Abstract
Atopic dermatitis (AD) is characterized by epidermal tight junction (TJ) defects and a propensity for Staphylococcus aureus (S. aureus) skin infections. S. aureus is sensed by many pattern recognition receptors including toll-like receptor (TLR) 2. We hypothesized that an effective innate immune response will include skin barrier repair and that this response is impaired in AD subjects. S. aureus-derived peptidoglycan (PGN) and synthetic TLR2 agonists enhanced TJ barrier and increased expression of TJ proteins, CLDN1, CLDN23, occludin and ZO-1 in primary human keratinocytes. A TLR2 agonist enhanced skin barrier recovery in human epidermis wounded by tape-stripping. Tlr2−/− mice had a delayed and incomplete barrier recovery following tape-stripping. AD subjects had reduced epidermal TLR2 expression as compared to nonatopic (NA) subjects, which inversely correlated (r= 0.654, P= 0.0004) with transepidermal water loss (TEWL). These observations indicate that TLR2 activation enhances skin barrier in murine and human skin and is an important part of a wound repair response. Reduced epidermal TLR2 expression observed in AD patients may play a role in their incompetent skin barrier.
INTRODUCTION
Recent findings have solidified the notion that skin barrier dysfunction plays a key role in the initiation of atopic dermatitis (AD). Dysfunction of the skin barrier in AD can occur through both genetic and acquired mechanisms. For example, AD subjects may have a loss-of-function mutation in filaggrin (Cork et al., 2006; Howell et al., 2009; Palmer et al., 2006), epidermal lipid abnormalities (Imokawa, 2001; Murata et al., 1996; Pilgram et al., 2001), altered protease activity (Cork et al., 2006; Vasilopoulos et al., 2007), more alkaline surface pH (Elias et al., 2008) as well as a defect in tight junction (TJ) function (De Benedetto et al., 2011), all of which can contribute to decreased skin barrier function.
The human epidermis is a multilayered structure that is made up of four progressively differentiated layers. Transient breaches in skin barrier such as might occur with topical exposure to organic solvents or detergents, scratching, endogenous or exogenous proteases (e.g. from allergens or microbes) can temporally disrupt our skin barrier. In healthy subjects, the barrier repair mechanisms normally prevent or minimize the potential for pathogens or allergens to penetrate the host and evoke an immune response. An inadequate barrier repair response would lead to enhanced and more prolonged allergen sensitization, microbial colonization and/or infection in addition to greater transepidermal water loss (TEWL) that leads to skin xerosis (Boguniewicz and Leung, 2011; De Benedetto et al., 2012; Grigoryev et al., 2010; Kubo et al., 2012). All of these are well-recognized features of AD.
The function of innate receptors, conventionally recognized for their antimicrobial effects has recently been extended to include epithelial barrier regulation (Cario et al., 2004, 2007; Rezaee et al., 2011; Yuki et al., 2011b). Toll-like receptor (TLR) 2 has received the greatest attention as it is important for immune responses to a number of microbes such as Staphylococcus aureus (S. aureus) and herpes simplex virus (HSV) (Bochud et al., 2007; De Benedetto et al., 2009; Sato et al., 2006; Takeuchi et al., 2000; Takeuchi et al., 1999; Zahringer et al., 2008) that more commonly colonize and/or infect the skin of AD patients (Boguniewicz and Leung, 2010; Ong and Leung, 2010). Several groups have shown that AD patients have reduced expression and/or function of TLR2 on their monocytes and macrophages (Hasannejad et al., 2007; Niebuhr et al., 2009). However, the role of TLR2 in modulating epidermal barrier in vitro and in vivo remains poorly understood. In this study, we observed a reduction of TLR2 expression in AD skin epithelium. In addition, we observed that TLR2 signaling enhances the TJ integrity in cultured keratinocytes, which is in part mediated by enhanced expression of key TJ proteins. This TLR2 barrier repair effect was also confirmed in the TLR2 deficient mouse and in a human wound model. We conclude that TLR2 is important for the maintenance of TJ integrity in response to barrier insults, and that this barrier repair mechanism may be defective in AD subjects. The consequence of this defect might explain why these patients are susceptible to microbial colonization and infections of the skin, which are thought to perpetuate their chronic skin inflammation.
RESULTS
TLR2 agonists enhance TJ function and increase expression of TJ proteins
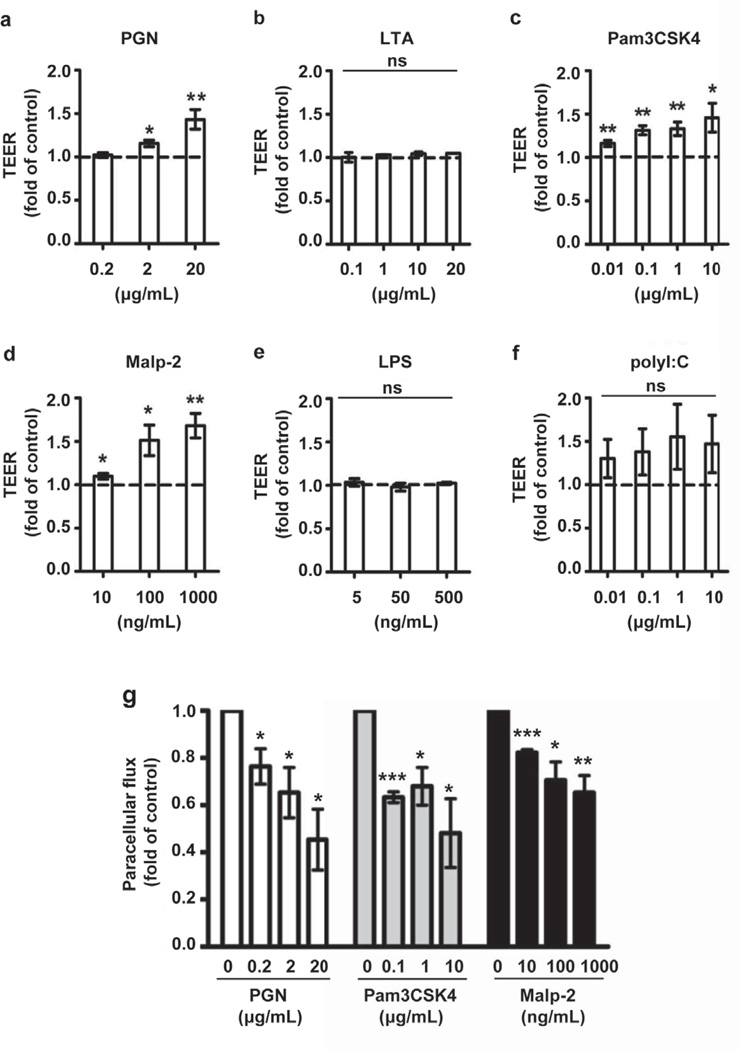
Several studies have suggested that the antimicrobial barrier and stratum corneum (SC) permeability barrier are coregulated (Aberg et al., 2008; Ahrens et al., 2011; Borkowski and Gallo, 2011; Grether-Beck et al., 2012). We hypothesized that the crosstalk between innate immunity and barrier integrity may extend to TJs, the other epidermal barrier structure important in regulating paracellular permeability of ions and macromolecules. Specifically, we wondered whether epidermal TJ permeability could be regulated by relevant cutaneous pathogens such as S. aureus. To test this hypothesis, PHK were stimulated with S. aureus-derived peptidoglycan (PGN) and lipoteichoic acid (LTA) (Figure 1a, b). PGN significantly increased the transepithelial electrical resistance (TEER) of PHK monolayers in a dose-dependent fashion. By contrast LTA had no effect on TJ barrier function despite evidence that it could enhance expression of TLR2, cluster of differentiation 14 (CD14), peptidoglycan recognition protein 3 (PGLYRP-3) and human β-defensin 2 (HBD-2) in human keratinocytes (data not shown). Using synthetic ligands targeting specific TLR2 heterodimers, Pam3CSK4 (TLR1/2 agonist) and Malp-2 (TLR2/6 agonist) we found that both increased keratinocyte TEER in dose-dependent manner (Pam3CSK4: P <0.05; Malp-2: P <0.05 - Figure 1c, d). In contrast LPS, a TLR4 agonist had no effect on TJ barrier integrity even at high concentrations (Figure 1e), which may reflect the low TLR4 expression on human PHK (data not shown) (Baker et al., 2003; Kollisch et al., 2005; Mempel et al., 2003). Interestingly, polyI:C a synthetic TLR3 agonist appeared to consistently enhance epidermal TJ barrier in a wide range of concentrations (0.01–10 µg/ml) but with substantial variability among KC preparations (Figure 1f). To examine whether TLR2 agonists affect epithelial permeability for larger molecules, we measured paracellular flux of fluorescein (Figure 1g). Incubation of PHK monolayers with natural (PGN) and synthetic TLR2 agonists (Pam3CSK4 and Malp-2) for 24 h significantly decreased their permeability to fluorescein. Collectively, the TEER and paracellular flux data demonstrate that TLR2 signaling enhances TJ barrier function, which nicely complements its other innate defense actions.
