved in integrin signaling (27). Furthermore, mice lacking podocalyxin also fail to form FPs and die soon after birth from renal failure (28). The podocyte transcription factor Kreisler (Table 1), whose targets include the SD proteins nephrin (25) and podocin (29), is also essential for FP formation (30). Moreover, disruption of Pod1 (also known as epicardin) leads to aberrant FP formation (Table 1) (31). These genetic studies reveal the importance of these molecules in podocyte development and, more specifically, in FP development. Table 1 summarizes transcription factors involved in the regulation of podocyte structure and function during development as well as under physiological and pathological conditions.
Table 1.
Transcription factors in podocyte development, physiology, and pathology
| Name | Putative target(s) | References |
|---|---|---|
| Wilm’s tumor protein 1 (WT1) | Podxl (podocalyxin), Nphs1 (nephrin) | 177–180 |
| LMX1B | α3 and α4 collagens, Nphs2 (podocin) | 115, 181, 182 |
| FOXC2 (Mfh2) | Nphs2, α3 and α4 collagens, MafB | 183 |
| Kreisler (MafB) | Nphs1, Nphs2, CD2AP | 30, 184 |
| Pax2 | WT1? | 185, 186 |
| ZHX1/ZHX2 | WT1? | 187, 188 |
| Pod1 (epicardin) | Nphs2, α3 collagen, MafB? | 31, 189 |
| ZEB2 | P/E cadherins | 190 |
| HIFα | CXCR4 | 191–193 |
| Rbpj (Notch pathway) | ? | 95 |
| NFAT | TRPC6 | 98, 138, 139 |
| β-Catenin | ? | 194, 195 |
Podocyte development is relevant to mechanisms of podocyte injury in the adult. Mature podocytes are unable to undergo cell division in vivo. The evidence for this hypothesis comes from studies showing that the number of podocyte nuclei does not increase during postnatal and hypertrophic kidney growth (32–36). The transition of podocytes from the S-shaped body stage to the capillary loop stage and their branching into specialized cell architecture involve a neuron-like complex cell differentiation process, which is mutually exclusive with cell division. Interestingly, in response to certain stimuli [e.g., fibroblast growth factor (FGF)-2], podocytes may reenter the cell cycle and undergo nuclear division but cannot complete cell division (33). The only exception to this phenomenon is noted in HIV nephropathy, in which podocytes undergo a tumor-like proliferation, albeit without reparative capacity (37–40). Although the underlying molecular mechanisms leading to the effective arrest of cytokinesis in vivo remain to be established, cyclins and cyclin-dependent kinases and their inhibitors have been implicated (reviewed in detail in Reference 41).
The inability of mature podocytes to undergo cell division and to replenish their population renders the glomerulus vulnerable to toxic cues, leading to significant podocyte loss, which is a hallmark in the development of chronic kidney failure (42). The search for a podocyte stem cell to replenish a diminishing podocyte pool has led to the notion that a population of cells in the parietal epithelium of the glomerulus may migrate into the glomerular tuft and differentiate into mature podocytes (43, 44). However, more recent evidence suggests that migrating parietal epithelial cells may be maladaptive for glomerular function (45). Further studies are likely to reveal if there is a population of cells capable of replenishing lost podocytes.
THE SLIT DIAPHRAGM: A MULTIPROTEIN SIGNALING HUB
The SD is a complex signal transduction unit with characteristics of a modified adherens junction that spans the 30–50-nm-wide filtration slits (15). The extracellular portion of the SD is made up of rod-like units that are thought to be composed of the extracellular domains of various transmembrane proteins such as nephrin and FAT (1). These rods are connected by a linear bar, forming a zipper-like pattern, which leaves pores the same size as or smaller than albumin (1). The SD’s cytoplasmic portion contains a region of Triton-X-100-resistant (1), electron-dense material, which is reminiscent of the highly insoluble specialization of the submembranous actin cytoskeleton in neurons known as the postsynaptic density (PSD) (46). The PSD contains multiple receptors and ion channels linked through a multitude of adaptor proteins to the cytoskeletal core, forming a large protein network (47). Similarly, at the SD, ion channels such as TRPCs (where TRPC denotes transient receptor potential canonical) (48), receptors, integrins, and other membrane proteins (Figure 2) are connected to actin via a variety of adaptor and effector proteins.
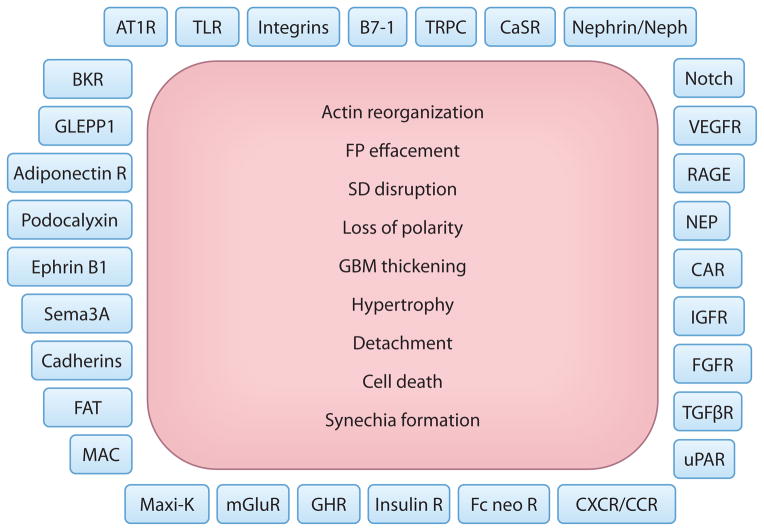
Figure 2.
Podocyte plasma membrane proteins and a canonical pattern of injury. Shown is an (incomplete) list of membrane proteins that have been implicated in the regulation of podocyte function in health and disease. Injured podocytes respond with a finite repertoire of changes, as depicted here. Our ability to repair the pathways initiated by the molecules on the podocyte plasma membrane to the precise cellular phenotypes listed here will provide not only novel insight but enormous opportunities for successful therapeutic interventions. Abbreviations: adiponectin R, adiponectin receptor; AT1R, angiotensin type 1 receptor; BKR, bradykinin receptor; CAR, Coxsackie and adenovirus receptor; CaSR, calcium-sensing receptor; CXCR/CCR, C-X-C/C-C chemokine receptor; FAT, protocadherin FAT1; Fc neo R, neonatal Fc receptor; FGFR, fibroblast growth factor receptor; FP, foot process; GBM, glomerular basement membrane; GHR, growth hormone receptor; GLEPP1, glomerular epithelial (podocyte) protein 1; IGFR, insulin growth factor receptor; insulin R, insulin receptor; MAC, membrane attack complex; Maxi-K, large-conductance calcium-activated potassium channel (also known as BK channel); mGluR, metabotropic glutamate receptor; NEP, neutral endopeptidase; RAGE, receptor for advanced glycation end products; SD, slit diaphragm; Sema3A, semaphorin-3A; TGF βR, transforming growth factor β receptor; TLR, Toll-like receptor; TRPC, transient receptor potential canonical; uPAR, urokinase receptor; VEGFR, vascular endothelial growth factor receptor.
The disruption of SD structure or function is a common theme in many kidney diseases arising at the podocyte level (49). The SD is thought to function as a key sensor for and as a regulator of adaptations in FP shape and length (7, 8). For example, the movement of each FP needs to be precisely coordinated with that of the FPs of neighboring podocytes to ensure the integrity of the filtration barrier. Such coordination is likely achieved through functional coupling of opposed FPs, which generates signaling cascades on both ends of the SD. This multiprotein network likely serves a far more complex role as a signaling platform and is not simply a physical sieve.
Nephrin is a well-known and widely studied SD membrane protein (8). Mutations in the NPHS1 gene encoding for nephrin have been identified as the cause of congenital nephrotic syndrome of the Finnish type (25). Nephrin has a single transmembrane domain; a short intracellular tail; and a long, immunoglobulin-like extracellular moiety, which is thought to align parallel to the extracellular domains of neighboring nephrin molecules. Through its intracellular domain, nephrin is connected to the actin cytoskeleton by several adapter proteins and plays a pivotal part in the regulation of podocyte actin dynamics (7, 50). Among other pathways (reviewed in detail in Reference 7), a recently discovered signaling pathway couples nephrin to the actin cytoskeleton via the adapter protein Nck (26, 51). After nephrin phosphorylation by Fyn (52), Nck binds to phospho-nephrin and to N-WASP (26, 51), activating the Arp2/3 complex, a major regulator of actin dynamics (7, 26, 50, 51). Recent work has also shown that Fyn phosphorylation of nephrin promotes activation of phosphoinositide 3 kinase and Rac1 activity (53; please also see below).
