Abstract
Objective
“Jitter” involves randomization of intervals between stimulus events. Compared with controls, individuals with ADHD demonstrate greater intrasubject variability (ISV) performing tasks with fixed interstimulus intervals (ISIs). Because Gaussian curves mask the effect of extremely slow or fast response times (RTs), ex-Gaussian approaches have been applied to study ISV.
Method
This study applied ex-Gaussian analysis to examine the effects of jitter on RT variability in children with and without ADHD. A total of 75 children, aged 9 to 14 years (44 ADHD, 31 controls), completed a go/no-go test with two conditions: fixed ISI and jittered ISI.
Results
ADHD children showed greater variability, driven by elevations in exponential (tau), but not normal (sigma) components of the RT distribution. Jitter decreased tau in ADHD to levels not statistically different than controls, reducing lapses in performance characteristic of impaired response control.
Conclusion
Jitter may provide a nonpharmacologic mechanism to facilitate readiness to respond and reduce lapses from sustained (controlled) performance.
Keywords: ADHD, executive function, attention, variability, response time, statistics, ex-Gaussian, jitter
Individuals with ADHD demonstrate deficient response control, manifested as slow and variable response times (RTs; Harris et al., 1995; Vaurio, Simmonds, & Mostofsky, 2009; Wodka et al., 2007). Over the past 15 years, impaired response inhibition has been considered the primary core feature of ADHD. More recently, however, studies point toward intrasubject variability (ISV), or moment-to-moment variability in each subject's RT series, as a more robust intermediate phenotype (Bidwell, Willcutt, Defries, & Pennington, 2007; Gomez-Guerrero et al., 2010). Individuals with ADHD demonstrate increased ISV compared with typically developing (TD) peers, suggesting inefficient response preparation and selection (Di Martino et al., 2008; Rommelse et al., 2008; Ryan, Martin, Denckla, Mostofsky, & Mahone, 2010).
Statistical methods have improved our approach toward the problem of RT variability. Traditional Gaussian analyses offer information regarding the mean and standard deviation of a given set of values. Because Gaussian curves can mask the effect of extremely slow or fast RTs, ex-Gaussian approaches have become increasingly popular among researchers investigating ADHD and other neuropsychiatric disorders (Hervey et al., 2006; Leth-Steensen, Elbaz, & Douglas, 2000; McAuley, Yap, Christ, & White, 2006; Vaurio et al., 2009). These ex-Gaussian methods model the mean (mu) of the normal distribution and the standard deviation (sigma) of the normal distribution, as well as the mean and standard deviation of the exponential component of the distribution (tau). Thus, for RT series, tau indicates extremely prolonged RTs, which are thought to result from “lapses” from on-task behavior. Several studies have shown tau to be increased in ADHD compared with TD individuals across a range of tasks involving response control (Epstein et al., 2011; Hervey et al., 2006; Leth-Steensen et al., 2000; Vaurio et al., 2009). Furthermore, increases in sigma were reported in tasks requiring higher response selection (Buzy, Medoff, & Schweitzer, 2009; Geurts et al., 2008; Vaurio et al., 2009). These findings suggest that ADHD is associated with increased RT variability represented in normal and exponential components of the distribution. As such, applying the ex-Gaussian approach may provide a more parsimonious representation of RT variability in children with ADHD.
Experimental paradigms that manipulate rate of event presentation and provision of incentives have demonstrated that ISV can be reduced in individuals with and without ADHD (Andreou et al., 2007; Kuntsi, Wood, van der Meere, & Asherson, 2009). Recent studies have applied interstimulus “jitter,” a technique involving randomization of time intervals between successive stimuli within a test series, to improve attentional and response control (Ryan et al., 2010; Wodka, Simmonds, Mahone, & Mostofsky, 2009). These studies compared performance under a fixed interstimulus interval (ISI) condition with that under a jittered (variable) ISI condition. In recently published findings by Ryan et al. (2010), under testing conditions of fixed ISI, individuals with ADHD showed increased ISV compared with controls. With the introduction of a moderate degree of jitter, ISV decreased in ADHD participants to levels not statistically different from controls.
The present study represents an extension and ex-Gaussian reanalysis of data presented in the Ryan et al. study, with 36 additional participants included in the present analysis, nearly doubling the sample size of the prior study. Because the standard summary variables produced in the Gaussian approach as performed in Ryan et al.'s study may not thoroughly represent the patterns of responses comprising the RT distribution curve (especially the exponential component), this reanalysis reflects a more comprehensive evaluation of RT variability in a larger cohort of children with ADHD. The aim of this study was to examine the effects of interstimulus jitter on the RTs of children with and without ADHD, examining the normal and exponential distributions. We hypothesized that, compared with TD controls, children with ADHD would show more variable RTs, with elevations in Gaussian (sigma) and exponential (tau) components, when performing a go/no-go task under a fixed ISI task condition. We also hypothesized that, with introduction of a moderate degree of interstimulus jitter, RT consistency would increase and the extreme lapses in attention would be reduced, as shown by decreases in both the variability in the normal (Gaussian) RT distribution (sigma) and exponential components of the RT distribution (tau).