Figure 1. TLR2 agonists enhance TJ function in PHK.
TEER was measured daily until day 8 in PHK stimulated with (a) S. aureus- derived peptidoglycan (PGN, n= 3–7), (b) S. aureus-derived lipoteichoic acid (LTA, n= 3), (c) Pam3CSK4, a TLR1/2 ligand (n= 3–6), (d) Malp-2, a TLR2/6 ligand (n= 5), (e) Lipopolysaccharide (LPS), a TLR4 ligand (n=3) and (f) poly I:C, a TLR3 ligand (n= 3–9). Data are presented as the mean ± SEM by analyzing the area under the TEER curve normalized to the mean values for the control group (media alone). (g) PHK grown on Transwell™ inserts were stimulated with the indicated concentrations of S. aureus-derived PGN, Pam3CSK4 and Malp-2 for 48 h and paracellular permeability measured and compared to media alone. Data are expressed as a fold of media alone group. n=3; *P <0.05; **P <0.01; ***P < .001; ns: not significant.
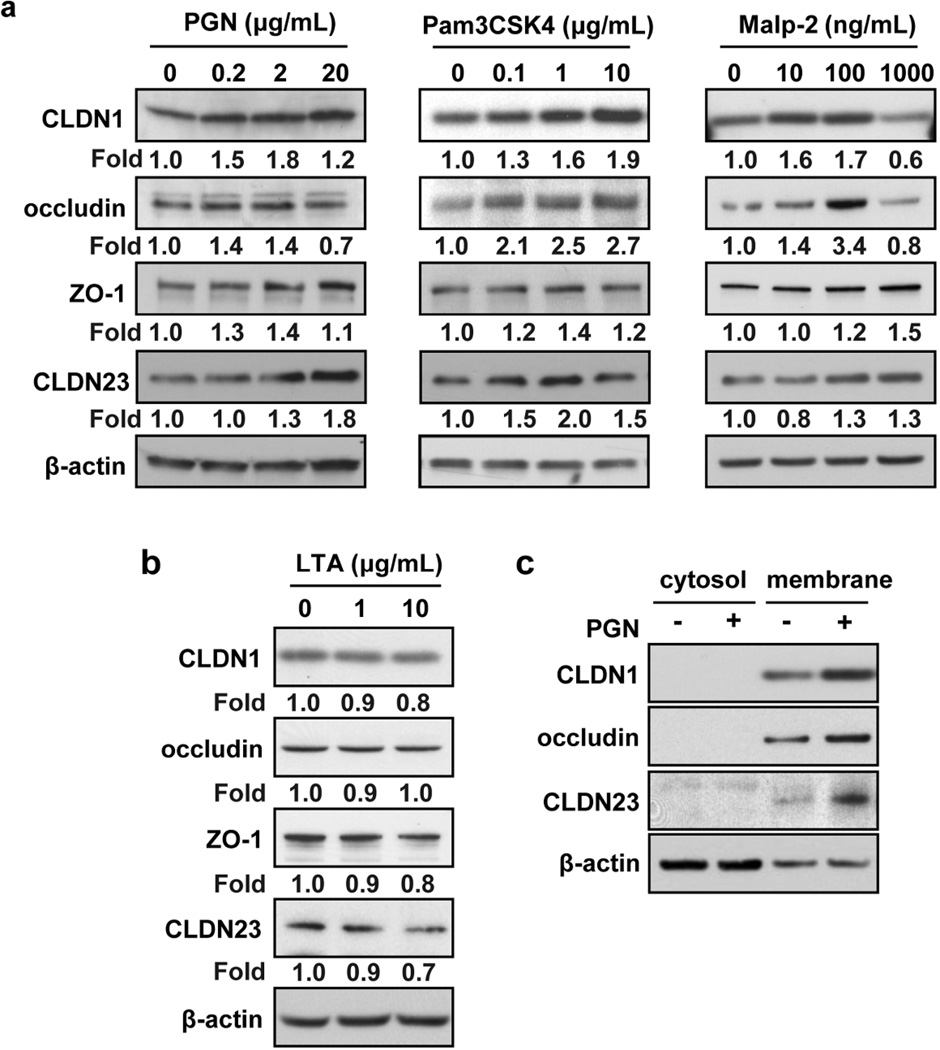
To examine if barrier-enhancing effects of TLR2 agonists are associated with altered expression of TJ proteins, we quantified expression of key transmembrane and cytosolic TJ components in cultured keratinocytes (Amagai et al., 2000; Baltes et al., 2004; Djalilian et al., 2006; Furuse et al., 2001; Furuse et al., 2002; Gareus et al., 2007; Kretz et al., 2004; Lucke et al., 1999; Ma et al., 2004; Ohnemus et al., 2008; Tunggal et al., 2005; Yuki et al., 2011a). We found that mRNA expression of CLDN1, CLDN23, occludin and ZO-1 was significantly (P <0.01) induced after 24 h stimulation of confluent PHK with PGN while expression of adherens junction, gap junction, and desmosomal proteins was not significantly affected (Figure S1). We confirmed these qPCR findings at the protein level by Western blot (Figure 2a). TLR2 synthetic agonists (Pam3CSK4 and Malp-2) also enhance the protein expression of CLDN1, occludin, ZO-1 and CLDN23. Of note, LTA, which had no effect on TJ function, also had no effect on the protein expression of key TJ components (Figure 2b). Quantified western blot results are shown in Figure S2.
Figure 2. TLR2 agonists induce TJ protein expression and subcellular localization.
PHK were treated with (a) S. aureus-derived PGN (0.2, 2, 20 µg/ml), Pam3CSK4 (0.1, 1, 10 µg/ml), Malp-2 (10, 100, 1000 ng/mL) and (b) LTA (1, 10 µg/ml) for 48 h and CLDN1, occludin, ZO-1 and CLDN23 protein levels were detected from whole cell lysates by Western blot. Quantitative protein expression was determined by densitometry of bands after normalization to the housekeeping protein (β-actin). (c) Membrane and cytosolic protein lysates were made and TJ proteins were detected by Western blot after treating PHK with PGN (20 µg/ml) for 48 h. Representative blot of n=3 experiments.
Integrity of epithelial barrier depends not only on the total level of TJ proteins but also on their ability to form complexes at the plasma membrane. Therefore, we performed cell fractionation to evaluate the effect of TLR2 signaling on localization of integral membrane TJ proteins (Marchiando et al., 2010; Utech et al., 2005). We found that, CLDN1, occludin and CLDN23 accumulated exclusively in the membrane fraction and their level at the membrane was enhanced by PGN stimulation (Figure 2c). The specificity of our cellular fractionation was confirmed using appropriate markers (Figure S3).