Notably, a large body of human genetic, animal, and physiological studies, including micro-puncture experiments, over more than four decades had established the podocyte FPs with the interposed SD as the final barrier to albumin (2, 8). However, some years ago, on the basis of live-imaging two-photon microscopy results, researchers developed the alternative hypothesis that nephrotic levels of albumin pass across the normal glomerulus filters and are subsequently retrieved by the proximal tubule (54). Through the use of the same two-photon microscopy approach, two recent independent studies convincingly refuted this leaky glomerular barrier hypothesis (55–57). Both Tanner (55) and Peti-Peterdi (57) directly confirmed the classical view that the glomerular filter is the primary barrier for albumin and that the glomerular sieving coefficient for albumin is extremely low (55–57), thereby corroborating the size and charge selectivity of the glomerular barrier (55, 58–60).
PODOCYTE INJURY IS THE HALLMARK OF PROTEINURIA AND GLOMERULAR DISEASE
Podocyte injury is the common denominator in many forms of human and experimental glomerular disease such as minimal change disease, focal segmental glomerulosclerosis (FSGS), membranous glomerulopathy, diabetic nephropathy (DN), and lupus nephritis (2, 4). The best-characterized pattern of injury involves a reorganization of the FP actin cytoskeleton that leads to FP effacement and to SD disruption (61, 62). The disruption of any of the three FP domains (Figure 1d), with the concomitant transformation of the actin cytoskeleton from parallel contractile bundles (3) into a dense network and loss of the normal interdigitating pattern (Figure 1e), leads to proteinuria (4).
There is a growing understanding of the sequence of events that mediates FP effacement and proteinuria (Figure 3a), followed over time by further phenotypic changes such as podocyte hypertrophy and ultimately podocyte detachment and loss (Figure 3b). The initial patterns of injury include (a) changes in SD structure or function (52, 63, 64), (b) interference with the GBM or the podocyte:GBM interaction (21, 65–70), (c) dysregulation of podocyte Ca2+ homeostasis (71, 72), (d) dysfunction of the podocyte actin cytoskeleton (50, 51, 62, 73–76), (e) modulation of the negative surface charge of podocytes (5, 77, 78), (f) activation of innate immunity pathways such as B7-1 signaling (79–81), (g) upregulation of CatL-mediated proteolysis (82–86), and (h) disturbances in the transcriptional regulation of podocyte function (87).
Figure 3.
Reversible and irreversible consequences of dysregulated podocyte signaling. (a) Dysregulated signaling at the plasma membrane may lead to reversible morphological changes. Therefore, proteinuria may arise, with or without foot process (FP) effacement (see text for details). However, if the upstream injurious signals are reversed, the cell morphology can revert back to physiological patterns. SD denotes slit diaphragm. (b) Persistence of podocyte injury is manifest in the activation of cellular processes that lead to irreversible changes such as loss of adhesion to the glomerular basement membrane (GBM), cell hypertrophy, transcriptional changes, disrupted metabolic pathways, autophagy, and cell cycle dysregulation. These irreversible changes can cause podocyte cell death or the detachment of podocytes from the GBM. Podocyte senescence may also be a manifestation of persistent injury, although less is known about this mechanism. The resulting loss of podocytes ultimately leads to irreversible glomerulosclerosis and kidney failure.
Importantly, a common theme to recent advances is the ever-growing significance of membrane proteins in the emergence of FP effacement and progressive podocyte injury (Figures 2 and 3). Growth factor receptors such as vascular endothelial growth factor (88, 89) and transforming growth factor β (90), GPCRs such as the angiotensin type 1 receptor (AT1R) (91–93), signaling through Notch (94, 95) or integrins (21–23, 96, 97), ion channels such as the TRPCs (48, 71, 98–100), and many other molecules (summarized in Figure 2) have been implicated in early podocyte injury. Although such molecules work in concert under physiological conditions, they become functionally uncoupled under disease conditions, leading to a disrupted cytoskeleton, the best known podocyte injury pattern to date.
Early podocyte injury is reversible if the actin cytoskeleton is repaired, allowing FPs to branch once again into their interdigitating pattern (Figure 3a). Sustained chronic podocyte injury can lead to the loss of glomerular and ultimately entire kidney function through three principal mechanisms: (a) the dysregulated pathway, (b) the inflammatory pathway, and (c) the degenerative pathway (101). First, in the dysregulated pathway, dedifferentiation of podocytes leads to podocyte proliferation within Bowman’s space and the collapse of the glomerular tuft, with GBM wrinkling and capillary loss. Thus, so-called collapsing glomerulopathy occurs, for example, in HIV-associated nephropathy. Second, inflammatory mechanisms can lead to the fixation of podocytes to the parietal basement membrane followed by the establishment of tuft adhesions to Bowman’s capsule (101). Further proliferation of podocytes and parietal cells results in the formation of cellular crescents. When the lesion heals by fibrosis, segmental glomerulosclerosis occurs (101). Finally, in the degenerative form, which is most commonly observed, the persistence of podocyte injury can cause cell body attenuation, podocyte hypertrophy, detachment from the GBM, and podocyte death followed by the formation of synechiae by the attachment of parietal epithelial cells to nude GBM (Figure 3b). This attachment results in misdirected filtration toward the interstitium (101, 102). Through a series of ensuing changes (reviewed in detail in Reference 101), the loss of podocytes ultimately leads to glomerulosclerosis and to kidney failure (101). The contribution of proteinuria to the progression of kidney disease is a matter of debate (103). Some investigators believe that proteinuria can induce podocyte damage (104) or tubulointerstitial inflammation and progressive injury (105). However, severe experimental protein loss across the glomerular filter caused by repeated injection of rats with the antinephrin antibody 5-1-6 did not lead to progressive renal failure (106). The role of proteinuria in the progression of kidney failure may depend on the route of protein loss. Significant podocyte loss per glomerulus, and thus misdirected filtration into the periglomerular interstitium, leads to tubular destruction and the progression of kidney failure (101, 107). In contrast, protein loss across the filtration barrier without significant podocyte loss does not lead to disease progression and may be reversible (Figure 3a) (101, 108).
GENETIC CAUSES OF PODOCYTE INJURY
Human genetics have fueled our progress toward a molecular understanding of the SD and the modulators of FP architecture. In the past 15 years, human genetic studies revealed that mutations in the genes encoding nephrin (25), podocin (29), phospholipase C ε (109), and coenzyme Q10 biosynthesis mono-oxygenase 6 (110) give rise to early-onset proteinuria. Additionally, adult-onset proteinuria such as FSGS is associated with mutations in the genes encoding α-actinin 4 (62), CD2AP (111), INF2 (112), TRPC6 (48, 100, 113), and synaptopodin (114). Mutations in LMX1B, which encodes a transcription factor for collagen, result in podocyte abnormalities due to impaired cell adhesion to the abnormal GBM (115). Similarly, mutations in the gene encoding laminin β2, another component of the GBM, lead to podocyte injury and proteinuria (116). Finally, recent exome sequencing as well as a whole-genome linkage analysis revealed MYO1E mutations in childhood proteinuric disease and FSGS; MYO1E encodes a mutant form of nonmuscle class I myosin (117, 118). Table 2 summarizes human mutations affecting podocyte structure and function. Mouse genetic studies have revealed that additional proteins regulating the plasticity of the podocyte actin cytoskeleton such as Rho GDP dissociation inhibitor α (Rho GDIα) (119), podocalyxin (6), FAT1 (120), Nck1/2 (26, 51), synaptopodin (121, 122), and cofilin (123) are also of critical importance for sustained function of the glomerular filtration barrier. Taken together, human and mouse genetics have revealed that the dysregulation of the highly specialized podocyte actin cytoskeleton is closely associated with disease phenotypes. At present, the regulation of the actin cytoskeleton is probably the best understood aspect of podocyte function (see below).
Table 2.
