Abstract
Background
Src kinases are activated in melanoma, and inhibition of Src kinase activity has preclinical anti-tumor effects. Targeting this pathway could therefore have therapeutic activity in patients with metastatic melanoma.
Patients and methods
We conducted a multi-center, open-label study of the Src kinase inhibitor saracatanib (AZD0530) in patients with metastatic melanoma. Twenty-three patients received saracatanib at a dose of 175 mg daily. The primary objectives were to determine whether this agent had clinical activity in patients with advanced melanoma and whether it increased progression free survival. Functional effects on circulating T cells were also assessed.
Results
Twenty-three patients received oral saracatanib on a continuous daily dosing regimen. There were no objective clinical responses. Saracatanib was generally well tolerated with few grade 3–4 adverse events. T cell function was inhibited in most patients, based on decreased superantigen-induced IL-2 production in post- versus pre-treatment samples.
Conclusions
Saracatanib has minimal clinical activity as a single agent in an unselected population of patients with advanced melanoma, as evidenced by a lack of objective responses in this study. Reduced T cell cytokine production in most treated patients suggests potential immune suppressive activity by this agent.
Keywords: Src, melanoma, AZD0530, clinical trials, IL-2
Introduciton
Melanoma is the 5th most commonly diagnosed cancer in the United States with an estimated 68,130 new cases in 2010 (1). Response rates to standard chemotherapy with dacarbazine are limited and survival for patients with advanced disease remains low (2). Recently, novel targeted therapies and immune therapies have demonstrated increased efficacy in melanoma. The recent FDA approval of the immune potentiating drug ipilimumab (3) was the first new drug approval for advanced melanoma in decades, and the approval of the BRAF inhibitor vemurafenib followed in late 2011 (4). Despite these recent advances, long-term disease control remains rare, and research into the biology of melanoma continues. In addition to the pursuit of combination therapies with these new drugs, active investigation of novel targets may lead to the development of additional new agents with activity in melanoma.
Src family kinases are non-receptor cytoplasmic protein mediators of signal transduction which act as proto-oncogenes by mediating tumor cell proliferation, adhesion and angiogenesis as well as invasion and metastasis (5). Src inhibition has preclinical activity in melanoma cell lines (6); inhibition of Src kinases decreases levels of phosphorylated Stat3 and promotes melanoma cell apoptosis (7). The role of Stat3 itself as both an oncogene and as a mediator of immune evasion in cancer makes it highly attractive to target in this disease. Saracatanib (AZD0530) is an orally available small molecule Src kinase inhibitor that is highly selective for non-receptor tyrosine kinases and has anti-tumor effects in several pre-clinical tumor models. We conducted a clinical study to evaluate whether continuous daily dosing with saracatanib would have activity as a single agent in metastatic melanoma. In addition, because T cell activation is critically dependent on the Src-family kinases Lck and Fyn (8), it seemed plausible that continuous daily dosing with saracatanib might inhibit T cell function. Given the concern that agents which are immunosuppressive could worsen outcomes in metastatic melanoma, T cell function, as determined by IL-2 production, was assayed in treated patients ex vivo. Understanding the effects of saracatanib on T cell activation in patients participating in early phase trials is critical for considering future combination therapies in melanoma, a disease in which both targeted agents and immune therapies can be effective.
Patients and methods
Eligibility
Patients 18 years of age or older with histologically or cytologically confirmed stage IV or non-resectable stage III melanoma, measurable disease, no more than one prior treatment regimen and ability to understand and willingness to sign a written informed consent document were eligible. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2, estimated life expectancy greater than three months, and adequate bone marrow, kidney and liver function with a creatinine and total bilirubin within the institutional limit of normal, SGOT and SGPT less than two and a half times the upper limit of normal, platelet count ≥ 100,000/µL, absolute neutrophil count ≥ 1,500/µL, white blood cell count ≥ 3,000/µL and hemoglobin ≥ 9.0 g/dL. Patients were not eligible if they had received prior treatment with any kinase inhibitor with activity against Src kinases for metastatic melanoma, treatment with luteinizing hormone-releasing hormone (LHRH) agonists within 4 weeks of study entry, or treatment with specifically prohibited CYP3A4-active agents within 7 days of receiving saracatanib. Patients receiving any other investigational agents were not eligible, nor were patients with a history of allergic reactions attributed to compounds of similar chemical or biologic composition to saracatanib, patients with greater than +1 proteinuria, QTc prolongation or other significant ECG abnormalities, uncontrolled hypertension, inability to swallow saracatanib tablets, known brain metastases, inter-current cardiac dysfunction, uncontrolled inter-current illness, pregnant women, HIV-positive patients or patients with concurrent malignancy within the previous 5 years. The protocol was reviewed by the institutional review board of all participating centers and all patients provided written informed consent.