Blocking TLR2 prevents PGN-mediated effect on TJ
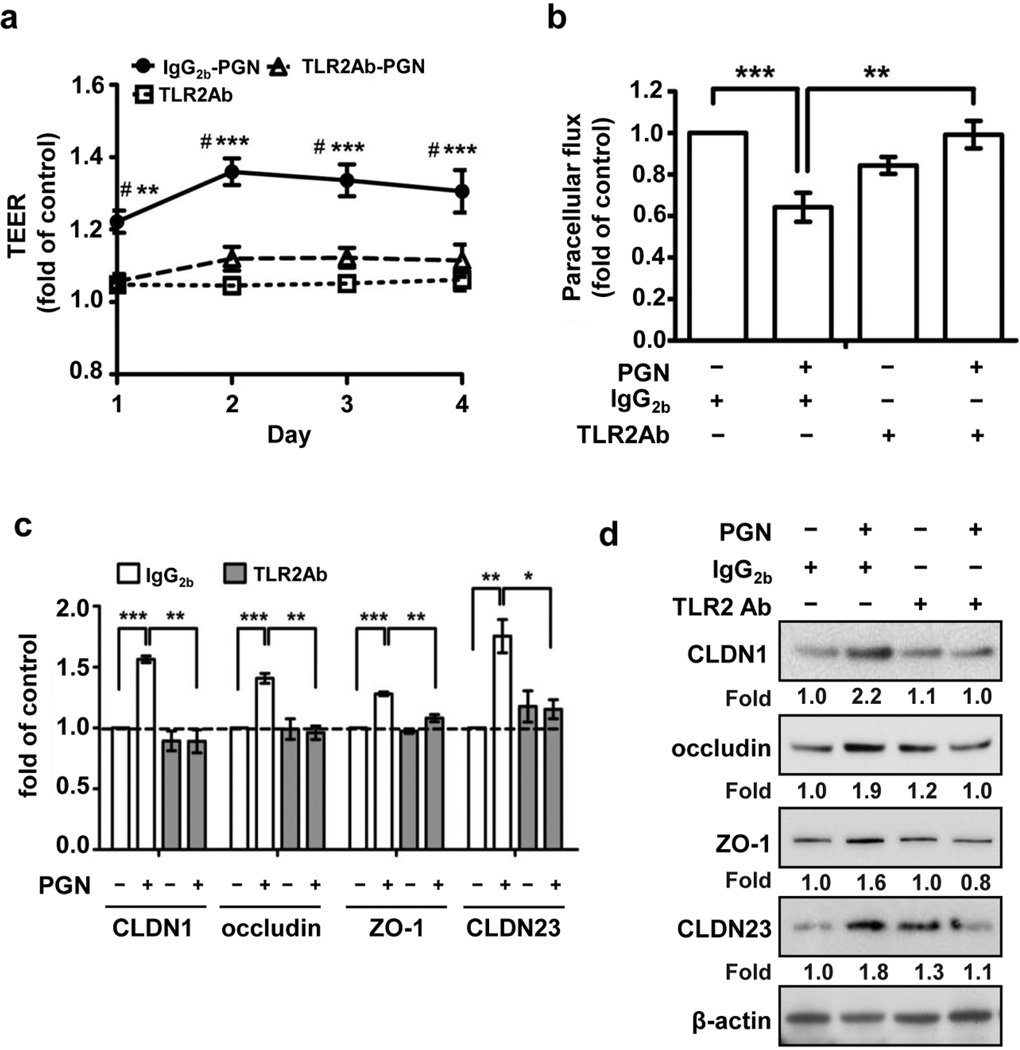
S. aureus-derived PGN can be sensed by several innate receptors including TLR2, NOD2 and PGLYRPs. To confirm that the enhanced barrier function and increased expression of TJ protein in PHK monolayers stimulated with PGN was TLR2-dependent, we performed blocking experiments using a specific TLR2-neutralizing antibody. As compared with isotype-matched control, the TLR2-neutralizing antibody completely inhibited the PGN-induced increase in TEER (P<0.01) (Figure 3a) and decrease in paracellular flux (P<0.01) (Figure 3b). Additionally, blocking TLR2 signaling abrogated the PGN-dependent upregulation of TJ components CLDN1, occludin, ZO-1 and CLDN23 both at the mRNA, (Figure 3c) and protein (Figure 3d, Figure S2b) level.
Figure 3. TLR2 neutralizing antibody inhibited S. aureus-derived PGN effect on TJ protein function and expression.
TEER (n=5) (a) and paracellular permeability (n=5) at 48 h (day 2) (b) measurement in PHK treated with PGN (20 µg/ml) and/or TLR2-neutralizing antibody (10 µg/ml). Data are normalized to the control group (IgG2b treatment). (c) TJ mRNA (n= 3) and (d) protein expression (representative blot of n=3 experiments) observed in response to treatment with PGN (20 µg/ml, 24 or 48 h, respectively) with or without TLR2-neutralizing antibody (5 µg/ml). Expression was quantified by densitometry of bands and normalization to the housekeeping protein (β-actin). *P <0.05; **P <0.01; ***P <0.001; #: compared IgG2b+PGN vs TLR2Ab+PGN.
A TLR2 agonist enhances human skin barrier repair
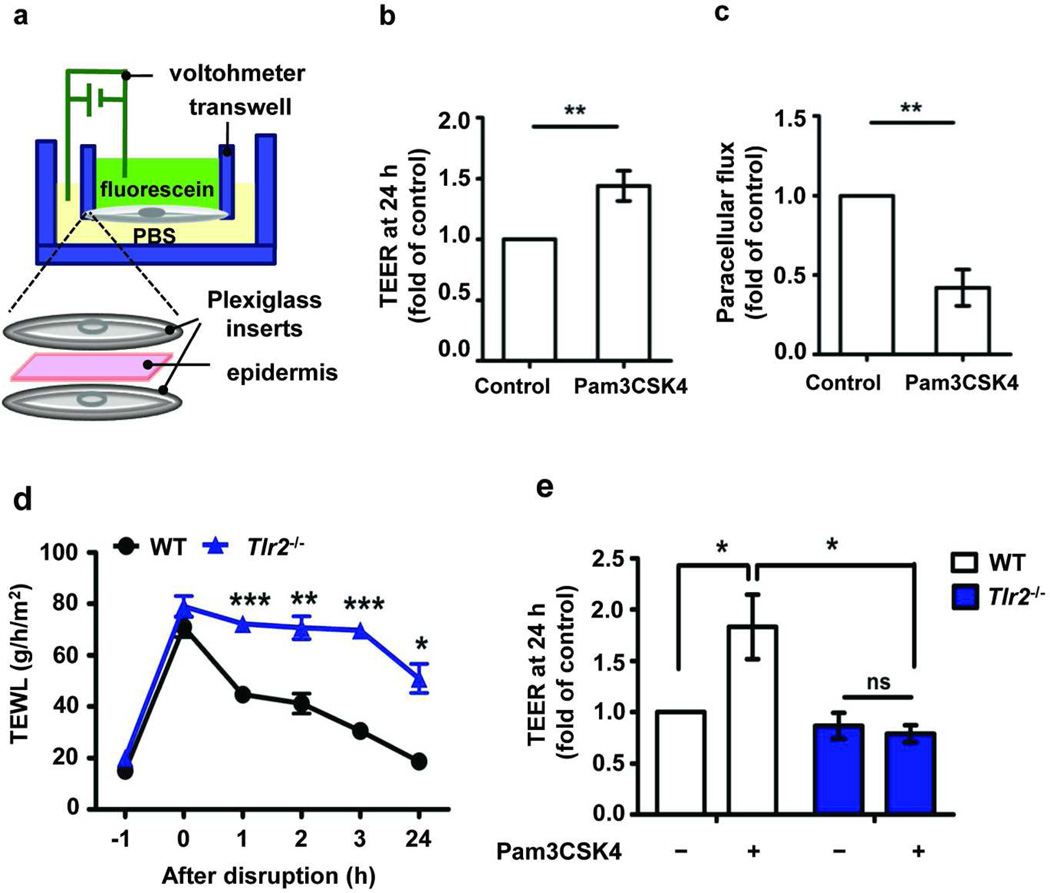
A classic hallmark of AD is a chronic itch/scratch cycle that leads to persistent barrier disruption. To mimic this mechanically induced barrier disruption (Koschwanez and Broadbent) (Taljebini et al., 1996), we performed tape-stripping in human discarded skins. TJ barrier recovery was monitored by measuring TEER and the paracellular fluorescein flux using the modified micro-Snapwell™ system with and without Pam3CSK4 treatment (see Methods and Figure 4a). Interestingly, treatment with the TLR1/2 agonist significantly enhanced TEER recovery (1.4 fold, P<0.004) (Figure 4b) and resulted in approximately 50% decrease in the paracellular flux (P=0.007) (Figure 4c) at 24 h after wounding.