Human mutations affecting podocyte structure and function
| Protein | Gene | Associated disease | Mode of inheritance | Clinical description | Reference |
|---|---|---|---|---|---|
| Nephrin | NPHS1 | Congenital nephrotic syndrome of the Finnish type (CNF) | Autosomal recessive (AR) | Often massive proteinuria in utero and nephrotic syndrome postnatally; resistant to treatment | 25 |
| Podocin | NPHS2 | Corticosteroid-resistant nephrotic syndrome (SRNS) | AR | Variable onset and severity of nephropathy; resistance to corticosteroid therapy | 29 |
| Coenzyme Q10 biosynthesis mono-oxygenase 6 | COQ6 | Corticosteroid-resistant nephrotic syndrome (SRNS) | AR | Early-onset SNRS with sensorineural deafness | 110 |
| PLCε1 | PLCE1 | Inherited nephrotic syndrome | AR | Nephrosis, diffuse mesangial sclerosis, and end-stage kidney disease (ESKD); may be reversible after early treatment | 109 |
| Laminin β2 | LAMB2 | Pierson’s syndrome Occasionally oligosymptomatic disease variants | AR | Onset of nephrosis postnatally, mesangial sclerosis, microcoria | 116 |
| α-Actinin-4 | ACTN4 | Focal segmental glomerulosclerosis (FSGS) | Autosomal dominant (AD) | Mild proteinuria in adolescence, slow progression to FSGS and ESKD in adulthood | 62 |
| TRPC6 | TRPC6 | FSGS | AD | Proteinuria in adolescence and early adulthood; progression to FSGS and ESKD; pediatric/sporadic cases reported | 48, 100 |
| MYH9 | MYH9 | Epstein syndrome Fechtner syndrome | AD | Thrombocytopenia, hearing defects, and progressive proteinuria | 126 |
| LMX1B | LMX1B | Nail-patella syndrome | AD | Variable penetrance, nephrotic syndrome, and skeletal/nail abnormalities in children | 115, 196 |
| WT1 | WT1 | Denys-Drash syndrome (DDS) Frasier’s syndrome (FS) | AD | Male pseudohermaphroditism with progressive nephropathy and ESKD; development of FSGS by 3 years (DDS) or later (FS) | 197, 198 |
| CD2AP | CD2AP | Sporadic FSGS | N/A | FSGS in African-American patients | 111 |
| Synaptopodin | SYNPO | Sporadic FSGS | N/A | FSGS in Chinese patients | 114 |
| Myosin 1E | MYO1E | Childhood FSGS | N/A | Progressive, steroid-resistant FSGS | 117, 118 |
| Apolipoprotein L-1 | APOL1 | Sporadic FSGS | N/A | Progressive proteinuria, FSGS, and ESKD in African-American patients | 127 |
| Glypican 5 | GPC5 | Acquired nephrotic syndrome | N/A | Progressive proteinuria and ESKD | 128 |
With the advent of genomics, large population studies have revealed common variations in a number of genes that predispose or confer susceptibility to acquired proteinuric kidney disease. These gene polymorphisms are not directly linked to podocyte-specific defects, but this is an area of active research at this time. A large locus containing numerous genes was recently identified in African-American populations (124, 125). Initial studies revealed MYH9 as a likely gene candidate in this locus. This was an attractive hypothesis, given previous work showing that MYH9 is responsible for two genetic causes of proteinuria: Epstein and Fechtner syndromes (Table 2) (126). Interestingly, further work revealed that the likely candidate gene conferring risk for kidney disease is APOL1. This gene encodes apolipoprotein L1, a molecule that is known for its trypanolytic properties and that confers an evolutionary advantage in African-Americans (127). Furthermore, common variations in GPC5 (which encodes glypican 5) are also associated with acquired nephrotic syndrome (128).
MOLECULAR REGULATORS OF PODOCYTE FUNCTION IN HEALTH AND DISEASE
The past decade has brought significant new knowledge about membrane proteins that initiate signaling pathways important for podocyte structure and function (Figure 2). These molecules act as sensors of the podocyte’s complex extracellular environment, receiving cues from the urinary (Bowman’s) space, the vascular (capillary) space, and the GBM. These signals are subsequently transduced to the intracellular environment, modulating the actin cytoskeleton, gene transcription, and cellular metabolism pathways, among many others (Figures 2 and 3). Here we focus on a few signaling cascades that multiple scientists have investigated: Ca2+ signaling and TRPC channels, angiotensin signaling, integrins, and a mammalian target of rapamycin (mTOR) cascade intersecting with autophagy pathways. The roles of numerous other molecules are not clearly understood. Figure 2, a schematic of a podocyte plasma membrane decorated by membrane proteins, represents our concerted effort to be inclusive of the proteins studied in podocytes to date.
CALCIUM SIGNALING IN PODOCYTES
In conjunction with mounting evidence that proteinuria and podocyte FP effacement are mediated by rearrangements of the actin cytoskeleton (7, 51, 129), disrupted Ca2+ signaling and homeostasis were postulated as early events in podocyte injury (130). Complement C5b-9 complex–mediated podocyte damage is associated with an increase in intracellular Ca2+ concentration ([Ca2+]i) (131). Protamine sulfate, which can cause FP effacement in vivo (132, 133), also increases [Ca2+]i in vitro in both cultured cells (134) and isolated glomeruli (72). Bradykinin and angiotensin II (Ang II) application on differentiated mouse and rat podocytes, respectively, increases [Ca2+]i (135, 136). A recent intriguing study also revealed that the Ca2+-sensing receptor has prosurvival and actin-stabilizing effects in podocytes (137). Importantly, Ang II evokes a nonselective cationic current recorded from podocytes of isolated rat glomeruli (93). Once TRPC6 channel mutations were found in patients with familial FSGS (48, 100), TRP channels emerged as prime candidates for this as-yet-uncharacterized conductance. Recently, detailed electrophysiology unveiled TRPC5 and TRPC6 as the channels downstream of the Ang II–evoked nonselective cationic conductance (71), initially identified more than a decade ago (93).
Activation of the Ca2+-dependent phosphatase calcineurin leads to cathepsin L–mediated cleavage of synaptopodin and to proteinuria (83). The calcineurin inhibitor cyclosporine A (CsA) prevents synaptopodin degradation in vitro, and mice resistant to cathepsin-mediated synaptopodin degradation are protected from proteinuria in vivo (83). Conversely, the activation of calcineurin in podocytes is sufficient to cause degradation of synaptopodin and proteinuria (83). Thus, the preservation of synaptopodin and the podocyte actin cytoskeleton provides an intriguing podocyte-specific, T cell– and NFAT-independent mechanism for the long-known antiproteinuric effect of calcineurin inhibition (83). However, NFAT-dependent mechanisms are also important in podocytes. Ca2+-conducting and FSGS-causing TRPC6 mutations, but not wild-type TRPC6, induce constitutive activation of calcineurin- and NFAT-dependent gene transcription (138). Importantly, the activation of NFAT signaling in podocytes is sufficient to cause glomerulosclerosis in mice (139). A recent study supports the notion of a positive feedback loop, whereby NFAT-mediated increases in TRPC6 channel transcription lead to proteinuria (98). Although not experimentally addressed in this study, the proposed mechanism of injury relates to excessive Ca2+ influx due to aberrantly high numbers of active TRPC6 channels on the podocyte plasma membrane. A similar feed forward cycle was demonstrated in a mouse model of cardiac hypertrophy: Calcineurin- and NFAT-mediated increases in TRPC6 transcription promote pathological cardiac remodeling (140). More recently, however, investigators showed that other TRPC channels mediate calcineurin-NFAT activation in cardiac myocytes (140–142), strongly suggesting that this pathway is not specific to TRPC6.
ANGIOTENSIN SIGNALING IN PODOCYTES
The podocyte-specific overexpression of the AT1R in rats is sufficient to cause proteinuria and FSGS-type lesions. This model may be one of the best rodent models of FSGS to date because the model mimics the human disease by exhibiting hyperlipidemia, hypoalbuminemia, and progressive kidney failure (91). At the cellular level, the activation of the AT1R transactivates the epidermal growth factor receptor (EGFR) in tubular epithelia (143) and in podocytes (144). Following an increase in cytosolic [Ca2+], AT1R-EGFR interactions also activate downstream serine/threonine kinases such as the mitogen-activated protein kinase pathway (145). Ang II induces membrane ruffling and the loss of stress fibers (92), similar to the depletion of synaptopodin (83, 122) or of TRPC6 (71; see section below). Intriguingly, the AT1R signals to TRPC5 and TRPC6 channels in podocytes (71; see section below). The most compelling evidence to study the effects of angiotensin in podocytes comes from human trials in which the inhibition of AT1R signaling through angiotensin-converting enzyme inhibitors or angiotensin receptor blockers delayed disease progression in patients with diabetic kidney disease (146, 147).
CALCIUM, TRPC CHANNELS, RHO GTPASES, AND THE REGULATION OF THE ACTIN CYTOSKELETON
Previous studies in many cell types, including fibroblasts and neurons, had established an intimate association between Ca2+ influx and the activation of the Rho GTPases, which are cytoskeleton master regulators (148, 149). Given the central role played by Ca2+ and the actin cytoskeleton in podocyte biology, it is not surprising that the Rho GTPases have been the subject of numerous recent studies in podocytes. At the cellular level, a stationary podocyte phenotype, which suggests intact FPs, is thought to be due to the relative predominance of RhoA activity. In contrast, a predominance of Cdc42/Rac1 activity mediates a disease-associated motile phenotype, suggesting unstable or retracted FPs (7). Synaptopodin has emerged as an important regulator of Rho GTPases in podocytes. Synaptopodin promotes RhoA signaling by preventing its ubiquitination and proteasomal degradation. This results in the preservation of stress fibers in vitro and in safeguarding against proteinuria in vivo (83, 122). Synaptopodin-depleted podocytes, in which RhoA is ubiquitinated, display loss of stress fiber formation and aberrant filopodia (121). Synaptopodin also suppresses Cdc42 signaling through the inhibition of Cdc42:IRSp53:Mena complexes (150). Recent studies thus support the notion that synaptopodin stabilizes the kidney filter by promoting RhoA and inhibiting Cdc42, thereby preventing the reorganization of the podocyte FP cytoskeleton into a migratory phenotype (83). The promotility GTPase Rac1 was also implicated in podocyte biology: Rho GDIα–null mice develop heavy albuminuria (119) due to increased, constitutively active Rac1 signaling in podocytes (75). Rac1 promotes the accumulation of mineralocorticoid receptor into the podocyte nucleus through p21-activated kinase phosphorylation (75). Pharmacological intervention with a Rac1-specific small-molecule inhibitor (NSC23766) diminishes mineralocorticoid receptor hyperactivity and ameliorates proteinuria and renal damage in this mouse model of proteinuria (75). Taken together, these studies suggest an important role for the RhoGTPases in podocyte health and disease.