Method
Participants
Participants were recruited from outpatient clinics at the Kennedy Krieger Institute, local schools, and pediatricians' offices, and enrolled as part of a larger study examining the brain mechanisms in ADHD. Informed consent was obtained for all study participants, in agreement with prior approval by the Johns Hopkins Medicine Institutional Review Board. Children between ages 9 and 14 years were screened by initial parent telephone interview to obtain demographic information, educational and developmental history, and medication status. Potential participants were excluded if there was a history of speech/language disorder or word reading difficulties identified either through telephone screening or prior school assessment completed within 1 year, evidence of visual or hearing impairment, history of other neurological disorder, or if they were taking psychotropic medications other than stimulants. Children who were taking stimulant medication were removed from medication on the day of and the day prior to testing. A total of 75 children (44 ADHD, 31 controls) were included in the present study.
Following telephone screening, parents were interviewed about their child using a structured psychiatric interview (i.e., Diagnostic Interview of Children and Adolescents–Fourth Edition [DICA-IV]). ADHD-oriented behavior rating scales, including the Conners' Parent Rating Scale–Revised/Conners' Teacher Rating Scale–Revised (CPRS-R/CTRS-R) and the ADHD Rating Scale–IV, were used to confirm diagnosis of ADHD. The diagnosis of ADHD was made using the following criteria: (a) Positive Diagnostic and Statistical Manual of Mental Disorders (4th ed., DSM-IV; American Psychiatric Association, 1994) ADHD diagnosis on DICA-IV, (b) T-scores greater than 65 on the DSM-IV Hyperactive/Impulsive or Inattentive scales of the CPRS-R or CTRS-R, and (c) six of nine DSM-IV symptoms met (item rating of 2 or 3) on the Hyperactive/Impulsive or Inattention scales of the ADHD Rating Scale–IV, Home or School Version. Children with DSM-IV diagnoses other than Oppositional Defiant Disorder and Specific Phobias were excluded. Parents of control participants completed the DICA-IV, CPRS-R, and ADHD Rating Scale–IV. Teachers of controls completed the CTRS-R and teacher form of the ADHD Rating Scale–IV. Controls with T-scores greater than 60 on either the DSM-IV Inattentive or Hyperactive/Impulsive scales of the CPRS-R or CTRS-R, or item ratings of 2 or greater for four or more symptoms of inattention or hyperactivity/impulsivity from the ADHD Rating Scale–IV (home or school), were also excluded. Children were excluded from the control group if they had a history of mental health services for behavior or emotional problems, history of academic problems requiring school-based intervention services, or history of defined primary reading or language-based learning disability. All participants were screened for word reading difficulties, which were defined as a score less than the 25th percentile on the Basic Reading Composite of the Woodcock Johnson–III (WJ-III) Tests of Achievement.
Children were administered the Wechsler Adult Intelligence Scale–IV (WISC-IV), the WJ-III reading tests, and the go/no-go test on the first day of assessment. The two conditions of the go/no-go test were administered in a counterbalanced sequence, with both groups experiencing the condition orders equally.
Demographics
Sample demographics are included in Table 1. The study included 75 participants: 44 with ADHD (27 male) and 31 controls (11 male), of whom 66.7% were Caucasian, 21.3% African American, 4.3% Asian, 1.3% Pacific Islander, and 6.7% mixed race. Within the ADHD group, there were 14 (31.8%) with Inattentive subtype, 0 with Hyperactive-Impulsive subtype, and 30 (68.2%) with Combined subtypes. DICA-IV assessments were obtained on 31controls and none had comorbidities. For children with ADHD (n = 44), 16 (36.4%) met criteria for Oppositional Defiant Disorder and 3 (6.8%) met criteria for Specific Phobia. Of the ADHD participants, 26 (59.0%) were on one or more stimulant medications at the time of enrollment in the study. For ADHD participants not on medication, time since last medication use was not reported. Participants ranged in age from 9 to 14 years, with an average age of 11.2 years. There were no significant differences in racial distribution, χ2(4) = 3.26, p = .516, between ADHD and control groups. In addition, there were no significant differences between groups in age or socioeconomic status (SES); however, control participants demonstrated higher Full Scale IQ (FSIQ) scores than the ADHD group (Table 1). There was also a higher proportion of boys in the ADHD group than in the control group, χ2(1) = 3.74, p = .053. Sex was not significantly correlated with any of the six outcome variables (mean RT—RT, standard deviation of RT—SD, and coefficient of variability–CV, mu, sigma, or tau) and, therefore, was not used as a covariate in analyses. In addition, given the overlap between components of FSIQ and the executive functions under investigation, especially those involving response preparation/processing speed, covarying for FSIQ was not considered appropriate when measuring group differences on components of the go/no-go test (Dennis et al., 2009). Furthermore, a recent meta-analysis of the effects of attention deficits on assessment of IQ noted that children with ADHD taking stimulant medications while being tested had a mean increase of 6 to 7 IQ points compared children with ADHD who were not taking stimulants during testing (such as those in the present study), suggesting that reduced IQ scores in our ADHD group may be driven by attentional problems and suboptimal test-taking behavior rather than reduced intelligence, per se (Jepsen, Fagerlund, & Mortensen, 2009). Therefore, we did not covary for FSIQ in the present analyses.
Table 1.