Study design and treatments
This was a multi-center, open-label, study of saracatanib in patients with advanced melanoma. All patients received saracatanib at a dose 175 mg per day orally continuously during each 28 day cycle. Patients were permitted to take saracatanib either with or without food. Patients remained on study until radiographic or clinical disease progression, unacceptable toxicity or withdrawal of consent. Full supportive care was provided as indicated. Dose reductions to 125 mg daily and 100 mg daily were permitted per the protocol. Patients were followed for 8 weeks after removal from study or until death, whichever occurred first.
Assessments
Evaluations before and during treatment consisted of a complete medical history, physical examinations, hematologic and metabolic laboratory profiles, ECOG performance status, urine protein and toxicity assessments according to the National Cancer Institute Common Toxicity Criteria version 3.0. An EKG was obtained at baseline only. Complete and partial responses and progressive disease were defined and assessed according to the Response Evaluation Criteria in Solid Tumors version 1 (9).
T cell function assay
T cell function was assayed in an ex-vivo study of patients treated with saracatanib by assessing IL-2 production in response to SEA both pre-treatment and during week 7 on drug. Briefly, cells were collected in 4 green-top tubes, cultured with the superantigen Staphylococcal enterotoxins A (SEA), or with Phorbol Myristate Acetate (PMA)/Ionomycin as a positive control, with or without the addition of a Src inhibitor in vitro. After an overnight culture, supernatants were analyzed for IL-2 content by ELISA using antibody pairs from Pharmingen. Changes in SEA-induced IL-2 production were assessed in post- versus pre-treatment samples. The coefficient of variance for this assay was less than10%. The assay was only performed on the patients in whom both pre- and on-treatment samples of PBMC were obtained that passed quality control.
Statistical methods
The primary endpoint of the study was to evaluate the objective response rate for AZD0530 in patients with advanced melanoma. The trial tested the null hypothesis that the true response rate was < 5% against the alternative hypothesis that it was > 15%. If two patients among the first 23 patients showed a clinical response, then the study would be opened to a second stage, including an additional 14 patients. A total of at least 4 responses out of 37 would warrant further investigation of AZD0530. This study design had a significance level of 0.10 and 78% power to detect a true response rate of greater than 15%. The secondary endpoint was median progression free survival (PFS). If the median PFS was at least 18 weeks, then this signal would also trigger opening to the second stage of accrual to include an additional 17 patients. If after 37 patients the observed median PFS continued to be at least 18 weeks, then the agent would also be considered sufficiently promising to warrant further study.
For evaluation of T cell function, a paired t-test was used to assess differences pre- and post- treatment.
Results
Patient characteristics
Twenty-three patients were enrolled at five sites in the United States between August, 2008 and September, 2009 and were included in the intent to treat analysis. All patients were Caucasian. 15 patients (65%) were male. Eighteen patients (78%) had received a prior therapy for advanced melanoma. The baseline demographics and disease characteristics of the patients are presented in Table 1.
Table 1.
Baseline Patient Characteristics
| Number | Percent (%) | |
|---|---|---|
| Median Age | 63 | |
| Range | 25–80 | |
| ECOG Performance Status | ||
| 0 | 13 | 57 |
| 1 | 10 | 43 |
| Sex | ||
| Male | 15 | 65 |
| Female | 8 | 35 |
| Mean Baseline LDH (U/L) | 238 | |
| Range | 123–573 | |
| Prior Therapy for Advanced Melanoma | 18 | 78 |
| Immunotherapy | 9* | 39 |
| Chemotherapy | 9** | 39 |
| Prior Adjuvant Interferon alpha | 6 | 26 |
Includes 1 patient treated with combination immune- and chemotherapy
Includes 2 patients treated on a trial of dacarbazine with either ipilimumab or placebo
Toxicity
Common grade 1/2 toxicities included fatigue, hyperglycemia, anemia, diarrhea, nausea, anorexia, increased AST/ALT, lymphopenia, and vomiting. Saracatanib was generally well tolerated and did not result in unexpected toxicities. One patient with grade 1 anemia at baseline developed grade 3 anemia during cycle 2. One patient with lymphopenia at baseline developed grade 3 lymphopenia during cycle 2. One patient developed grade 3 hypophosphatemia during cycle 1. A patient with Grade 1 anorexia at baseline developed grade 3 anorexia, fatigue, lipase increase and nausea during cycle 1 and required a dose reduction. There were no other dose reductions orother treatment related grade 3–5 adverse events. 2 additional patients required dose interruptions for toxicity without subsequent dose reduction. The remainder of the patients received full dose saracatinib daily on >75% of treatment days during each cycle until removal from the study. Table 2 summarizes the clinically relevant observed adverse events.