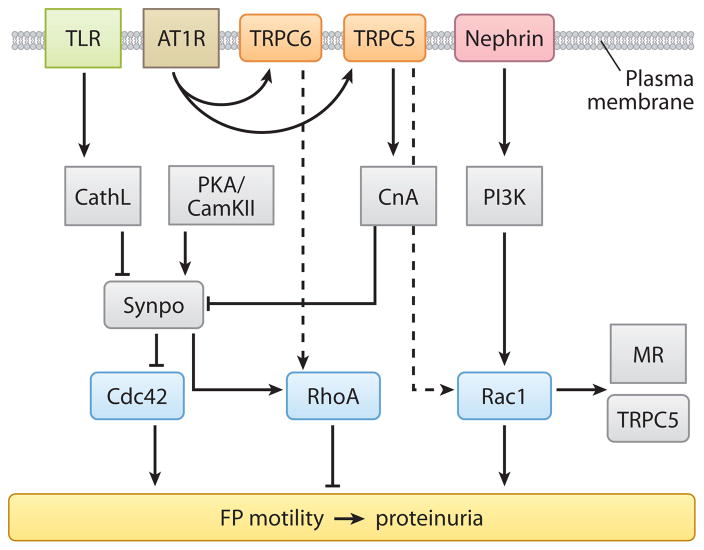
Since the discovery of gain-of-function TRPC6 mutations in familial FSGS (48, 100), the molecular mechanisms involving TRPC channels have become a central question in podocyte biology. Recently, AT1R-activated TRPC5 and TRPC6 channels were unveiled as antagonistic regulators of actin dynamics and cell motility in podocytes through the regulation of Rac1 and RhoA, respectively (Figure 4) (71). The latter study showed that TRPC6 depletion results in the loss of stress fibers, Rac1 activation, and increased motility, all of which are rescued by constitutively active RhoA (71). Conversely, TRPC5 depletion leads to enhanced stress fiber formation, RhoA activation, and decreased motility, all of which are reversed by constitutively active Rac1 (Figure 4) (71). Two distinct signaling microdomains emerged: TRPC5 specifically interacts with and activates Rac1, whereas TRPC6 specifically interacts with and activates RhoA (Figure 4) (71). Consistent with previous studies (83), CsA restored synaptopodin expression in TRPC6-depleted cells (71). In contrast, synaptopodin expression was preserved in TRPC5-depleted podocytes (71). These results significantly extended our mechanistic understanding of TRPC channelopathies and provided a mechanistic link between AT1R activation, Ca2+ signaling, TRPC channels, and Rho GTPase activity in podocytes.
Figure 4.
An example of pathways at the intersection of AT1R and TRPC signaling at the plasma membrane as they converge on synaptopodin and Rho GTPase signaling in the cytosol to effect critical cytoskeletal remodeling. Dashed arrows indicate indirect effects between molecules. Abbreviations: TLR, Toll-like receptor; AT1R, angiotensin type 1 receptor; TRPC6/5, transient receptor potential canonical channels 6 and 5; CathL, cathepsin L; PKA/CamKII, protein kinase A/calcium-calmodulin-dependent protein kinase II; CnA, calcineurin; PI3K, phosphoinositide 3 kinase; Synpo, synaptopodin; MR, mineralocorticoid receptor; FP, foot process.
Mice overexpressing wild-type TRPC6 and TRPC6 gain-of-function mutants develop albuminuria and FSGS-type lesions (151). Although proteinuria in these mice was modest with low and variable penetrance and structural abnormalities were not observed before 6–8 months of age (151), these results are consistent with Ca2+ overload–mediated cellular injury and death, ultimately leading to FSGS. In keeping with cell culture data showing RhoA activation downstream of TRPC6 activity (71), recent work revealed that mice overexpressing constitutively active RhoA in a podocyte-specific manner also developed proteinuria and FSGS-type lesions (152). Taken together, these studies suggest that an unopposed or overactive TRPC6-RhoA pathway may cause irreversible injury, podocyte loss, and kidney failure. In keeping with this, a recent study reported that TRPC6-null mice were significantly protected from the proteinuric effects of Ang II (99). The observed protective role of TRPC6 deletion is surprising, given that TRPC6-null mice were initially reported to be hypertensive at baseline due to (over)compensation by TRPC3 for the loss of TRPC6 (153). In the light of the antagonistic effects of TRPC5 and TRPC6 on podocyte actin dynamics (71), future studies will be needed to address possible compensatory or antagonistic relationships between TRPC channels in TRPC6 or other TRPC knockout mice. The development of podocyte-specific, inducible TRPC knockout mice is also likely to illuminate our understanding of in vivo TRPC channel function in podocytes.
INTEGRIN SIGNALING IN PODOCYTES
Integrins are heterodimeric cell adhesion receptors whose function is central to inflammation, immunity, tumor progression, development, and the maintenance of normal tissue architecture in mature organs (154, 155). Cell adhesion and spreading as well as remodeling of the extracellular matrix (ECM) involve bidirectional signaling and physical linkage between the ECM, integrins, and the cytoskeleton (154, 155). The actin cytoskeleton of podocyte FPs is linked to the GBM by α3β1 integrin (23, 96, 156), αvβ3 integrin (64, 157), and α-/β-dystroglycans (65, 158). Podocytes also express β4 integrin, and mutations in the gene encoding β4 integrin are associated with congenital FSGS and skin disease (159). Genetic inactivation of α3 (21) or β1 (22, 23) integrin causes podocyte FP effacement and kidney failure in newborn mice, thereby underscoring the critical role of α3β1 integrin in the development and maintenance of the glomerular filter. A recent study (160) suggested that soluble uPAR (suPAR) may be a factor responsible for the development of recurrent FSGS (161). According to this study, suPAR leads to recurrent FSGS through the activation of β3 integrin in podocytes (160). Of note, these data are in contrast with previous studies identifying β3 integrin as a mediator of protective osteopontin signaling in podocytes (157). Future studies will be required to address the precise function of β3 integrin signaling in podocytes. In addition, the relative contribution of β3 integrin activation versus loss of sphingomyelin phosphodiesterase acid–like 3b protein expression (162) to the pathogenesis of recurrent FSGS remains to be resolved.
The β1 integrin–binding protein integrin-linked kinase (ILK) is another mediator of progressive podocyte damage (163). Podocyte-specific deletion of the ILK gene in mice causes progressive FSGS and renal failure (22, 164, 165). Activation of ILK in podocytes induces Wnt signaling, which in turn leads to the reduction of CD2AP and P-cadherin expression, podocyte detachment, and proliferation (166). Moreover, overexpression of ILK causes the rearrangement of the podocyte actin cytoskeleton, presumably via ILK-mediated phosphorylation of α-actinin (163). ILK serves as an adaptor that biochemically and functionally connects the GBM with the SD: It interacts with nephrin, α-actinin, PINCH, and α-parvin, playing a crucial role in podocyte adhesion, morphology, and survival (165, 167). A recent study also brought to light the importance of focal adhesion kinase (FAK) in podocytes, showing that its inhibition protects against proteinuria and FP effacement (168). This study concluded that podocytes isolated from conditional, podocyte-specific FAK knockout mice demonstrated reduced spreading and migration. How the FAK, the ILK, and other integrin-mediated pathways intersect to mediate adaptive or maladaptive podocyte adhesion to the GBM will undoubtedly be the subject of many future inquiries.
The role of B7-1 as a bidirectional regulator of T cell activation and tolerance is well established and involves the binding of B7-1 to its cognate receptors CD28, CTLA, or PD-L1 (169). Most interestingly, however, B7-1 is also an inducible mediator of podocyte injury and proteinuria (81). Initially identified due to its significant upregulation in α3 integrin knockout mice, it is also up-regulated in podocytes in human and experimental lupus nephritis and in nephrin knockout mice (81). The clinical significance of these results was underscored by the observation that podocyte expression of B7-1 correlates with the severity of human lupus nephritis. The latter study (81) also found that in vivo exposure to low-dose lipopolysaccharide (LPS) rapidly upregulates B7-1 in podocytes of wild-type and SCID mice, thereby leading to proteinuria. In contrast, mice lacking B7-1 are protected from LPS-induced proteinuria, demonstrating a functional link between podocyte B7-1 expression and proteinuria (81). LPS signaling through Toll-like receptor-4 reorganizes the podocyte actin cytoskeleton. Moreover, the activation of B7-1 in cultured podocytes leads to the reorganization of the vital SD proteins nephrin and CD2AP (81). Thus, the upregulation of B7-1 in podocytes by LPS contributes to the pathogenesis of proteinuria by disrupting the glomerular filter (81). Given the initial observation that B7-1 is upregulated in the absence of α3 integrin in vivo, future studies will be required to address how B7-1 might intersect with integrin-mediated signaling in podocytes.