Sample Demographic Characteristics
| Control (n = 31) |
ADHD (n = 44) |
|||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | |
| Age (years) | 11.23 | 1.44 | 11.16 | 1.57 | 0.183 | .855 |
| SES | 47.99 | 14.92 | 45.76 | 16.10 | 0.603 | .548 |
| FSIQ | 116.13 | 10.01 | 99.52 | 13.34 | 5.859 | < .001 |
| VCI | 112.68 | 13.37 | 101.55 | 12.06 | 3.764 | < .001 |
| CPRS-R/DSM-IV Inattentive (T) | 47.42 | 7.42 | 72.16 | 8.47 | −12.30 | < .001 |
| CPRS-R/DSM-IV Hyperactive/Impulsive (T) | 47.31 | 6.20 | 72.47 | 13.24 | −9.08 | < .001 |
| CPRS-R/DSM-IV total score (T) | 47.35 | 7.46 | 74.35 | 9.76 | −12.11 | < .001 |
Note: SES = socioeconomic status; FSIQ = Full Scale IQ;VCI = Verbal Comprehension Index; CPRS-R = Conners' Parent Rating Scale–Revised; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders (4th ed.); T = T-score.
Measures
Go/no-go test. Participants completed a computer-based go/no-go paradigm in which red and green spaceships were presented. Each participant was instructed to use his or her dominant hand index finger to push a button immediately in response to green spaceships only. Familiar color elements such as green for “go” and red for “no-go” minimized the working memory load during the test. For a detailed description of the paradigm, the reader is referred to Ryan et al. (2010) and Wodka et al. (2007). Two conditions of the go/no-go test were administered. In the fixed ISI condition, cues appeared on the screen for 300 ms and were presented once every 1,500 ms. Presentation cues were weighted toward green spaceships at a ratio of 3:1 (162 “go” cues and 54 “no-go” cues), intensifying the need to inhibit a habituated motor response. In the jittered ISI condition, stimuli were presented with a variable ISI, using a moderate (33.3%) level of jitter in which five ISIs were presented randomly, ranging from 1,000 to 2,000 ms (i.e., 1,000, 1,250, 1,500, 1,750, and 2,000). Higher or lower levels of jitter would contain longer or shorter ISI, respectively. The presentations were administered under the same sequence to all participants. The total time for each go/no-go condition was 6 min 30 s. Only “go” RTs were used, and RTs on failed inhibition trials were omitted from the analysis.
Data Analysis
Ex-Gaussian analyses were performed for the RT series to estimate the distribution parameters: mu, sigma, and tau. The analyses were performed using the egfit function in MATLAB (Lacouture & Cousineau, 2008). Only accurate responses (i.e., correct hits) were used in analyses of reaction time data (i.e., omitted responses or commission errors were not included). Repeated measures ANOVAs were used to examine the moderating effect of jitter on go/no-go test performance, and to compare performance between control and ADHD groups for accuracy (omissions and commissions), mu, sigma, and tau, as well as for CV and standard (Gaussian) mean RT components.
Results
There were no significant sex differences on any of the eight outcome variables (p > .24 for all comparisons). Therefore, data from boys and girls were combined for all subsequent analyses.
Children's performance across task conditions is presented in Table 2. For percent omissions, there was a significant effect for group (ADHD vs. control), such that children with ADHD had more omission errors than controls, F(1, 72) = 5.17, p = .026, ηp2 = 0.067. The effects of condition (fixed vs. jittered), F(1, 72) = 0.70, p = .792, ηp2 = 0.001, and the group-by-condition interaction, F(1, 72) = 2.06, p = .156, ηp2 = 0.028, were not significant. For percent commission errors, a similar pattern emerged. The effect for group was significant, such that children with ADHD had more commission errors than controls, F(1, 72) = 7.20, p = .009, ηp2 = 0.091. The effects of condition, F(1, 72) = 0.75, p = .389, ηp2 = 0.010, and the group-by-condition interaction, F(1, 72) = 0.009, p = .926, ηp2 = 0.0001, were not significant. Thus, children with ADHD perform worse than controls with regard to task accuracy, regardless of condition (fixed vs. jittered).
Table 2.
Errors, Gaussian, and Ex-Gaussian RT (ms) Values Across Condition by Group
| Control (n = 31) |
ADHD (n = 44) |
|||
|---|---|---|---|---|
| Fixed (SD) | Jittered (SD) | Fixed (SD) | Jittered (SD) | |
| Omissions | 1.93 (2.43) | 2.54 (2.89) | 4.62 (5.71) | 3.74 (4.36) |
| Commissions | 22.11 (16.17) | 23.16 (18.34) | 33.36 (20.71) | 34.67 (19.22) |
| Mean RT | 455.23 (135.74) | 464.35 (133.97) | 435.66 (97.92) | 431.95 (70.74) |
| CV | 0.30 (0.16) | 0.26 (0.70) | 0.42 (0.17) | 0.31 (0.11) |
| Mu | 354.36 (130.74) | 369.27 (103.93) | 296.04 (93.98) | 324.05 (52.95) |
| Sigma | 59.46 (31.84) | 55.53 (32.75) | 63.38 (27.10) | 55.08 (25.75) |
| Tau | 100.88 (49.48) | 95.08 (38.19) | 139.62 (62.65) | 107.90 (49.59) |
| RT SD | 133.97 (59.37) | 118.65 (42.85) | 178.14 (68.96) | 135.35 (60.01) |
Note: RT = response time; SD = standard deviation; Omissions = percent omission errors; Commissions = percent commission errors; CV = coefficient of variability (RT SD/mean RT); Mu = mean, ex-Gaussian; Sigma = SD, ex-Gaussian;Tau = mean and SD, ex-Gaussian.