Figure 4. TLR2 agonist enhances skin barrier repair.
(a) Schema of modified Snapwell™ system used to measure TEER and paracellular flux in full thickness human or mouse epidermis. Epidermal sheets from normal subjects were placed in modified Snapwell following tape-stripping and treated with Pam3CSK4 (10 µg/mL) for 24 h. TEER (n=7/group) (b) and paracellular flux (n=3/group) (c) were measured at 24 h. (d) TEWL recovery curve after tape-stripping mouse skin. (WT, n= 4; Tlr2−/−, n= 4; Time -1 h= baseline TEWL before tape-stripping). (e) TLR2 agonist effect on TEER restoration at 24 h measured by modified Snapwell™ after murine barrier disruption by tape-stripping (WT, n= 5; Tlr2−/−, n= 5). TEER absolute value at 24 h in control group is 227±27 ohms × cm2 (human epidermis) and 241± 44 ohms × cm2 (WT mice epidermis). ns: not significant; *P <0.05; **P <0.01; *** P <0.001.
TLR2 knockout (Tlr2−/−) mice have a delayed and incomplete recovery of skin barrier function
To further clarify the importance of TLR2 in epidermal barrier repair in vivo, we utilized a tape-stripping wound model in WT and Tlr2−/− mice, and monitored barrier recovery by measuring transepidermal water loss (TEWL). In WT mice the skin barrier fully recovered 24 h after tape-stripping, whereas Tlr2−/− mice had a substantially slower recovery rate and did not reach baseline values even 24 h after wounding (Figure 4d). Two hours after skin barrier disruption, mRNA expression of cldn1 and zo-1 were slightly but significantly increased in WT but not in Tlr2−/− mice, while cldn2, a pore-forming TJ protein was increased in Tlr2−/− mice (Figure S4). Because TEWL measurement is an in vivo barrier assay that may reflect the integrity of both epidermal barrier structures (SC and TJ) as well as dermal blood flow, we used our developed micro-Snapwell™ system to clarify whether the changes we observed in TEWL were at least in part reflected in functional changes in TJ ex vivo. To determine whether TLR2 agonists affect TJ integrity in this wound model, we measured TEER using the modified micro-Snapwell™ system in murine epidermis with and without Pam3CSK4 treatment. Pam3CSK4 significantly enhanced TEER over media alone 24 h after wounding in the WT mice (P=0.029). This effect was not observed in wounded epidermis from Tlr2−/− mice (Figure 4e). Collectively, these results indicate that TLR2 agonist enhances TJ barrier recovery in human and murine epidermal wound models.
AD subjects have reduced epidermal expression of TLR1 and 2
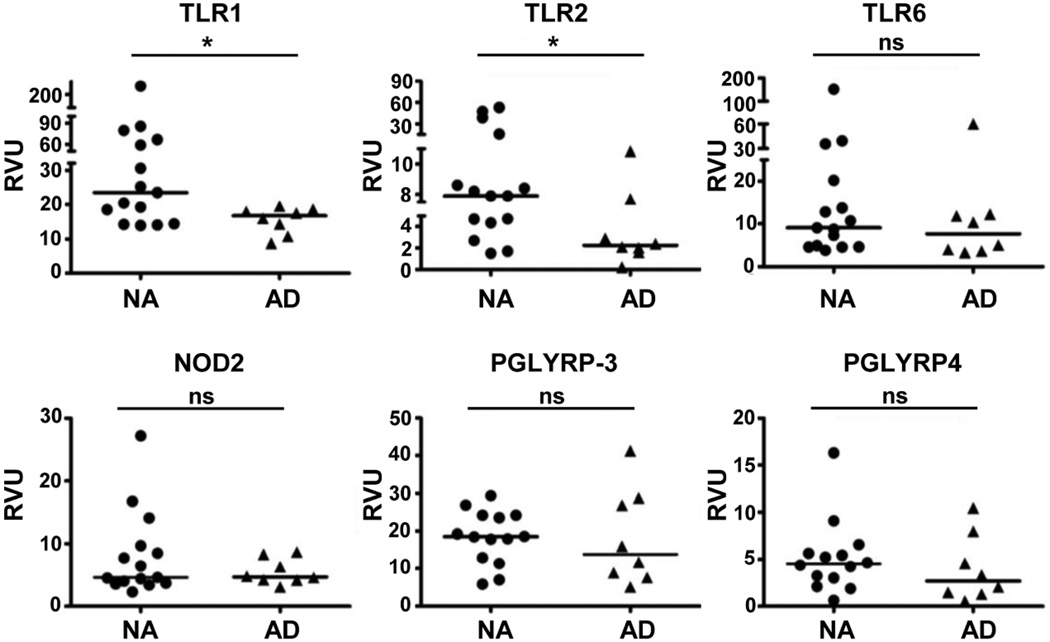
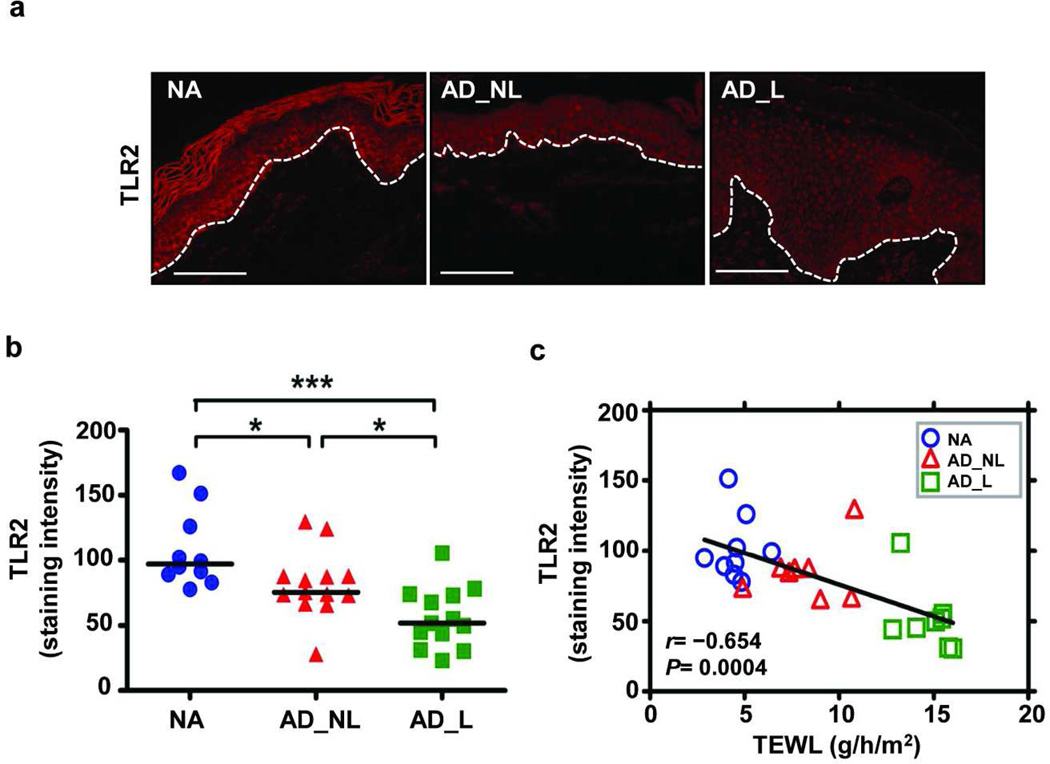
Keratinocytes respond to S. aureus using multiple innate receptors that reside on the cell membrane (TLR1, 2, 6), are intracellular (NOD2) or are secreted (PGLYRP-3, PGLYRP-4). We hypothesized that expression of one or more of these key receptors may be reduced in AD, which could explain altered barrier repair responses and AD subjects’ susceptibility to S. aureus colonization. To test this hypothesis, epidermal samples were taken from non-sunexposed, volar forearms to control for anatomical differences and photo-induced changes from well-characterized subjects with AD and nonatopic (NA) controls. We initially quantified mRNA expression for different innate receptors in epidermal samples using qPCR. Both epidermal TLR1, which heterodimerizes with TLR2, and TLR2 mRNA were significantly decreased (P= 0.03 and 0.04, respectively) in AD subjects. Expression levels of TLR6, NOD2, PGLYRP-3 and PGLYRP-4 were similar in the two groups (Figure 5). The reduced TLR2 expression was confirmed at the protein level by immunofluorescence staining (Figure 6a). Epidermal TLR2 staining was significantly reduced in all AD samples (non-lesional and lesional; median intensity: 76 and 52, P= 0.01, P= 0.0005, respectively) as compared to NA (median intensity: 96; Figure 6b). Interestingly, PHKs isolated from AD epidermis have a reduced inflammatory response (TNF-α, IL-6 and HBD-2; P≤ 0.032) to PGN (Figure S7).