PODOCYTE MAMMALIAN TARGET OF RAPAMYCIN SIGNALING AND AUTOPHAGY
The mTOR signaling cascade regulates a wide array of cellular processes, including cell growth, proliferation, and autophagy, in response to nutrients such as glucose, amino acids, and growth factors. mTOR is an evolutionarily conserved protein kinase and forms two functional complexes termed mTOR complex 1 (mTORC1) and mTORC2. mTORC1 is a rapamycin-sensitive protein kinase that senses nutrient availability (170). mTOR signaling in podocytes has come to the forefront for two reasons. First, the mTOR inhibitor rapamycin, which is clinically used for immunosuppression after organ transplantation, can induce proteinuria, although the underlying molecular mechanisms are poorly understood. The idea that rapamycin can impair podocyte function is supported by the observation that its application on cultured podocytes decreases the protein abundance of nephrin, TRPC6, and Nck and reduces cell adhesion and motility (171). Nephrin expression is also reduced in glomeruli of kidney-transplanted patients undergoing mTOR inhibition therapy (172). However, rapamycin can prevent the protamine sulfate–induced and Ca2+-dependent disruption of the podocyte actin cytoskeleton, suggesting a potential beneficial effect of rapamycin on proteinuria (72).
In a second independent line of research, two recent studies revealed the importance of mTOR signaling in podocyte function and diabetic nephropathy in humans and mice. Genetic deletion of mTORC1 in mouse podocytes induced proteinuria and progressive glomerulosclerosis, whereas simultaneous deletion of both mTORC1 and mTORC2 from mouse podocytes aggravated the glomerular lesions (173). In contrast, increased mTOR activity accompanied human diabetic nephropathy, characterized by early glomerular hypertrophy and hyperfiltration. Curtailing mTORC1 signaling in mice by genetically reducing mTORC1 copy number in podocytes prevented glomerulosclerosis and ameliorated glomerular disease progression in diabetic nephropathy (173). Similarly, the activity of the mTOR complex was enhanced in podocytes of diabetic animals (174). Furthermore, podocyte-specific mTORC1 activation induced by ablation of an upstream negative regulator (PcKOTsc1) recapitulated many DN features, including podocyte loss, GBM thickening, mesangial expansion, and proteinuria, in nondiabetic young and adult mice (174). Abnormal mTORC1 activation caused mislocalization of SD proteins and endoplasmic reticulum (ER) stress in podocytes. Conversely, reduction of ER stress with a chemical chaperone significantly protected against both the podocyte phenotypic switch and podocyte loss in PcKOTsc1 mice. Finally, genetic reduction of podocyte-specific mTORC1 in diabetic animals suppressed the development of DN (174). Although protein synthesis and autophagic degradation are regulated in an opposite manner by mTOR (170), such regulation could be beneficial under certain conditions if these two events occurred in unison to handle rapid protein turnover. In keeping with this notion, a recent study revealed that spatial coupling of mTOR and autophagy augments the secretory phenotype of podocytes and macrophages (175). Narita and colleagues (175) observed a distinct cellular compartment at the trans side of the Golgi apparatus, the TOR-autophagy spatial coupling compartment (TASCC), where (auto)lysosomes and mTOR accumulated during Ras-induced senescence. mTOR recruitment to the TASCC was amino acid dependent and Rag guanosine triphosphatase dependent, and disruption of mTOR localization to the TASCC suppressed interleukin-6/8 synthesis. TASCC formation was observed during macrophage differentiation and in podocytes; both macrophages and podocytes displayed increased protein secretion. The spatial coupling of cells’ catabolic and anabolic machinery could augment their respective functions and facilitate the mass synthesis of secretory proteins (175). Collectively, these data underscore the critical role of mTOR signaling in podocytes for glomerular function in health and disease. These results demonstrate the requirement for tightly balanced mTOR activity in podocyte homeostasis and suggest that mTOR inhibition may protect podocytes and prevent progressive diabetic nephropathy (173). Given the detrimental effect of prolonged rapamycin treatment on podocyte function, it will be important to test if reduction of podocyte mTOR activity can be harnessed as a potential therapeutic strategy to treat DN.
OUTLOOK AND FUTURE DIRECTIONS
In recent decades, the podocyte has been firmly established as the cell responsible for proteinuria and kidney damage. Yet much remains to be learned about this complex cell. In this review, we take a membrane biologist’s view, examining membrane receptors, ion channels, and other signaling molecules implicated in podocyte biology. This approach may be fruitful in understanding the podocyte and its unique geographical properties because it brings a new perspective to the many challenges that lie ahead. Although we have made significant inroads in identifying the molecular components of this complex cell, many central questions remain. One challenge is to gain a further understanding of how these molecules fit together and work in unison under physiological conditions and how the system is perturbed after a noxious insult. A membrane biology perspective may help in this regard if we consider the plasma membrane as the beginning of Ariadne’s thread, which can help us navigate our way through what is truly a signaling labyrinth. Although disrupted cytoskeletal dynamics have emerged as a central mechanism for podocyte injury, another challenge is to go beyond the cytoskeleton in an effort to assign a molecular signature to pathways that may mediate the propagation of injury, such as metabolic dysregulation and autophagy. Indeed, little is known about the molecular determinants of pathological progression in podocytes. For example, does podocyte cell death precede detachment or vice versa? The future study of appropriate animal models of disease progression is also likely to be instrumental in this area. Finally, transmembrane receptors and ion channels have proven to be successful drug targets in other areas (176) and therefore should garner significant attention as putative drug targets for antiproteinuric therapies. The challenge will be to discern which of the multitude of podocyte membrane proteins, many of which we discuss here, will be the critical target(s) of choice.
Acknowledgments
We apologize to our colleagues whose work we were not able to cite in this review due to space limitations. Reviews were often quoted at the expense of original work. We thank W. Kriz and M.A. Arnaout for helpful discussions. A.G. is funded by a NephCure Young Investigator Grant, the ASN Gottschalk Award, and NIH grant DK083511; P.M., by NIH grants DK57683 and DK062472.
Footnotes
Errata
An online log of corrections to Annual Review of Physiology articles may be found at http://physiol.annualreviews.org/errata.shtml
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Anna Greka, Email: greka.anna@mgh.harvard.edu.
Peter Mundel, Email: mundel.peter@mgh.harvard.edu.