For mean RT, the effects for group, F(1, 73) = 1.28, p = .26, ηp2 = 0.017; condition, F(1, 73) = 0.10, p = .75, ηp2 = 0.001; and the group-by-condition interaction, F(1, 73) = 0.590, p = .44, ηp2 = 0.008, were not significant (Figure 1a). For the standard deviation of RT (SD), there were significant effects for group (ADHD > control), F(1, 73) = 6.99, p = .01, ηp2 = 0.087, and condition (fixed > jittered), F(1, 73) = 13.32, p < .001, ηp2 = 0.154. The group-by-condition interaction was not significant, F(1, 73) = 2.97, p = .089, ηp2 = 0.039.
Figure 1.
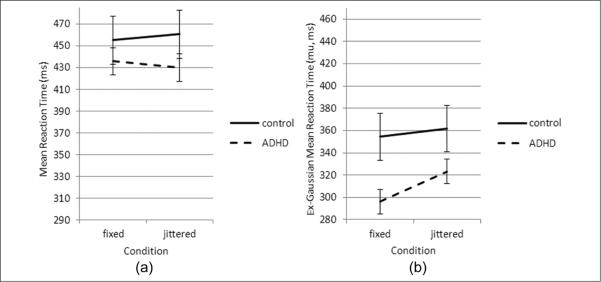
Group differences in mean response time (ms) by group across task conditions
Note: (a) The mean reaction time in ADHD is faster compared with controls under both conditions, but these effects did not reach statistical significance. (b) Ex-Gaussian analysis demonstrates that under the fixed condition ADHD was faster than controls, but there was no significant effect for groups by condition. Bars represent standard error.
Mu represents the mean of the normal component of the RT distribution. For mu, the repeated measures ANOVA revealed a significant effect for group (ADHD faster than controls), F(1, 73) = 6.10, p = .016, ηp2 = 0.077, and task condition (fixed faster than jittered), F(1, 73) = 7.16, p = .009, ηp2 = 0.089, but not for the group-by-condition interaction, F(1, 73) = 0.667, p = .42, ηp2 = 0.009 (Figure 1b).
ISV was calculated as the coefficient of variation (CV), which is the standard deviation/mean (Stuss, Murphy, Binns, & Alexander, 2003). For CV, the repeated measures ANOVA revealed significant main effects for group (ADHD more variable than controls), F(1, 73) = 11.74, p = .001, ηp2 = 0.139, and task condition (fixed more variable than jittered), F(1, 73) = 15.96, p < .001, ηp2 = 0.179, without a significant group-by-condition interaction, F(1, 73) = 2.42, p = .12, ηp2 = 0.032 (Figure 2a).
Figure 2.
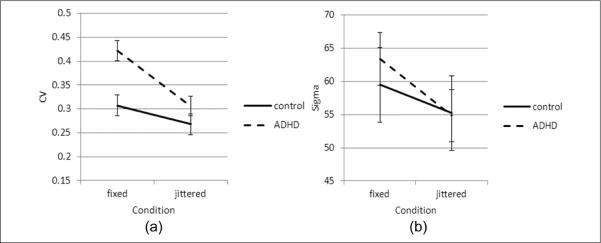
Intrasubject variability of response time by group across conditions
Note: CV = coefficient of variability. (a) ADHD subjects showed significantly higher CV than controls under the fixed condition, but there was no significant group by condition interaction. (b) Under the fixed condition, both groups showed higher sigma, but there was no effect between groups, or interaction for groups by condition. Bars represent standard error.
Sigma measures the standard deviation of the normal component of the RT distribution. For sigma, the repeated measures ANOVA revealed significant effects for task condition (jittered more variable than fixed), F(1, 73) = 3.85, p = .054, ηp2 = 0.050, but no significant effects for group (ADHD vs. controls), F(1, 73) = 0.082, p = .775, ηp2 = 0.001, or group-by-condition interaction, F(1, 73) = 0.490, p = .486, ηp2 = 0.007 (Figure 2b).
Tau measures the mean and standard deviation of the exponential component of the RT distribution. For tau, the repeated measures ANOVA revealed significant effects for group (ADHD > control), F(1, 73) = 6.02, p = .017, ηp2 = 0.076; task condition (fixed > jittered), F(1, 73) = 9.50, p = .003, ηp2 = 0.115; and group-by-condition interaction, F(1, 73) = 4.54, p = .037, ηp2 = 0.59. To explore the significant group-by-condition interaction for tau, repeated measures ANOVAs were completed separately within each group, revealing significant effects for condition within the ADHD group (fixed > jittered), F(1, 44) = 14.48, p < .001, ηp2 = 0.252, but not among controls, F(1, 31) = 0.48, p = .494, ηp2 = 0.016. In addition, between-group comparisons were made within each condition, and revealed significant group differences in tau in the fixed condition (ADHD > controls), F(1, 74) = 8.23, p = .005, ηp2 = 0.101, but not in the jittered condition, F(1, 74) = 1.46, p = .231, ηp2 = 0.020 (Figure 3).
Figure 3.