Figure 5. Reduced mRNA expression of TLR1 and 2 in epidermal sheets from AD subjects.
Nonlesional AD epidermis (n= 8) was compared with epidermis from nonatopic healthy control (NA; n= 14–15) obtained from the same anatomical location. The relative mRNA expression level was normalized to GAPDH (Mann-Whitney t-test). RVU= relative value unit. *P <0.05; ns: not significant.
Figure 6. The reduced TLR2 expression observed in AD epithelium inversely correlates with measures of barrier integrity.
(a) Representative paraffin-embedded skin biopsy samples from a nonatopic healthy control (NA; n= 10), non-lesional AD (AD_NL; n= 13), and lesional AD (AD_L; n= 13) subjects stained for TLR2 are shown. The white dotted lines denote the epidermal-dermal junction. Bar= 100 µm. Epidermal TLR2 staining intensity is shown in (b) (Mann-Whitney t-test). (c) The line representing the linear least square fit for TLR2 epidermal staining intensity versus TEWL (Spearman nonparametric coorelation, [n= 25]; r= 0.654, P= 0.0004).
TLR2 protein expression inversely correlated with TEWL
To address whether TLR2 epidermal expression might be a determinant of skin barrier function, we evaluated the relationship between TLR2 staining intensity and TEWL, a functional measurement of skin barrier integrity (Figure 6C). Epidermal TLR2 immunoreactivity demonstrated a significant inverse correlation with TEWL (r= 0.676, P= 0.0002). We also observed that mRNA expression for both TLR1 and TLR2 correlated inversely with TEWL (Figure S5). In summary, these observations strongly suggest that the decreased expression of key innate receptors TLR1 and TLR2 may adversely affect integrity of the epidermal barrier.
DISCUSSION
Epidermal TJs form the barrier preventing paracellular movement between different cell layers of the stratum granulosum. TJ are very dynamic structures, loosening and tightening in response to endogenous signals that originate from epithelial and subepithelial compartments, as well as exogenous agents such as bacterial-derived products and proteolytic allergens. TJ are composed of transmembrane proteins such as occludin and the claudin family members that directly mediate cell-cell adhesion and contribute to the development of the paracellular barrier. These transmembrane constituents are clustered and stabilized by cytosolic plaque proteins, such as ZO-1. Changes in expression of any one of these TJ components can have significant effects on TJ integrity (Niessen, 2007). We have observed reduced expression of CLDN1 and CLDN23 in nonlesional skin from AD subjects where TJ function is significantly impaired (De Benedetto et al., 2011). Silencing of CLDN1 in human keratinocytes as well as mice genetically deficient in CLDN1 confirm the importance of this protein for a competent TJ and skin barrier (De Benedetto et al., 2011; Furuse et al., 2002). Results reported herein arose from experiments designed to identify pathways that would enhance TJ function in hopes that these might be used to repair AD barrier defects. We found that TJ function in keratinocytes was enhanced by activation of the S. aureus-responsive innate immune receptor, TLR2 (Figure 1). This TLR2 activation was accompanied by increased expression of several TJ components including those that were reduced in AD epidermis (CLDN1 and CLDN23; Figures 2 and 3). Similarly, exfoliative toxin-negative S. aureus enhanced expression of TJ molecules (occludin and ZO-1) in human keratinocytes, but had no effect on the adherens junction proteins (Ohnemus et al., 2008), suggesting that TLR2-mediated effects on TJ integrity in the skin are not mediated by changes in the expression of other intercellular junctional proteins. In keeping with these observations, we found that the Tlr2−/− mouse had a delayed and incomplete repair response to epidermal injury (Figure 4) suggesting that this innate immune receptor mediates an important barrier repair pathway that is likely operative in AD subjects who suffer from a chronic itch-scratch cycle. This observation corroborates a previous finding that dysregulation of the wound repair response in skin might contribute to the severe phenotype in patients with AD (Grigoryev et al., 2010). Furthermore, we hypothesize that this repair pathway may be defective in AD subjects who express less epidermal TLR2 than controls (Figures 5 and 6). Collectively, these data establish a previously under-appreciated role for innate receptors expressed on epidermal cells in skin barrier maintenance and recovery.
Earlier studies demonstrated that Tlr2−/− mice also develop more severe intestinal disease in a dextran sulfate sodium (DSS)-mediated ulcerative colitis model, which was due to an impaired intestinal TJ barrier repair response (Cario et al., 2007). Importantly Tlr4−/− mice did not have the same phenotype suggesting that this was not a global TLR effect in intestinal epithelial cells (Cario et al., 2007). In line with this finding, our results indicate that TLR4 agonist, LPS had no effect on TEER in human keratinocytes (Figure 1e), which is concordant with our unpublished data and that of other groups demonstrating little to no TLR4 expression in human keratinocytes (Baker et al., 2003; Kollisch et al., 2005). On the other hand, TLR3 ligands appeared to have an enhancing effect on TJ function but with tremendous variability from donor to donor (Figure 1f). In contrast, we have shown that TLR3 induces TJ disassembly in bronchial epithelium (Rezaee et al., 2011), suggesting that the functions of these receptors may depend more on the epithelial cell phenotype than the receptor itself. Interestingly, S. aureus-derived LTA, which is considered activation through TLR2, had no effect on TJ function (Figure 1b and 2b), but did induce inflammatory gene expression in human keratinocytes (Menzies and Kenoyer, 2006; Olaru and Jensen, 2010). This suggests that the TLR2 signaling pathways may be different for inflammatory mediator release versus TJ barrier repair. It is also unclear whether LTA is a specific ligand for TLR2 (Schroder et al., 2003; Takeuchi et al., 1999). It has been shown that macrophages isolated from Tlr2−/− mice stimulated with LTA have similar induction of IL-6 and TNF-α as compared to WT mice. Whereas macrophages from Tlr4−/− mice stimulated with LTA have severely impaired cytokine production (Takeuchi et al., 1999). This suggests that LTA might act through TLR4. In our studies we found that TLR4 had no effect on TJ function (Figure 1e), and collectively this may explain why we observed no effect with LTA stimulation.