LITERATURE CITED
- 1.Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol. 1995;192:385–97. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- 2.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–35. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 3.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Investig. 1988;59:673–82. [PubMed] [Google Scholar]
- 4.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Investig. 2001;108:1583–87. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Investig. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmieder S, Nagai M, Orlando RA, Takeda T, Farquhar MG. Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J Am Soc Nephrol. 2004;15:2289–98. doi: 10.1097/01.ASN.0000135968.49899.E8. [DOI] [PubMed] [Google Scholar]
- 7.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–37. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 9.Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–92. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 10.Sorokin L, Ekblom P. Development of tubular and glomerular cells of the kidney. Kidney Int. 1992;41:657–64. doi: 10.1038/ki.1992.101. [DOI] [PubMed] [Google Scholar]
- 11.Reeves W, Caulfield JP, Farquhar MG. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Investig. 1978;39:90–100. [PubMed] [Google Scholar]
- 12.Schnabel E, Dekan G, Miettinen A, Farquhar MG. Biogenesis of podocalyxin—the major glomerular sialoglycoprotein—in the newborn rat kidney. Eur J Cell Biol. 1989;48:313–26. [PubMed] [Google Scholar]
- 13.Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–63. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Nuclear localization of the protein encoded by the Wims’ tumor gene WT1 in embryonic and adult tissues. Development. 1993;119:1329–41. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- 15.Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 16.Hartleben B, Schweizer H, Lubben P, Bartram MP, Moller CC, et al. Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem. 2008;283:23033–38. doi: 10.1074/jbc.M803143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber TB, Hartleben B, Winkelmann K, Schneider L, Becker JU, et al. Loss of podocyte aPKCλ/ι; causes polarity defects and nephrotic syndrome. J Am Soc Nephrol. 2009;20:798–806. doi: 10.1681/ASN.2008080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose T, Satoh D, Kurihara H, Kusaka C, Hirose H, et al. An essential role of the universal polarity protein, aPKCλ, on the maintenance of podocyte slit diaphragms. PLoS ONE. 2009;4:e4194. doi: 10.1371/journal.pone.0004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mundel P, Gilbert P, Kriz W. Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J Histochem Cytochem. 1991;39:1047–56. doi: 10.1177/39.8.1856454. [DOI] [PubMed] [Google Scholar]
- 20.Holthofer H, Miettinen A, Lehto VP, Lehtonen E, Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Investig. 1984;50:552–29. [PubMed] [Google Scholar]
- 21.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–47. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 22.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, et al. Integrin β1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313:584–93. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, et al. β1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316:288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciani L, Patel A, Allen ND, Ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23:3575–82. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–82. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 26.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–23. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 27.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–33. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, et al. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med. 2001;194:13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–54. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 30.Sadl V, Jin F, Yu J, Cui S, Holmyard D, et al. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol. 2002;249:16–29. doi: 10.1006/dbio.2002.0751. [DOI] [PubMed] [Google Scholar]
- 31.Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, et al. The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis. Development. 1999;126:5771–83. doi: 10.1242/dev.126.24.5771. [DOI] [PubMed] [Google Scholar]
- 32.Pabst R, Sterzl RB. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983;24:626–31. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- 33.Kriz W, Elger M, Kretzler M, Uiker S, Koeppen-Hagemann I, et al. The role of podocytes in the development of glomerular sclerosis. Kidney Int. 1994;45:S64–72. [PubMed] [Google Scholar]
- 34.Nagata M, Yamaguchi Y, Komatsu Y, Ito K. Mitosis and the presence of binucleate cells among glomerular podocytes in diseased human kidneys. Nephron. 1995;70:68–71. doi: 10.1159/000188546. [DOI] [PubMed] [Google Scholar]
- 35.Rasch R, Norgaard JO. Renal enlargement: comparative autoradiographic studies of 3H-thymidine uptake in diabetic and uninephrectomized rats. Diabetologia. 1983;25:280–87. doi: 10.1007/BF00279944. [DOI] [PubMed] [Google Scholar]
- 36.Fries JW, Sandstrom DJ, Meyer TW, Rennke HG. Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab Investig. 1989;60:205–18. [PubMed] [Google Scholar]
- 37.Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 38.Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. 2000;58:137–43. doi: 10.1046/j.1523-1755.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 39.Husain M, Gusella GL, Klotman ME, Gelman IH, Ross MD, et al. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J Am Soc Nephrol. 2002;13:1806–15. doi: 10.1097/01.asn.0000019642.55998.69. [DOI] [PubMed] [Google Scholar]
- 40.Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D’Agati V, Alpers CE. Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: role in podocyte proliferation and maturation. Kidney Int. 2000;58:674–83. doi: 10.1046/j.1523-1755.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- 41.Shankland SJ, Wolf G. Cell cycle regulatory proteins in renal disease: role in hypertrophy, proliferation, and apoptosis. Am J Physiol Ren Physiol. 2000;278:515–29. doi: 10.1152/ajprenal.2000.278.4.F515. [DOI] [PubMed] [Google Scholar]
- 42.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int. 1998;54:687–97. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 43.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, et al. Tracing the origin of glomerular extra-capillary lesions from parietal epithelial cells. J Am Soc Nephrol. 2009;20:2604–15. doi: 10.1681/ASN.2009010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–43. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, et al. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:1262–74. doi: 10.1681/ASN.2010090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kennedy MB. The postsynaptic density. Curr Opin Neurobiol. 1993;3:732–37. doi: 10.1016/0959-4388(93)90145-o. [DOI] [PubMed] [Google Scholar]
- 47.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 48.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–44. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15:1–7. doi: 10.1097/01.mnh.0000199012.79670.0b. [DOI] [PubMed] [Google Scholar]
- 50.Tryggvason K, Pikkarainen T, Patrakka J. Nck links nephrin to actin in kidney podocytes. Cell. 2006;125:221–24. doi: 10.1016/j.cell.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Investig. 2006;116:1346–59. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, et al. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278:20716–23. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 53.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73:556–66. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 54.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–13. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 55.Tanner GA. Glomerular sieving coefficient of serum albumin in the rat: a two-photon microscopy study. Am J Physiol Ren Physiol. 2009;296:1258–65. doi: 10.1152/ajprenal.90638.2008. [DOI] [PubMed] [Google Scholar]
- 56.Tanner GA, Rippe C, Shao Y, Evan AP, Williams JC., Jr Glomerular permeability to macromolecules in the Necturus kidney. Am J Physiol Ren Physiol. 2009;296:1269–78. doi: 10.1152/ajprenal.00371.2007. [DOI] [PubMed] [Google Scholar]
- 57.Peti-Peterdi J. Independent two-photon measurements of albumin GSC give low values. Am J Physiol Ren Physiol. 2009;296:1255–57. doi: 10.1152/ajprenal.00144.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haraldsson B, Jeansson M. Glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18:331–35. doi: 10.1097/MNH.0b013e32832c9dba. [DOI] [PubMed] [Google Scholar]
- 59.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–87. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 60.Navar LG. Glomerular permeability: a never-ending saga. Am J Physiol Ren Physiol. 2009;296:1266–68. doi: 10.1152/ajprenal.00152.2009. [DOI] [PubMed] [Google Scholar]
- 61.Tryggvason K, Wartiovaara J. Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens. 2001;10:543–49. doi: 10.1097/00041552-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–56. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 63.Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, et al. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol. 2001;159:1069–77. doi: 10.1016/S0002-9440(10)61782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei C, Moller CC, Altintas MM, Li J, Schwarz K, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 65.Regele HM, Fillipovic E, Langer B, Poczewki H, Kraxberger I, et al. Glomerular expression of dystroglycans is reduced in minimal change nephrosis but not in focal segmental glomerulosclerosis. J Am Soc Nephrol. 2000;11:403–12. doi: 10.1681/ASN.V113403. [DOI] [PubMed] [Google Scholar]
- 66.Raats CJ, Bakker MA, van den Born J, Berden JH. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272:26734–41. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- 67.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin β2: nephrosis despite molecular compensation by laminin β1. Nat Genet. 1995;10:400–6. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- 68.Kretzler M, Teixeira VP, Unschuld PG, Cohen CD, Wanke R, et al. Integrin-linked kinase as a candidate downstream effector in proteinuria. FASEB J. 2001;15:1843–45. doi: 10.1096/fj.00-0832fje. [DOI] [PubMed] [Google Scholar]
- 69.Lorenzen J, Shah R, Biser A, Staicu SA, Niranjan T, et al. The role of osteopontin in the development of albuminuria. J Am Soc Nephrol. 2008;19:884–90. doi: 10.1681/ASN.2007040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, et al. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175:33–39. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vassiliadis J, Bracken C, Matthews D, O’Brien S, Schiavi S, Wawersik S. Calcium mediates glomerular filtration through calcineurin and mTORC2/Akt signaling. J Am Soc Nephrol. 2011;22:1453–61. doi: 10.1681/ASN.2010080878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smoyer WE, Mundel P. Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med. 1998;76:172–83. doi: 10.1007/s001090050206. [DOI] [PubMed] [Google Scholar]