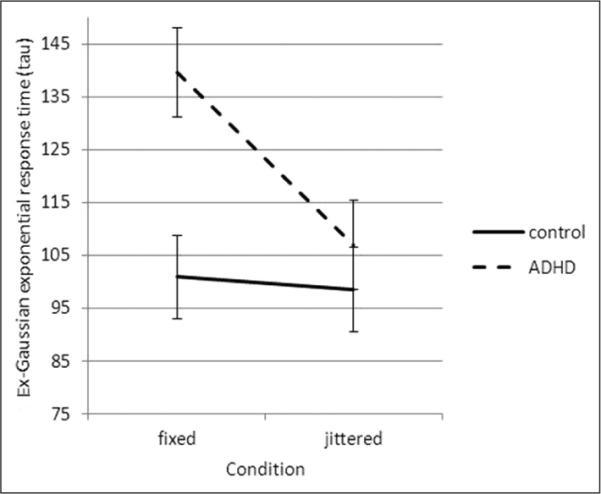
Group differences in tau across task conditions
Note: Bars represent standard error.
Across task conditions, the magnitude of group difference was greatest for CV (ηp2 = 0.139; ADHD more variable than controls) and smallest for sigma (ηp2 = 0.001). Individual logistic regression analyses examining group discrimination for each variable yielded a similar pattern of results in which CV was the strongest discriminating variable—CV: χ2(1) = 8.530, p = .003, odds ratio (OR) = 75.98; tau: χ2(1) = 8.527, p = .003, OR = 1.013; SD: χ2(1) = 8.279, p = .004, OR = 1.011; sigma: χ2(1) = 0.338, p = .561, OR = 1.005; mean RT: χ2(1) = 0.53, p = .465, OR = 0.999; and mu: χ2(1) = 4.98, p = .026, OR = 0.995.
Given the finding of reduced mu and increased errors (omissions and commissions) among children with ADHD, relative to controls, the issue of a speed-accuracy trade-off appeared salient. Therefore, we examined the associations among mu, omissions, and commissions in both test conditions. In the fixed condition, mu and omissions were significantly correlated among children with ADHD (r = −.403, p = .007), but not among controls (r = −.137, p = .462), whereas in the jittered condition, the associations between omissions and mu were not significant in either group (ADHD: r = −.183, p = .234; controls: r = −.245, p = .185). A different pattern emerged for commission errors such that significant speed/accuracy associations were observed in both groups in both conditions. In the fixed condition, the correlation between mu and commissions was significant in both groups (ADHD: r = −.661, p < .001; controls: r = −.497, p = .004); in the jittered condition, the association was also significant in both groups (ADHD: r = −.617, p < .001; controls: r = −.528, p = .002), suggesting that increased commission errors were associated with faster RTs, regardless of condition or group.
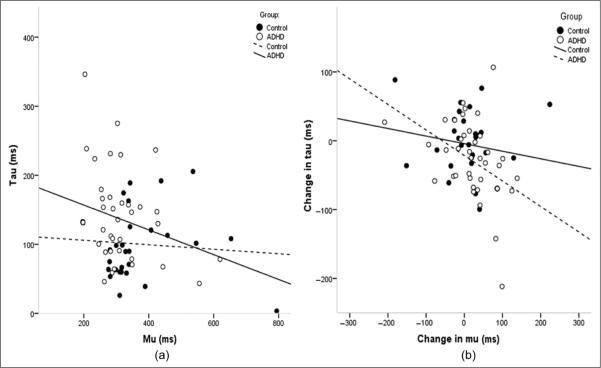
Given the finding of increased tau among children with ADHD, we also examined the relationship between mu and tau, and the relative changes in these variables associated with the jittered condition (compared with the fixed condition). The associations between mu and tau are depicted in Figure 4. In the fixed condition, mu and tau were not significantly correlated among controls (r = −.086, p = .645); however, the association was stronger (approaching significance) among children with ADHD (r = −.269, p = .077), suggesting that in children with ADHD, performance lapses tend to increase with faster responses. Furthermore, among children with ADHD, change in tau (i.e., the reduction in tau between fixed and jittered conditions) was significantly associated with change in mu (i.e., the increase in mu between fixed and jittered conditions; r = −.431, p = .003), suggesting that improvement in tau occurred in conjunction with slower responses. Both groups also showed a significant positive correlation for sigma and tau under the fixed condition (control: p = .006, r = .486; ADHD: p = .005, r = .418), but under the jittered condition, only the ADHD group had a significant correlation (p = .005, r = .413).
Figure 4.
Scatterplots of correlation between mu and tau by group and condition: (a) In the fixed condition, the correlation between mu (mean response time) and tau (outlier responses) was nonsignificant across groups (ADHD: p = .07, r = −.269; control: p = .645, r = −.086) and (b) change in tau (the difference between test conditions: jittered minus fixed) was significantly correlated with change in mu for ADHD but not controls (ADHD: p = .003, r = −.431; control: p = .348, r = −.174)
Discussion
The present investigation examined the impact of interstimulus jitter on go/no-go accuracy and RT variability in children with and without ADHD. Consistent with our first hypothesis, we found that children with ADHD showed increased variability (i.e., CV), relative to controls on go/no-go task. Importantly, the findings from the present investigation suggest that this increase in variability is driven by elevation in the exponential (tau), but not the normal (sigma) component of the RT distribution (Andreou et al., 2007; Kuntsi et al., 2009; Vaurio et al., 2009).