To evaluate the biological relevance of TLR2 in skin barrier repair we evaluated Tlr2−/− mice using an established mechanical epidermal wound model (i.e. repetitive tape-tripping). Tape-stripping liberates several danger associated molecular patterns such as heat shock proteins and hyaluronic acid some of which are recognized as TLR2 ligands (Dickel et al., 2010; Ozinsky et al., 2000; Schwandner et al., 1999; Tsan and Gao, 2004; Yoshimura et al., 1999). Additionally in this model murine commensal bacteria are also activating epidermal TLR2. In this model, we observed that Tlr2−/− mice had a markedly delayed and incomplete barrier recovery in response to a mechanically induced epidermal wound as measured by TEWL, an in vivo measurement that may reflect changes in either or both epidermal barrier structures, SC and/or TJ (Figure 4d). We found that TLR2 agonists help repair epidermal barrier in human and WT mice skin after tape-stripping (Figure 4b, c and e). To determine whether TLR2 agonist are acting on epidermal TJs, we developed a method to study human and murine epidermal TJ function (i.e. TEER and paracellular flux) using the micro-Snapwell™ system. We took advantage of the fact that prolonged epidermal hydration (i.e. 24 hr) results in disruption of intercellular lamellar lipid bilayers, degradation of corneodesmosomes, and formation of amorphous regions within the intercellular lipid (Warner et al., 1999; Warner et al., 2003), which would arguably significantly compromise SC barrier function. This observation combined with the fact that the skin samples had undergone repetitive tape-stripping, lead to our assertion that we are primarily measuring TJ function in this micro-Snapwell™ assay. We cannot entirely rule out the possibility that this significantly disturbed SC may contribute to our readouts but we think that is highly unlikely. In summary, we have found that TLR2 agonists enhance TJ functions in full thickness epidermal samples from both mice and humans that reaffirm our observations made in keratinocyte monolayers. We observed a delay in barrier repair in our Tlr2−/− murine wound model which we think is explained at least in part to an impairment in TJ recovery.
We speculate that the enhanced expression of TLR2 observed in adjacent epidermal cells following barrier disruption (Jin et al., 2009; Schauber et al., 2007) serves two purposes. The first is to respond to and contain pathogens that proliferate in wounded skin. The second function is to promote early TJ formation in keratinocytes that form a monolayer over the dermal wound base. The early formation of this TJ would prevent further serum leakage and thereby limit the nutrients and adhesion molecules that would promote microbial overgrowth, and additionally this would also minimize insensible water loss preventing wound desiccation. We have observed that TLR2 is only expressed on keratinocytes in the cellular layers below TJs (unpublished data). Therefore, the development of a competent TJ would also serve to limit TLR2-mediated inflammation that might be harmful at these later stages of wound repair.
Recently, Yuki et al. found that adding TLR2 agonists to highly differentiated adult human keratinocytes enhances TJ barrier function within 3 h but no change in TJ protein expression was observed (Yuki et al., 2011b)). There are several critical differences in their methodology as compared to ours. They used primary keratinocytes isolated from adult skin samples and differentiated them in high calcium media (1.8mM) for 4 days before treating with TLR2 agonist, while in our study we stimulated our neonatal primary keratinocytes with TLR2 ligands at the point the cells were placed in high calcium media. Interestingly, we found that TLR2 agonists had little effects when they were added to highly differentiated keratinocytes (unpublished data). This most likely reflects the reduced expression of TLR2 observed in highly differentiated keratinocytes (Figure S6) and suggests that TLR2 signaling plays a major role not in a steady-state maintenance of epidermal barrier integrity but rather in epidermal repair. These methodological differences may explain why they did not observe any changes in TJ protein expression. Additionally, we utilized both TEER measurement and a paracellular flux assay, which is more sensitive to assess changes in TJ integrity. Moreover, we evaluated this finding in full thickness human epidermis and murine skins both of which were wounded by tape-stripping and observed this same TLR2-mediated effect. Tape-stripping is a model for the chronic itch-scratch cycle commonly observed in subjects with AD and therefore our findings provide more direct evidence that this is a clinically relevant effect. The other discrepancy between our findings is that they observed that LPS treatment (10 µg/mL) induced TEER, whereas we used lower doses of LPS (5–500 ng/mL) and did not observe an effect on TJ function. It is worth noting that high concentration of LPS (1µg/mL) have been shown to induce TLR2 expression and its’ downstream signaling (NF-κB activation) (Liu et al., 2001). Additionally, the concentrations we employed in our experiments of both LTA and PGN (µg range) as well as of LPS (ng range) are comparable when they are transposed to bacterial equivalents. Based on this, the doses of LPS used in Yuki et al., paper are potentially super-physiologic. Therefore it is possible that the TEER effect they observed in LPS may be through TLR2 signaling.
Lastly, keratinocytes from AD subjects have reduced mRNA expression of TNF-α and IL-6 upon PGN stimulation (Figure S7). Interestingly, it has been shown that Il-6−/− mice (Wang et al., 2004) and Tnfr1−/− mice (Jensen et al., 1999) have delayed TEWL recovery after tape-stripping, suggesting that IL-6 and TNF-α might be critical downstream mediators of TLR2 mediated skin barrier repair. Studies are ongoing to understand the mechanism by which TLR2 improves TJ barrier function. It is possible that the reduced response to PGN observed in AD keratinocytes is due in part to the reduced TLR2 expression observed in AD epidermis (Figure 5, 6a and 6b). This finding is also consistent with the observation that TLR2 expression is decreased in circulating monocytes from AD subjects (Antiga et al., 2011; Niebuhr et al., 2009), suggesting that AD subjects may have a genetic or epigenetic defect that affects expression and/or function of TLR2 on multiple cell types (Antiga et al., 2011; Hasannejad et al., 2007; Mrabet-Dahbi et al., 2008; Niebuhr et al., 2009). Indeed, we examined 11 haplotype-tagging single nucleotide polymorphisms in the TLR2 gene, and found none of these were significantly associated, after correction for multiple comparisons, with AD in two independent groups of patients with AD (Caucasians: n=258; African Americans: n=176) and healthy control subjects (n=156; n= 176, respectively) (unpublished data). This suggests that the observed changes in TLR2 expression in AD subjects likely develop as the consequence of local inflammatory mediators or on an epigenetic basis. Recent reports indicate that several microRNAs (miRNA) could inhibit TLR2 protein translation and thereby decrease its expression and likely also its function (Benakanakere et al., 2009; Jurkin et al., 2010; Liu et al., 2009; Nahid et al., 2011). Studies are ongoing to evaluate this important hypothesis.
In conclusion, keratinocytes provide the first line of defense against microbes and dynamically respond to tissue injury. This involves the production of a tightly orchestrated inflammatory response aimed at enhancing epidermal barrier recovery and limiting microbial overgrowth. Our data demonstrate that the innate immune function of the keratinocytes also includes the preservation of TJ integrity. Therefore, in healthy subjects TLR2 will have a reparative effect on disrupted TJ, and we hypothesize that this barrier protective response is diminished in AD subjects who express less TLR2. Defective TLR2 responses would also enable pathogenic bacteria like S. aureus to persist on the skin surface and perpetuate inflammation. Strategies that boost TLR2 expression or function may hold promise in restoring epidermal integrity in AD.
MATERIALS AND METHODS
Transepithelial Electric Resistance (TEER)
Primary human keratinocytes (PHK) were isolated from discarded neonatal foreskins (also see Supplementary materials). PHK were plated in K-SFM in 24-well Costar® Transwell inserts (polyester membranes, 0.4-µm pore size; Corning Life Sciences, Corning, NY). After cells were confluent, media were switched to DMEM media allowing PHK differentiation and TJ formation. At the same time, TLR ligands were placed in upper wells for 8 days. Culture media was changed every other day. TEER was measured as previously described (De Benedetto et al., 2011). TEER absolute values at day 1 in control groups range from 87±15 to 305±120 ohms × cm2. The study was approved by the Research Subject Review Board at the University of Rochester Medical Center and was conducted according to Declaration of Helsinki Principles.
Paracellular flux assay
PHK were seeded in Transwell inserts and treated as described above. After 48 h, 0.02% fluorescein sodium (Sigma-Aldrich) in PBS was added to the upper well, while PBS alone was added to the lower well. Samples were collected from the lower well after 30 mins. The amount of fluorescein sodium (florescein) that diffused from across the filter was measured with the iQ5 Multicolor real-time PCR detection system (Bio-Rad). Paracellular flux was presented as follows: Paracellular flux (fold of control)= fluorescein intensity of treatment groups/ fluorescein intensity of control group.