- 74.Kos CH, Le TC, Sinha S, Henderson JM, Kim SH, et al. Mice deficient in α-actinin-4 have severe glomerular disease. J Clin Investig. 2003;111:1683–90. doi: 10.1172/JCI17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–76. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 76.Lu TC, He JC, Wang ZH, Feng X, Fukumi-Tominaga T, et al. HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem. 2008;283:8173–82. doi: 10.1074/jbc.M708920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, et al. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 2001;12:1589–98. doi: 10.1681/ASN.V1281589. [DOI] [PubMed] [Google Scholar]
- 78.Galeano B, Klootwijk R, Manoli I, Sun M, Ciccone C, et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Investig. 2007;117:1585–94. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–93. doi: 10.1161/01.HYP.0000222003.28517.99. [DOI] [PubMed] [Google Scholar]
- 80.Navarro-Munoz M, Ibernon M, Perez V, Ara J, Espinal A, et al. Messenger RNA expression of B7-1 and NPHS1 in urinary sediment could be useful to differentiate between minimal change disease and focal segmental glomerulosclerosis in adult patients. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr128. In press. [DOI] [PubMed] [Google Scholar]
- 81.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Investig. 2004;113:1390–97. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asanuma K, Shirato I, Ishidoh K, Kominami E, Tomino Y. Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiated podocytes. Kidney Int. 2002;62:822–31. doi: 10.1046/j.1523-1755.2002.00539.x. [DOI] [PubMed] [Google Scholar]
- 83.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–38. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and α3 integrin. J Biol Chem. 2004;279:34827–32. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 85.Ronco P. Proteinuria: Is it all in the foot? J Clin Investig. 2007;117:2079–82. doi: 10.1172/JCI32966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sever S, Altintas MM, Nankoe SR, Moller CC, Ko D, et al. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Investig. 2007;117:2095–104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quaggin SE. Transcriptional regulation of podocyte specification and differentiation. Microsc Res Tech. 2002;57:208–11. doi: 10.1002/jemt.10076. [DOI] [PubMed] [Google Scholar]
- 88.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Investig. 2003;111:707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–36. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Böttinger EP, Bitzer M. TGF-β signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–10. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 91.Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15:1475–87. doi: 10.1097/01.asn.0000127988.42710.a7. [DOI] [PubMed] [Google Scholar]
- 92.Hsu HH, Hoffmann S, Endlich N, Velic A, Schwab A, et al. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med. 2008;86:1379–94. doi: 10.1007/s00109-008-0399-y. [DOI] [PubMed] [Google Scholar]
- 93.Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, et al. Angiotensin II depolarizes podocytes in the intact glomerulus of the rat. J Clin Investig. 1997;99:2772–81. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waters AM, Wu MY, Onay T, Scutaru J, Liu J, et al. Ectopic Notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–57. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–98. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 96.Kreidberg JA. Functions of α3β1 integrin. Curr Opin Cell Biol. 2000;12:548–53. doi: 10.1016/s0955-0674(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 97.Clement LC, Avila-Casado C, Mace C, Soria E, Bakker WW, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–22. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011;179:1719–32. doi: 10.1016/j.ajpath.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, et al. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol. 2011;22:526–35. doi: 10.1681/ASN.2010050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–4. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 101.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases—insights from animal models. Kidney Int. 2005;67:404–19. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 102.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–15. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 103.Zandi-Nejad K, Eddy AA, Glassock RJ, Brenner BM. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int. 2004;66:S76–89. doi: 10.1111/j.1523-1755.2004.09220.x. [DOI] [PubMed] [Google Scholar]
- 104.Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, et al. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–20. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilmer WA, Rovin BH, Hebert CJ, Rao SV, Kumor K, Hebert LA. Management of glomerular proteinuria: a commentary. J Am Soc Nephrol. 2003;14:3217–32. doi: 10.1097/01.asn.0000100145.27188.33. [DOI] [PubMed] [Google Scholar]
- 106.Kikuchi H, Kawachi H, Ito Y, Matsui K, Nosaka H, et al. Severe proteinuria, sustained for 6 months, induces tubular epithelial cell injury and cell infiltration in rats but not progressive interstitial fibrosis. Nephrol Dial Transplant. 2000;15:799–810. doi: 10.1093/ndt/15.6.799. [DOI] [PubMed] [Google Scholar]
- 107.Kriz W, Hartmann I, Hosser H, Hahnel B, Kranzlin B, et al. Tracer studies in the rat demonstrate misdirected filtration and peritubular filtrate spreading in nephrons with segmental glomerulosclerosis. J Am Soc Nephrol. 2001;12:496–506. doi: 10.1681/ASN.V123496. [DOI] [PubMed] [Google Scholar]
- 108.Gross ML, Hanke W, Koch A, Ziebart H, Amann K, Ritz E. Intraperitoneal protein injection in the axolotl: the amphibian kidney as a novel model to study tubulointerstitial activation. Kidney Int. 2002;62:51–59. doi: 10.1046/j.1523-1755.2002.00402.x. [DOI] [PubMed] [Google Scholar]
- 109.Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet. 2006;38:1397–405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 110.Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Investig. 2011;121:2013–24. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298–300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 112.Brown EJ, Schlondorff JS, Becker DJ, Tsukaguchi H, Uscinski AL, et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 2010;42:72–76. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heeringa SF, Moller CC, Du J, Yue L, Hinkes B, et al. A novel TRPC6 mutation that causes childhood FSGS. PLoS ONE. 2009;4:e7771. doi: 10.1371/journal.pone.0007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dai S, Wang Z, Pan X, Wang W, Chen X, et al. Functional analysis of promoter mutations in the ACTN4 and SYNPO genes in focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2010;25:824–35. doi: 10.1093/ndt/gfp394. [DOI] [PubMed] [Google Scholar]
- 115.Morello R, Zhou G, Dreyer SD, Harvey SJ, Ninomiya Y, et al. Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome. Nat Genet. 2001;27:205–8. doi: 10.1038/84853. [DOI] [PubMed] [Google Scholar]
- 116.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, et al. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–32. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 117.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med. 2011;365:295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanna-Cherchi S, Burgess KE, Nees SN, Caridi G, Weng PL, et al. Exome sequencing identified MYO1E and NEIL1 as candidate genes for human autosomal recessive steroid-resistant nephrotic syndrome. Kidney Int. 2011;80:389–96. doi: 10.1038/ki.2011.148. [DOI] [PubMed] [Google Scholar]
- 119.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIα. Oncogene. 1999;18:5373–80. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 120.Moeller MJ, Soofi A, Braun GS, Li X, Watzl C, et al. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J. 2004;23:3769–79. doi: 10.1038/sj.emboj.7600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, et al. Synaptopodin regulates the actin-bundling activity of α-actinin in an isoform-specific manner. J Clin Investig. 2005;115:1188–98. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–91. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 123.Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, et al. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem. 2010;285:22676–88. doi: 10.1074/jbc.M110.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–92. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–84. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arrondel C, Vodovar N, Knebelmann B, Grunfeld JP, Gubler MC, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65–74. doi: 10.1681/ASN.V13165. [DOI] [PubMed] [Google Scholar]
- 127.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–45. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Okamoto K, Tokunaga K, Doi K, Fujita T, Suzuki H, et al. Common variation in GPC5 is associated with acquired nephrotic syndrome. Nat Genet. 2011;43:459–63. doi: 10.1038/ng.792. [DOI] [PubMed] [Google Scholar]
- 129.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol. 2007;27:8698–712. doi: 10.1128/MCB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kerjaschki D. Polycation-induced dislocation of slit diaphragms and formation of cell junctions in rat kidney glomeruli. The effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab Investig. 1978;39:430–40. [PubMed] [Google Scholar]
- 131.Cybulsky AV, Bonventre JV, Quigg RJ, Lieberthal W, Salant DJ. Cytosolic calcium and protein kinase C reduce complement-mediated glomerular epithelial injury. Kidney Int. 1990;38:803–11. doi: 10.1038/ki.1990.274. [DOI] [PubMed] [Google Scholar]
- 132.Seiler MW, Rennke HG, Venkatachalam MA, Cotran RS. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab Investig. 1977;36:48–61. [PubMed] [Google Scholar]
- 133.Seiler MW, Venkatachalam MA, Cotran RS. Glomerular epithelium: structural alterations induced by polycations. Science. 1975;189:390–93. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- 134.Rudiger F, Greger R, Nitschke R, Henger A, Mundel P, Pavenstadt H. Polycations induce calcium signaling in glomerular podocytes. Kidney Int. 1999;56:1700–9. doi: 10.1046/j.1523-1755.1999.00729.x. [DOI] [PubMed] [Google Scholar]
- 135.Mundel P, Reiser J, Kriz W. Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol. 1997;8:697–705. doi: 10.1681/ASN.V85697. [DOI] [PubMed] [Google Scholar]
- 136.Henger A, Huber T, Fischer KG, Nitschke R, Mundel P, et al. Angiotensin II increases the cytosolic calcium activity in rat podocytes in culture. Kidney Int. 1997;52:687–93. doi: 10.1038/ki.1997.383. [DOI] [PubMed] [Google Scholar]