Among children with ADHD, prolonged RTs within the exponential distribution (i.e., elevated tau) likely reflect excessive performance lapses or off-task behaviors (Vaurio et al., 2009). Indeed, ADHD-associated difficulty with sustaining performance, particularly in the context of a repetitive (and boring) go/no-go task, has been hypothesized to be due to difficulty inhibiting attention to more attractive stimuli. Experiments exploring the effects of variations in reward magnitude and immediacy show that individuals with ADHD demonstrate excessive “temporal discounting” with a decrease of subjective reward value as a function of increasing delay (Barkley, Edwards, Laneri, Fletcher, & Metevia, 2001; Bitsakou, Psychogiou, Thompson, & Sonuga-Barke, 2009; Scheres et al., 2006). Incentives improve performance in individuals with ADHD on go/no-go test by decreasing mean RT and variability (Uebel et al., 2010). Therefore, performance lapses (reflected in increased tau) among nonmedicated children with ADHD may reflect a tendency to discount the long-term goal of doing well on the task, and preferentially attend to a more immediately attractive reward, including environmental stimuli or one's own thoughts. Alternatively, increased tau may be due (at least in part) to the fact that the sustained effort required for the go/no-go task is more unpleasant for children with ADHD, such that the performance lapses reflect an escape from the aversive (boring) task.
We also hypothesized that the introduction of a moderate degree of interstimulus jitter would decrease response variability—especially among children with ADHD. The data also supported this hypothesis. Across groups, introduction of a moderate degree of jitter decreased response variability, including normal (sigma) and exponential (tau) components of the RT distribution. Furthermore, when considering the component of response variability that appears to be driven by performance (or motivational) lapses (i.e., tau), the introduction of jitter served to reduce these lapses among children with ADHD to a much greater degree than among controls, such that in the jittered test condition, there was no significant difference in tau among children with and without ADHD. Conversely, this same pattern of differential response to jitter among children with ADHD was not observed for the normal component of the RT distribution (sigma). Recent work by Epstein et al. (2011) and Uebel et al. (2010) suggest that event rate manipulation does not significantly affect ADHD performance differentially when compared with controls; however, these studies applied a fixed ISI (with faster or slower rates), rather than manipulating the ISI via jittering throughout an entire trial. The present findings imply that variability in stimulus presentation increases consistency of motor responses, resulting in decreased variability within the normal RT distribution curve and reduced lapses in performance (i.e., decreased tau) for individuals with ADHD. In sum, we found that jittering ISI decreased sigma and tau, resulting in greater consistency between RTs and a reduction in extreme lapses in on-task behavior. The effect of jitter was particularly strong for children with ADHD, as decreases in variability are principally caused by a reduction in the number of the outlier responses characteristic of poor response control in ADHD under fixed testing conditions. To our knowledge, this is the first study to apply an ex-Gaussian approach to demonstrate the effect of moderate ISI jitter in improving response control deficits in children with ADHD.
The Gaussian analysis of mean RTs does not identify any significant differences between groups (ADHD vs. controls), test conditions (fixed vs. jittered), or between conditions within each group, perhaps because the mean RT variable included outlier responses. In contrast, the ex-Gaussian analysis revealed lower mean RTs in the normal distribution (mu) for the ADHD group compared with controls when outlier responses were controlled. It therefore appears that after accounting for the increased occurrence of very slow RTs, children with ADHD actually show faster responses than controls on these go/no-go tasks. This faster response speed is associated with increased omission errors (suggesting a speed-accuracy trade-off), but only in the (boring) fixed ISI condition, and not when the ISI is jittered. Conversely, stimulus jittering does not affect the manifestation of the speed-accuracy trade-off for commission errors, as a significant association between faster RT and increased commissions was observed in both groups, regardless of task condition. Jittering may, however, produce a speed-variability trade-off, such that jittering induces decreased variability and fewer outlier RTs (lower tau), resulting in slower mean RT in the normal distribution (higher mu). Under the fixed condition, children with ADHD show a bias toward speed as opposed to consistency, with faster RTs in the normal distribution (lower mu), but greater variability with excessive outlier RTs (higher tau) as compared with controls. Thus, among children with ADHD, jittering appears to shift the bias in response control, increasing consistency by minimizing outlier RTs while decreasing mean RT.
Jittering appears to provide a nonpharmacologic mechanism to facilitate readiness to respond to a stimulus and reduce extreme momentary lapses from on-task behavior. This effect may be a function of increased activity of dopaminergic and/or noradrenergic circuits that maintain frontal networks critical to attention and response control. Both “top-down” mechanisms mediated by prefrontal circuitry and “bottom-up” mechanisms facilitated by external manipulation in tasks designed to increase vigilance through activation of noradrenergic projections of the locus coeruleus have been proposed (Berridge et al., 2006; Krawczyk, 2002; Sergeant, Geurts, Huijbregts, Scheres, & Oosterlaan, 2003). Jitter may also reduce variability through dopaminergic projections. There is substantial evidence that ADHD behaviors are associated with dysfunction of dopaminergic reward systems (Borger & van der Meere, 2000; Groom et al., 2010; Liddle et al., 2010; Rubia et al., 2009; Volkow et al., 2010). The current pattern of findings suggests that jitter may increase the novelty of stimulus presentation, thus increasing phasic dopamine release in anticipation of a reward, and for individuals with hypoactive dopaminergic reward systems (such as in ADHD). This effect is particularly beneficial for normalizing performance on a response-time task.