Table 2.
Observed Grade 1–2 Toxicities
| Grade 1–2 Toxicity | Number | Percent (%) |
|---|---|---|
| Fatigue | 18 | 78 |
| Hyperglycemia | 17 | 74 |
| Anemia | 14 | 61 |
| Diarrhea | 13 | 57 |
| Anorexia | 10 | 43 |
| Hypocalcemia | 10 | 43 |
| Nausea | 10 | 43 |
| Hyponatremia | 9 | 39 |
| Increased AST/ALT | 7 | 30 |
| Lymphopenia | 6 | 26 |
| Thrombocytopenia | 5 | 22 |
| Vomiting | 5 | 22 |
Efficacy
Twenty-three patients received treatment during a total of 50 cycles of saracatanib. There were no objective clinical responses; only 2 patients had stable disease and received an additional 2 cycles of therapy for a total of four cycles before developing progressive disease. The trial was therefore terminated after the first stage of enrollment.
Effects on T cell function
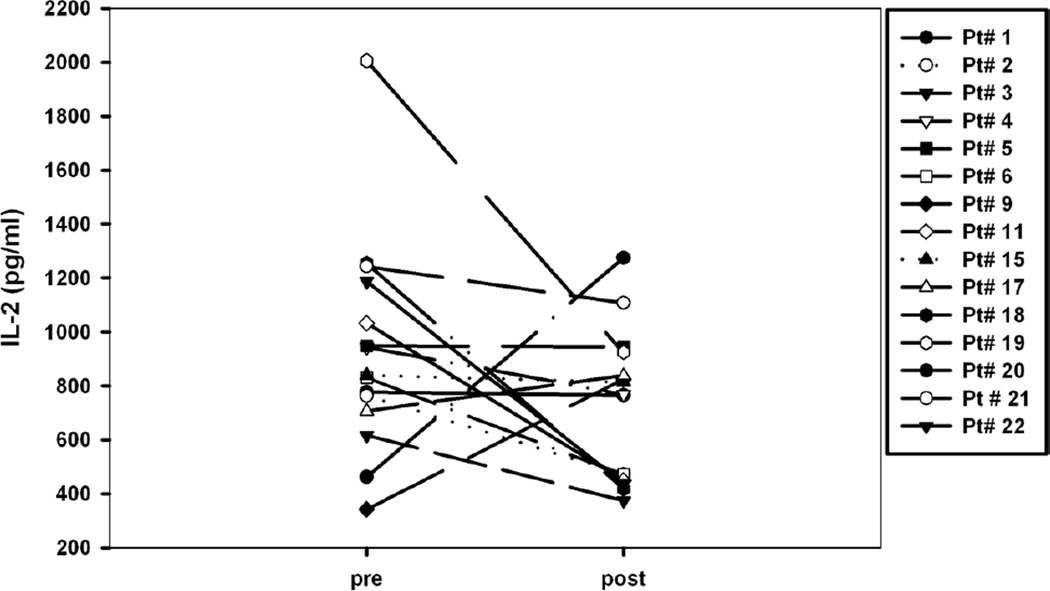
An ex-vivo assay of T cell function demonstrated inhibition of IL-2 production in a subset of patients after starting treatment with AZD0530. Overall, there was a trend towards decreased IL-2 production for the 15 subjects for which paired pre- and post-treatment viable PBMC samples were available for the assay (Figure 1; p=0.07
Figure 1.
Effects of AZD0530 on T cell function measured ex vivo. PBMC were cryopreserved and tested in batch fashion. Briefly, cells were stimulated with the polyclonal T cell activator SEA and IL-2 production was measured in supernatants by ELISA. Each symbol represents an individual patient. P=0.07 as measured using a paired student’s t-test.