Barrier disruption of murine skin
Age-matched (12–16 weeks old), female Tlr2−/− mice (C57BL6 background; kindly provided by Dr. Jian-Dong Li) (Shuto et al., 2002) and WT mice (C57BL6; purchased from National Cancer Institute) were anesthetized with ketamine/xylazine, back hair was shaved and depilatory applied for 3 min (Veet, Reckitt Benckiser Inc; NJ) to completely remove all hair. To disrupt the skin barrier, Cellotape (Nichiban, Tokyo, Japan) was pressed firmly against the skin, removed, and repeated with a new piece of tape each time until TEWL reached the same value (~75 ± 10 g/h/m2) on both mice, and then barrier recovery was monitored by TEWL measurement (Tewameter 300; Courage-Khazaka Electronics, Koln, Germany) at 0, 1, 2, 3 and 24 h. Animal experiments were approved by the University Committee on Animal Resources at the University of Rochester Medical Center.
Micro-Snapwell™ barrier function assay on ex vivo mouse and human skin
The Snapwell™ system was used to measure TEER from human and murine epidermis with and without exposure to the TLR2 agonist, Pam3CSK4. The protocol used was a modification of one previously described (El Asmar et al., 2002). In brief, skin from the back was harvested from mice immediately following tape-stripping (time 0 h), and rinsed in PBS. For human skins, full thickness epidermis was obtained from discarded skin samples after fifteen tape-strippings, using a Weck blade (Goulian Skin Graft Knife Set, George Tiemann & Co. Hauppauge, NY) and then rinsed in PBS. Skin samples were mounted on filter supports (Whatman Nuclepore Track-Etch Membrane; GE Healthcare Biosciences, Pittsburgh, PA) with the epidermal side oriented upward, and sandwiched between two sterile Plexiglas discs (Rohm & Haas Co., Philadelphia, PA) with an opening of 3 mm and placed in modified Snapwell™ chambers (Corning, Corning, NY, USA). Samples were submerged in DMEM complete media and kept at 37°C, 5% CO2 for 30 min. Pam3CSK4 (10 µg/mL) or media alone were then added to both sides of the transwell, and TEER was measured at 0 and 24 h by using Endohm with a planar electrode (Endohm-Snap; World Precision Instruments, Sarasota, FL, USA). TEER (ohms × cm2)= (measured value) × (transwell area; 0.07 cm2). The percentage change in TEER between time 0 (100 percent) and time 24 h was expressed as follows: (TEER24h /TEER0h) × 100. Data were normalized to control group, and presented as fold of control. Paracellular flux of fluorescein in human epidermis was measured at 24 h as described above. Using human discarded skins was approved by the Research Subject Review Board at the University of Rochester Medical Center. This study was conducted according to Declaration of Helsinki Principles.
Statistical Analysis
Data are expressed as mean ± standard error (SEM) and are representative of at least three separate experiments. One-way or two-way analysis of variance (ANOVA) was used to determine significance unless otherwise stated. Mann-Whitney t-test was used to compare gene expression and immunofluorescence intensity between AD and NA skin. Spearman nonparametric correlation was used to evaluate the correlation between TLR2 staining intensity and TEWL. The statistical analysis was performed using GraphPad Prism 4 (GraphPad Software, Inc, San Diego, CA). P < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by Atopic Dermatitis Research Network (contract HHSN272201000020C and HHSN272201000017C) (L.A.B., R.L.G., D.Y.L. and K.C. DHHS/PHS/NIH 5 T32 AR007472-21 (A.D.B.), National Eczema Association research grant (A.D.B. and L.A.B.), Dermatology Foundation research grant (A.D.B.), National Institute of Diabetis Digestive and Kidney Diseases grants DK083968 and DK084953 A.I.I.) and NIH R01 HL071933 (S.N.G) and NIEHS P30 ES01247 (S.N.G). Authors thankful to the Department of Pathology at the University of Rochester Medical Center for providing discarded skin samples for the study. We also thank Dr. Jian-Dong Li, PhD, Dr. Jae Hyang Lim, PhD at Georgia State University (Atlanta, GA), and Dr. Xiangbin Xu, PhD at the University of Rochester Medical Center (Rochester, NY) provided the Tlr2−/− mice.
Abbreviations
- AD
Atopic Dermatitis
- CLDN1
Claudin-1
- CLDN23
Claudin-23
- LTA
Lipoteichoic acid
- LPS
Lipopolysaccharide
- Malp-2
Macrophage-activating lipopeptide-2
- NA
Nonatopic
- NOD2
Nucleotide-binding oligomerization domain containing 2
- Pam3CSK4
N-palmitoyl-S-[2,3-bis(palmitoyl)-(2RS)-propyl]-(R)cysteinyl-alanyl-glycine
- PGN
Peptidoglycan
- PGLYRP-3
Peptidoglycan recognition protein 3
- PGLYRP-4
Peptidoglycan recognition protein 4
- PolyI:C
Polyinosinic-polycytidylic acid
- PHK
Primary human keratinocytes
- qPCR
Quantitative PCR
- TJ
Tight junction
- TEER
Transepithelial electrical resistance
- TEWL
Transepidermal water loss
- TLR
Toll-like receptor
- ZO-1
Zonulae occludens 1
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of Interest.
REFERENCES
- Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens K, Schunck M, Podda GF, Meingassner J, Stuetz A, Schroder JM, et al. Mechanical and metabolic injury to the skin barrier leads to increased expression of murine beta-defensin-1,-3, and-14. J Invest Dermatol. 2011;131:443–452. doi: 10.1038/jid.2010.289. [DOI] [PubMed] [Google Scholar]
- Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med. 2000;6:1275–1277. doi: 10.1038/81385. [DOI] [PubMed] [Google Scholar]
- Antiga E, Volpi W, Torchia D, Fabbri P, Caproni M. Effects of tacrolimus ointment on Toll-like receptors in atopic dermatitis. Clinical and experimental dermatology. 2011;36:235–241. doi: 10.1111/j.1365-2230.2010.03948.x. [DOI] [PubMed] [Google Scholar]
- Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–679. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- Baltes S, Nau H, Lampen A. All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev Growth Differ. 2004;46:503–514. doi: 10.1111/j.1440-169x.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- Benakanakere MR, Li Q, Eskan MA, Singh AV, Zhao J, Galicia JC, et al. Modulation of TLR2 protein expression by miR-105 in human oral keratinocytes. J Biol Chem. 2009;284:23107–23115. doi: 10.1074/jbc.M109.013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochud PY, Magaret AS, Koelle DM, Aderem A, Wald A. Polymorphisms in TLR2 are associated with increased viral shedding and lesional rate in patients with genital herpes simplex virus Type 2 infection. The Journal of infectious diseases. 2007;196:505–509. doi: 10.1086/519693. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010;125:4–13. doi: 10.1016/j.jaci.2009.11.027. quiz 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunological reviews. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski AW, Gallo RL. The coordinated response of the physical and antimicrobial peptide barriers of the skin. J Invest Dermatol. 2011;131:285–287. doi: 10.1038/jid.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. quiz 2–3. [DOI] [PubMed] [Google Scholar]
- De Benedetto A, Agnihothri R, McGirt LY, Bankova LG, Beck LA. Atopic dermatitis: a disease caused by innate immune defects? J Invest Dermatol. 2009;129:14–30. doi: 10.1038/jid.2008.259. [DOI] [PubMed] [Google Scholar]
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949–963. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–786. e1–e7. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickel H, Gambichler T, Kamphowe J, Altmeyer P, Skrygan M. Standardized tape stripping prior to patch testing induces upregulation of Hsp90, Hsp70, IL-33, TNF-alpha and IL-8/CXCL8 mRNA: new insights into the involvement of 'alarmins'. Contact dermatitis. 2010;63:215–222. doi: 10.1111/j.1600-0536.2010.01769.x. [DOI] [PubMed] [Google Scholar]
- Djalilian AR, McGaughey D, Patel S, Seo EY, Yang C, Cheng J, et al. Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J Clin Invest. 2006;116:1243–1253. doi: 10.1172/JCI27186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareus R, Huth M, Breiden B, Nenci A, Rosch N, Haase I, et al. Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation. Nat Cell Biol. 2007;9:461–469. doi: 10.1038/ncb1560. [DOI] [PubMed] [Google Scholar]