In summary, ADHD is a neurobiological abnormality associated with genetic and/or environmental influences (Castellanos et al., 2002). Mechanisms remain elusive because of the complex nature of these cognitive processes. Several critical executive function skills, including sustained attention, inhibition of off-task behavior, and preparedness to respond are intermediate steps between stimulus perception and the choice of when and how to respond (Denckla, 1996). Nonpharmacologic modulation of these interconnected networks may provide an alternative to pharmacologic therapy or serve as adjunct therapy. Many questions remain, including whether synergy exists between pharmacologic and nonpharmacologic interventions, and whether jittering affects RT variability in other disorders of cognition. The mechanisms underlying the jitter effect remain rich areas of study. Expanded models of the jitter paradigm include varying the degree of jitter and application of jitter to education-based learning strategies. Further studies should explore the effect of jitter on improving sustained attention (e.g., reading fluency) and other ADHD functional impairments.
Strengths of this study include the application of ex-Gaussian methodology, relevance for current biological models of ADHD, and the potential for application in the academic setting. Limitations of this study include the use of single-degree “moderate” jitter, rather than higher or lower degree of jitter, which may broaden the scope of applicable variability between presented stimuli. In addition, the ex-Gaussian approach to analysis of RT data does not take into account the impact of response accuracy on RT variability and, as such, may not fully characterize the psychological processes (attentional lapses) that it is intended to represent (Matzke & Wagenmakers, 2009). Finally, the present sample was relatively restrictive in its operationalization of ADHD, thus the generalizability of the present study's findings to children with ADHD and commonly cooccurring conditions such as language disorder or learning disability is questionable. The present sample was also not taking stimulant medication at the time of testing—Future research should investigate the impact of stimulant medication use on attentional lapses such as those identified in this study. Revealing abnormalities in the neural systems coordinating response preparation is critical for understanding the biological basis of ADHD. Although increasingly complex, our knowledge describing human cognition and behavior is advancing. The ex-Gaussian method represents one comprehensive approach for studying RT variability. As reported in this study, interstimulus jitter may be a good candidate for a nonpharmacologic approach to improve response preparation efficiency in ADHD. As our understanding of the influences underlying the genetic and physiologic substrates of attention and response control improves, application of novel test paradigms such as jitter may result in improved outcomes for individuals with ADHD.
Acknowledgment
The authors would like to thank Lisa Ferenc for her assistance with data management and recruitment.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health Grants P50 HD 52121, P30 HD 24061 (Intellectual and Developmental Disabilities Research Center), R01 MH078160, R01 MH085328, and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program, UL1-RR025005.
Biographies
Ryan W. Y. Lee, MD, is a faculty instructor in the Department of Neurology and Developmental Medicine at the Kennedy Krieger Institute and Johns Hopkins University School of Medicine. He received his MD at the John A. Burns School of Medicine, University of Hawaii, and completed residencies in pediatrics at the Kapiolani Medical Center for Women and Children, University of Hawaii, and Child Neurology and Neurodevelopmental Disabilities at the Kennedy Krieger Institute and Johns Hopkins Hospital. His primary interest is the study of neural underpinnings of cognitive delay, with a focus on neuroimaging approaches to study the impact of interventions on neurogenetic disorders.
Lisa A. Jacobson, PhD, is a clinical neuropsychologist and licensed psychologist in the Department of Neuropsychology at the Kennedy Krieger Institute in Baltimore, Maryland. She is also an instructor in the Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine. She received her PhD from the University of Virginia, Curry School of Education in 2008, and completed her postdoctoral fellowship in pediatric neuropsychology at the Kennedy Krieger Institute. She is also a nationally certified school psychologist. Her clinical and research interests focus on childhood disorders affecting the development of executive functions, particularly with regard to the effect of executive dysfunction on daily functioning within academic settings.
Alison E. Pritchard, PhD, is the director of the Neuropsychology Research Lab at the Kennedy Krieger Institute and a faculty instructor in the Department of Psychiatry and Behavioral Sciences at the Johns Hopkins University School of Medicine. Her research interests center on the effective identification and treatment of ADHD and learning disabilities among school-age children as well as the utility of classroom and testing accommodations for these populations of students.
Matthew S. Ryan, MS, is a psychology associate in the Department of Neuropsychology at the Kennedy Krieger Institute. He received his bachelor's degree from Washington and Jefferson College and master's degree in developmental psychology from the Johns Hopkins University. His areas of research include the neurobiology of ADHD, its effects on reading, and the investigation of early signs of ADHD.
Qilu Yu, PhD, is a research associate in the Division of Geriatrics and Gerontology at the Johns Hopkins Center on Aging and Health. Her research interests focus on the development and application of statistical methods for longitudinal data and population-based studies related to aging research, especially those with ignorable or nonignorable missing data.
Martha B. Denckla, MD, is the director of the Department of Developmental Cognitive Neurology and the Batza Family Endowed chair at the Kennedy Krieger Institute. She is also professor of neurology, pediatrics, and psychiatry at the Johns Hopkins University School of Medicine and professor of education at the Johns Hopkins University School of Education. She has 35 years clinical and research experience in working with children with ADHD, and has been among the pioneers in the use of neurobehavioral assessment and neuroimaging to study brain development in ADHD.