Discussion
Saracatanib was generally well tolerated in this study and could be administered at a dose of 175 mg p.o. daily to patients with advanced melanoma. Adverse events observed in this study were similar to those observed in a reported phase I trial of saracatanib (10) and primarily included fatigue, hyperglycemia, anemia, lymphopenia, gastrointestinal symptoms, and increased aminotransferases. The results of this study indicate that saracatanib has minimal clinical activity as a single agent in patients with metastatic melanoma.
A limitation of the study is that pre-treatment and on-treatment biopsy samples were not collected for assessment of target inhibition. Inhibition of Src kinases in vitro has been shown to decrease levels of phosphorylated Stat3 and promote melanoma cell apoptosis with downregulated expression of the antiapoptotic proteins Bcl-x(L) and Mcl-1 (7) as well as decreased phosphorylated FAK and paxillin, (11) which are critical mediators of cell migration and invasion. Inhibition of tumor Src activity has been demonstrated in a phase I trial of saracatanib as evidenced by reduced p-FAK and p-PAX in serial biopsy samples (10). Serial biopsy samples to assay for these Src inhibition-related changes in tumor tissue would have added information to the current study regarding whether the lack of clinical efficacy was related to insufficient target activity of saracatanib in this study population.
Given the involvement of Src in pathways of cell invasion, migration and metastasis, it was of interest to ascertain whether saracatanib may have had any clinical effect on decreasing the incidence of new metastatic lesions. Although no responses were seen in this study, most of the patients came off study due to progression of target lesions, rather than the development of new sites of metastatic disease. While the study was too small to draw any conclusions about the role of Src inhibition in preventing new metastatic disease, this may be a hypothesis worth pursuing in the future.
Interestingly, administration of saracatanib resulted in diminished T cell cytokine production in a subset of patients as assessed in an ex-vivo assay of IL-2, suggesting a potential immune suppressive effect of Src inhibition. While the clinical significance of this finding is unknown, it is theoretically possible that this immune modulation decreased or negated clinical evidence of anti-tumor activity. In addition, we do not know whether some patients may have had pre-existing immune responses contributing to tumor control at baseline. The potential for Src inhibitors to act as immune suppressive drugs should be considered in future studies.
In summary, the study did not meet the pre-specified clinical response criteria to warrant further investigation of saracatanib as a single agent in patients with advanced melanoma. The decrease in T cell function observed with Src inhibition highlights the importance of evaluating potential immune interaction effects in the development of targeted signal transduction inhibitors for melanoma.
Acknowledgments
The investigators thank Karen Matijevich for research nursing support, Jeffrey Bozeman for data management, Yuanyuan Zha for help with correlative assays, Theodore Karrison for statistical assistance, and all the members of the University of Chicago Phase II clinical trial consortium. This work was supported by N01-CM-62201from the National Cancer Institute.
Grant support
This study was supported by the National Cancer Institute Early Therapeutics Development with Phase II emphasis [grant number N01-CM-62201]
Footnotes
The authors do not have any relationships that they believe could be construed as resulting in an actual, potential, or perceived conflict of interest with regard to the manuscript submitted for review.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. JCO. 1999;17(9):2745–2751. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. NEJM. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. 30. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. NEJM. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. 30; Epub 2011 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. NatRev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 6.Buettner R, Mesa T, Vultur A. Inhibition of Src Family Kinases with Dasatinib Blocks Migration and Invasion of Human Melanoma Cells. Mol Cancer Res. 2008;6:1766–1774. doi: 10.1158/1541-7786.MCR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 8.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23(48):7990–8000. doi: 10.1038/sj.onc.1208074. 18. [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Baselga J, Cervantes A, Martinelli E, et al. Phase I Safety, Pharmacokinetics, and Inhibition of Src Activity Study of Saracatinib in Patients with Solid Tumors. Clin Cancer Res. 2010;16:4876. doi: 10.1158/1078-0432.CCR-10-0748. [DOI] [PubMed] [Google Scholar]
- 11.Rajeshkumar NV, Tan AC, De Oliveira E, et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. 2009;15(12):4138–4146. doi: 10.1158/1078-0432.CCR-08-3021. 15. [DOI] [PubMed] [Google Scholar]