- Grether-Beck S, Felsner I, Brenden H, Kohne Z, Majora M, Marini A, et al. Urea Uptake Enhances Barrier Function and Antimicrobial Defense in Humans by Regulating Epidermal Gene Expression. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryev DN, Howell MD, Watkins TN, Chen YC, Cheadle C, Boguniewicz M, et al. Vaccinia virus-specific molecular signature in atopic dermatitis skin. J Allergy Clin Immunol. 2010;125:153–159. e28. doi: 10.1016/j.jaci.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasannejad H, Takahashi R, Kimishima M, Hayakawa K, Shiohara T. Selective impairment of Toll-like receptor 2-mediated proinflammatory cytokine production by monocytes from patients with atopic dermatitis. J Allergy Clin Immunol. 2007;120:69–75. doi: 10.1016/j.jaci.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124:R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Imokawa G. Lipid abnormalities in atopic dermatitis. J Am Acad Dermatol. 2001;45:S29–S32. doi: 10.1067/mjd.2001.117020. [DOI] [PubMed] [Google Scholar]
- Jensen JM, Schutze S, Forl M, Kronke M, Proksch E. Roles for tumor necrosis factor receptor p55 and sphingomyelinase in repairing the cutaneous permeability barrier. J Clin Invest. 1999;104:1761–1770. doi: 10.1172/JCI5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kumar L, Mathias C, Zurakowski D, Oettgen H, Gorelik L, et al. Toll-like receptor 2 is important for the T(H)1 response to cutaneous sensitization. The Journal of allergy and clinical immunology. 2009;123:875–882. e1. doi: 10.1016/j.jaci.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkin J, Schichl YM, Koeffel R, Bauer T, Richter S, Konradi S, et al. miR-146a is differentially expressed by myeloid dendritic cell subsets and desensitizes cells to TLR2-dependent activation. J Immunol. 2010;184:4955–4965. doi: 10.4049/jimmunol.0903021. [DOI] [PubMed] [Google Scholar]
- Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–541. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschwanez HE, Broadbent E. The use of wound healing assessment methods in psychological studies: a review and recommendations. Br J Health Psychol. 16:1–32. doi: 10.1348/135910710X524633. [DOI] [PubMed] [Google Scholar]
- Kretz M, Maass K, Willecke K. Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants. Eur J Cell Biol. 2004;83:647–654. doi: 10.1078/0171-9335-00422. [DOI] [PubMed] [Google Scholar]
- Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. The Journal of clinical investigation. 2012;122:440–447. doi: 10.1172/JCI57416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106:15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Yamakuchi M, Isowaki S, Nagata E, Kanmura Y, et al. Upregulation of toll-like receptor 2 gene expression in macrophage response to peptidoglycan and high concentration of lipopolysaccharide is involved in NF-kappa b activation. Infection and immunity. 2001;69:2788–2796. doi: 10.1128/IAI.69.5.2788-2796.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke T, Choudhry R, Thom R, Selmer IS, Burden AD, Hodgins MB. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J Invest Dermatol. 1999;112:354–361. doi: 10.1046/j.1523-1747.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel M, Voelcker V, Kollisch G, Plank C, Rad R, Gerhard M, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003;121:1389–1396. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- Menzies BE, Kenoyer A. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human beta-defensin 3 in skin keratinocytes. Infect Immun. 2006;74:6847–6854. doi: 10.1128/IAI.00389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrabet-Dahbi S, Dalpke AH, Niebuhr M, Frey M, Draing C, Brand S, et al. The Toll-like receptor 2 R753Q mutation modifies cytokine production and Toll-like receptor expression in atopic dermatitis. The Journal of allergy and clinical immunology. 2008;121:1013–1019. doi: 10.1016/j.jaci.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Murata Y, Ogata J, Higaki Y, Kawashima M, Yada Y, Higuchi K, et al. Abnormal expression of sphingomyelin acylase in atopic dermatitis: an etiologic factor for ceramide deficiency? J Invest Dermatol. 1996;106:1242–1249. doi: 10.1111/1523-1747.ep12348937. [DOI] [PubMed] [Google Scholar]
- Nahid MA, Satoh M, Chan EK. Mechanistic role of microRNA-146a in endotoxin-induced differential cross-regulation of TLR signaling. J Immunol. 2011;186:1723–1734. doi: 10.4049/jimmunol.1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr M, Lutat C, Sigel S, Werfel T. Impaired TLR-2 expression and TLR-2-mediated cytokine secretion in macrophages from patients with atopic dermatitis. Allergy. 2009;64:1580–1587. doi: 10.1111/j.1398-9995.2009.02050.x. [DOI] [PubMed] [Google Scholar]
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- Ohnemus U, Kohrmeyer K, Houdek P, Rohde H, Wladykowski E, Vidal S, et al. Regulation of epidermal tight-junctions (TJ) during infection with exfoliative toxin-negative Staphylococcus strains. The Journal of investigative dermatology. 2008;128:906–916. doi: 10.1038/sj.jid.5701070. [DOI] [PubMed] [Google Scholar]
- Olaru F, Jensen LE. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1alpha signaling loop. J Invest Dermatol. 2010;130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong PY, Leung DY. The infectious aspects of atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:309–321. doi: 10.1016/j.iac.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Pilgram GS, Vissers DC, van der Meulen H, Pavel S, Lavrijsen SP, Bouwstra JA, et al. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001;117:710–717. doi: 10.1046/j.0022-202x.2001.01455.x. [DOI] [PubMed] [Google Scholar]
- Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, et al. Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. The Journal of allergy and clinical immunology. 2011;128:1216–1224. e11. doi: 10.1016/j.jaci.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. The Journal of biological chemistry. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Shuto T, Imasato A, Jono H, Sakai A, Xu H, Watanabe T, et al. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J Biol Chem. 2002;277:17263–17270. doi: 10.1074/jbc.M112190200. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Taljebini M, Warren R, Mao-Oiang M, Lane E, Elias PM, Feingold KR. Cutaneous permeability barrier repair following various types of insults: kinetics and effects of occlusion. Skin Pharmacol. 1996;9:111–119. doi: 10.1159/000211406. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, et al. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilopoulos Y, Cork MJ, Teare D, Marinou I, Ward SJ, Duff GW, et al. A nonsynonymous substitution of cystatin A a cysteine protease inhibitor of house dust mite protease, leads to decreased mRNA stability and shows a significant association with atopic dermatitis. Allergy. 2007;62:514–519. doi: 10.1111/j.1398-9995.2007.01350.x. [DOI] [PubMed] [Google Scholar]
- Wang XP, Schunck M, Kallen KJ, Neumann C, Trautwein C, Rose-John S, et al. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. J Invest Dermatol. 2004;123:124–131. doi: 10.1111/j.0022-202X.2004.22736.x. [DOI] [PubMed] [Google Scholar]
- Warner RR, Boissy YL, Lilly NA, Spears MJ, McKillop K, Marshall JL, et al. Water disrupts stratum corneum lipid lamellae: damage is similar to surfactants. The Journal of investigative dermatology. 1999;113:960–966. doi: 10.1046/j.1523-1747.1999.00774.x. [DOI] [PubMed] [Google Scholar]
- Warner RR, Stone KJ, Boissy YL. Hydration disrupts human stratum corneum ultrastructure. The Journal of investigative dermatology. 2003;120:275–284. doi: 10.1046/j.1523-1747.2003.12046.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- Yuki T, Hachiya A, Kusaka A, Sriwiriyanont P, Visscher MO, Morita K, et al. Characterization of tight junctions and their disruption by UVB in human epidermis and cultured keratinocytes. J Invest Dermatol. 2011a;131:744–752. doi: 10.1038/jid.2010.385. [DOI] [PubMed] [Google Scholar]
- Yuki T, Yoshida H, Akazawa Y, Komiya A, Sugiyama Y, Inoue S. Activation of TLR2 enhances tight junction barrier in epidermal keratinocytes. J Immunol. 2011b;187:3230–3237. doi: 10.4049/jimmunol.1100058. [DOI] [PubMed] [Google Scholar]
- Zahringer U, Lindner B, Inamura S, Heine H, Alexander C. TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213:205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.