- 137.Oh J, Beckmann J, Bloch J, Hettgen V, Mueller J, et al. Stimulation of the calcium-sensing receptor stabilizes the podocyte cytoskeleton, improves cell survival, and reduces toxin-induced glomerulosclerosis. Kidney Int. 2011;80:483–92. doi: 10.1038/ki.2011.105. [DOI] [PubMed] [Google Scholar]
- 138.Schlondorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol. 2009;296:58–69. doi: 10.1152/ajpcell.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, et al. Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2010;21:1657–66. doi: 10.1681/ASN.2009121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Investig. 2006;116:3114–26. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–16. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA. 2010;107:7000–5. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen J, Chen JK, Neilson EG, Harris RC. Role of EGF receptor activation in angiotensin II-induced renal epithelial cell hypertrophy. J Am Soc Nephrol. 2006;17:1615–23. doi: 10.1681/ASN.2005111163. [DOI] [PubMed] [Google Scholar]
- 144.Flannery PJ, Spurney RF. Transactivation of the epidermal growth factor receptor by angiotensin II in glomerular podocytes. Nephron Exp Nephrol. 2006;103:e109–18. doi: 10.1159/000092196. [DOI] [PubMed] [Google Scholar]
- 145.Inagami T, Eguchi S, Numaguchi K, Motley ED, Tang H, et al. Cross-talk between angiotensin II receptors and the tyrosine kinases and phosphatases. J Am Soc Nephrol. 1999;10(Suppl 11):57–61. [PubMed] [Google Scholar]
- 146.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–69. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 147.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 148.Aspenstrom P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377:327–37. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 150.Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young Choi H, et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–27. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, et al. Podocyte-specific overexpression of wild type or mutant Trpc6 in mice is sufficient to cause glomerular disease. PLoS ONE. 2010;5:e12859. doi: 10.1371/journal.pone.0012859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:1621–30. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–89. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 155.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 156.Kerjaschki D, Ojha PP, Susani M, Horvat R, Binder S, et al. A β1-integrin receptor for fibronectin in human kidney glomeruli. Am J Pathol. 1989;134:481–89. [PMC free article] [PubMed] [Google Scholar]
- 157.Schordan S, Schordan E, Endlich K, Endlich N. αv-Integrins mediate the mechanoprotective action of osteopontin in podocytes. Am J Physiol Ren Physiol. 2011;300:119–32. doi: 10.1152/ajprenal.00143.2010. [DOI] [PubMed] [Google Scholar]
- 158.Raats CJ, van Den Born J, Bakker MA, Oppers-Walgreen B, Pisa BJ, et al. Expression of agrin, dystroglycan, and utrophin in normal renal tissue and in experimental glomerulopathies. Am J Pathol. 2000;156:1749–65. doi: 10.1016/S0002-9440(10)65046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kambham N, Tanji N, Seigle RL, Markowitz GS, Pulkkinen L, et al. Congenital focal segmental glomerulosclerosis associated with β4 integrin mutation and epidermolysis bullosa. Am J Kidney Dis. 2000;36:190–96. doi: 10.1053/ajkd.2000.8293. [DOI] [PubMed] [Google Scholar]
- 160.Wei C, El Hindi S, Li J, Fornoni A, Goes N, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–60. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–83. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 162.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Blattner SM, Kretzler M. Integrin-linked kinase in renal disease: connecting cell-matrix interaction to the cytoskeleton. Curr Opin Nephrol Hypertens. 2005;14:404–10. doi: 10.1097/01.mnh.0000172730.67746.5b. [DOI] [PubMed] [Google Scholar]
- 164.El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, et al. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol. 2006;17:1334–44. doi: 10.1681/ASN.2005090921. [DOI] [PubMed] [Google Scholar]
- 165.Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, et al. Essential role of integrin-linked kinase in podocyte biology: bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006;17:2164–75. doi: 10.1681/ASN.2006010033. [DOI] [PubMed] [Google Scholar]
- 166.de Paulo V, Teixeira C, Blattner SM, Li M, Anders HJ, et al. Functional consequences of integrin-linked kinase activation in podocyte damage. Kidney Int. 2005;67:514–23. doi: 10.1111/j.1523-1755.2005.67108.x. [DOI] [PubMed] [Google Scholar]
- 167.Yang Y, Guo L, Blattner SM, Mundel P, Kretzler M, Wu C. Formation and phosphorylation of the PINCH-1-integrin linked kinase-α-parvin complex are important for regulation of renal glomerular podocyte adhesion, architecture, and survival. J Am Soc Nephrol. 2005;16:1966–76. doi: 10.1681/ASN.2004121112. [DOI] [PubMed] [Google Scholar]
- 168.Ma H, Togawa A, Soda K, Zhang J, Lee S, et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol. 2010;21:1145–56. doi: 10.1681/ASN.2009090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 171.Vollenbroker B, George B, Wolfgart M, Saleem MA, Pavenstadt H, Weide T. mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes. Am J Physiol Ren Physiol. 2009;296:418–26. doi: 10.1152/ajprenal.90319.2008. [DOI] [PubMed] [Google Scholar]
- 172.Biancone L, Bussolati B, Mazzucco G, Barreca A, Gallo E, et al. Loss of nephrin expression in glomeruli of kidney-transplanted patients under m-TOR inhibitor therapy. Am J Transplant. 2010;10:2270–78. doi: 10.1111/j.1600-6143.2010.03259.x. [DOI] [PubMed] [Google Scholar]
- 173.Godel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Investig. 2011;121:2197–209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Inoki K, Mori H, Wang J, Suzuki T, Hong S, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Investig. 2011;121:2181–96. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–70. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ashcroft FM. From molecule to malady. Nature. 2006;440:440–47. doi: 10.1038/nature04707. [DOI] [PubMed] [Google Scholar]
- 177.Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell. 1990;60:509–20. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- 178.Gessler M, Poustka A, Cavenee W, Neve RL, Orkin SH, Bruns GA. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990;343:774–78. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- 179.Huang A, Campbell CE, Bonetta L, McAndrews-Hill MS, Chilton-MacNeill S, et al. Tissue, developmental, and tumor-specific expression of divergent transcripts in Wilms tumor. Science. 1990;250:991–94. doi: 10.1126/science.2173145. [DOI] [PubMed] [Google Scholar]
- 180.Bonetta L, Kuehn SE, Huang A, Law DJ, Kalikin LM, et al. Wilms tumor locus on 11p13 defined by multiple CpG island-associated transcripts. Science. 1990;250:994–97. doi: 10.1126/science.2173146. [DOI] [PubMed] [Google Scholar]
- 181.Miner JH, Morello R, Andrews KL, Li C, Antignac C, et al. Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Investig. 2002;109:1065–72. doi: 10.1172/JCI13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rohr C, Prestel J, Heidet L, Hosser H, Kriz W, et al. The LIM-homeodomain transcription factor Lmx1b plays a crucial role in podocytes. J Clin Investig. 2002;109:1073–82. doi: 10.1172/JCI13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, et al. Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J. 2006;25:1160–74. doi: 10.1038/sj.emboj.7601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol. 2006;26:5715–27. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dehbi M, Ghahremani M, Lechner M, Dressler G, Pelletier J. The paired-box transcription factor, PAX2, positively modulates expression of the Wilms’ tumor suppressor gene (WT1) Oncogene. 1996;13:447–53. [PubMed] [Google Scholar]
- 186.Ryan G, Steele-Perkins V, Morris JF, Rauscher FJ, 3rd, Dressler GR. Repression of Pax-2 by WT1 during normal kidney development. Development. 1995;121:867–75. doi: 10.1242/dev.121.3.867. [DOI] [PubMed] [Google Scholar]
- 187.Clement LC, Liu G, Perez-Torres I, Kanwar YS, Avila-Casado C, Chugh SS. Early changes in gene expression that influence the course of primary glomerular disease. Kidney Int. 2007;72:337–47. doi: 10.1038/sj.ki.5002302. [DOI] [PubMed] [Google Scholar]
- 188.Liu G, Clement LC, Kanwar YS, Avila-Casado C, Chugh SS. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J Biol Chem. 2006;281:39681–92. doi: 10.1074/jbc.M606664200. [DOI] [PubMed] [Google Scholar]
- 189.Cui S, Li C, Ema M, Weinstein J, Quaggin SE. Rapid isolation of glomeruli coupled with gene expression profiling identifies downstream targets in Pod1 knockout mice. J Am Soc Nephrol. 2005;16:3247–55. doi: 10.1681/ASN.2005030278. [DOI] [PubMed] [Google Scholar]
- 190.Kumar PA, Kotlyarevska K, Dejkhmaron P, Reddy GR, Lu C, et al. Growth hormone (GH)-dependent expression of a natural antisense transcript induces zinc finger E-box-binding homeobox 2 (ZEB2) in the glomerular podocyte: a novel action of GH with implications for the pathogenesis of diabetic nephropathy. J Biol Chem. 2010;285:31148–56. doi: 10.1074/jbc.M110.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ding M, Cui S, Li C, Jothy S, Haase V, et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12:1081–87. doi: 10.1038/nm1460. [DOI] [PubMed] [Google Scholar]
- 192.Brukamp K, Jim B, Moeller MJ, Haase VH. Hypoxia and podocyte-specific Vhlh deletion confer risk of glomerular disease. Am J Physiol Ren Physiol. 2007;293:1397–407. doi: 10.1152/ajprenal.00133.2007. [DOI] [PubMed] [Google Scholar]
- 193.Freeburg PB, Robert B, St John PL, Abrahamson DR. Podocyte expression of hypoxia-inducible factor (HIF)-1 and HIF-2 during glomerular development. J Am Soc Nephrol. 2003;14:927–38. doi: 10.1097/01.asn.0000059308.82322.4f. [DOI] [PubMed] [Google Scholar]
- 194.Heikkila E, Juhila J, Lassila M, Messing M, Perala N, et al. β-Catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant. 2010;25:2437–46. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- 195.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dreyer SD, Zhou G, Baldini A, Winterpacht A, Zabel B, et al. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47–50. doi: 10.1038/ng0598-47. [DOI] [PubMed] [Google Scholar]
- 197.Pelletier J, Bruening W, Kashtan CE, Mauer SM, Manivel JC, et al. Germline mutations in the Wilm’s tumor suppressor gene are associated with abnormal urogential development in Denys-Drash syndrome. Cell. 1991;67:437–47. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- 198.Pelletier J, Bruening W, Li FP, Haber DA, Glaser T, Housman DE. WT1 mutations contribute to abnormal genital system development and hereditary Wilms’ tumour. Nature. 1991;353:431–34. doi: 10.1038/353431a0. [DOI] [PubMed] [Google Scholar]