Stewart Mostofsky, MD, is the director of the Laboratory for Neurocognitive and Imaging Research and the medical director of the Center for Autism and Related Disorders at Kennedy Krieger Institute. In addition, he is an associate professor of Neurology and Psychiatry at Johns Hopkins University School of Medicine. For nearly two decades, he has applied behavioral, imaging, and electrophysiology approaches to examining the neural basis of, and treatment approaches for, developmental disorders, in particular ADHD and autism, with a particular focus on sensorimotor function.
E. Mark Mahone, PhD, ABPP, is the director of neuropsychology at the Kennedy Krieger Institute and an associate professor of psychiatry and behavioral sciences at the Johns Hopkins University School of Medicine. He received his PhD from the State University of New York at Albany in 1990 and completed his child neuropsychology residency at Boston Children's Hospital/Harvard Medical School in 1993. His professional career has emphasized clinical services for young children with neurodevelopmental disorders, training and mentoring of psychologists and physicians in working with young children, and research involving investigation of brain-behavior relationships in young children with and without neurodevelopmental disorders.
Footnotes
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, Kuntsi J. Reaction time performance in ADHD: Improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) Journal of Abnormal Child Psychology. 2001;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, Defries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJ. Delay aversion in attention deficit/hyperactivity disorder: An empirical investigation of the broader phenotype. Neuropsychologia. 2009;47:446–456. doi: 10.1016/j.neuropsychologia.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Borger N, van der Meere J. Motor control and state regulation in children with ADHD: A cardiac response study. Biological Psychology. 2000;51:247–267. doi: 10.1016/s0301-0511(99)00040-x. [DOI] [PubMed] [Google Scholar]
- Buzy WM, Medoff DR, Schweitzer JB. Intra-individual variability among children with ADHD on a working memory task: An ex-Gaussian approach. Child Neuropsychology. 2009;15:441–459. doi: 10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;228:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Biological correlates of learning and attention: What is relevant to learning disability and attention deficit/hyperactivity disorder? Journal of Developmental & Behavioral Pediatrics. 1996;17:1–6. [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, Castellanos FX. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;64:607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Anonini TN, Altaye M. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Grasman RP, Verté S, Oosterlaan J, Roeyers H, van Kammen SM, Sergeant JA. Intra-individual variability in ADHD, autism spectrum disorders and Tourette's syndrome. Neuropsychologia. 2008;46:3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Gomez-Guerrero L, Martin CD, Mairena MA, Di Martino A, Wang J, Mendelsohn AL, Castellanos FX. Response-time variability is related to parent ratings of inattention, hyperactivity, and executive function. Journal of Attention Disorders. 2010;15:572–582. doi: 10.1177/1087054709356379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom MJ, Scerif G, Liddle PF, Batty MJ, Liddle EB, Roberts KL, Hollis C. Effects of motivation and medication on electrophysiological markers of response inhibition in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;67:624–631. doi: 10.1016/j.biopsych.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EL, Schuerholz LJ, Singer HS, Reader MJ, Brown JE, Cox C, Denckla MB. Executive function in children with Tourette syndrome and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 1995;1:511–516. doi: 10.1017/s1355617700000631. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychology. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Jepsen JR, Fagerlund B, Mortensen EL. Do attention deficits influence IQ assessment in children and adolescents with ADHD? Journal of Attention Disorders. 2009;12:551–562. doi: 10.1177/1087054708322996. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience & Biobehavioral Reviews. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, van der Meere J, Asherson P. Why cognitive performance in ADHD may not reveal true potential: Findings from a large population-based sample. Journal of the International Neuropsychological Society. 2009;15:570–579. doi: 10.1017/S135561770909081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture Y, Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology. 2008;4:35–45. [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, Liddle PF. Task-related default mode network modulation and inhibitory control in ADHD: Effects of motivation and methylphenidate. Journal of Child Psychology and Psychiatry. 2010;52:761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D, Wagenmakers E-J. Psychological interpretation of the ex-Gaussian and shifted Wald parameters: A diffusion model analysis. Psychonomic Bulletin & Review. 2009;16:798–817. doi: 10.3758/PBR.16.5.798. [DOI] [PubMed] [Google Scholar]
- McAuley T, Yap M, Christ SE, White DA. Revisiting inhibitory control across the life span: Insights from the ex-Gaussian distribution. Developmental Neuropsychology. 2006;29:447–458. doi: 10.1207/s15326942dn2903_4. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Altink ME, Oosterlaan J, Beem L, Buschgens CJ, Buitelaar J, Sergeant JA. Speed, variability, and timing of motor output in ADHD: Which measures are useful for endophenotypic research? Behavioral Genetics. 2008;38:121–132. doi: 10.1007/s10519-007-9186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalizes activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Ryan M, Martin R, Denckla MB, Mostofsky SH, Mahone EM. Interstimulus jitter facilitates response control in children with ADHD. Journal of the International Neuropsychological Society. 2010;16:388–393. doi: 10.1017/S1355617709991305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: Effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. On the top and bottom of ADHD: A neuropsychological perspective. Neuroscience & Biobehavioral Reviews. 2003;27:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: The frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Börger NA, Butler L, Chen W, Banaschewski T. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, Swanson JM. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Molecular Psychiatry. 2010;16:1147–1154. doi: 10.1038/mp.2010.97. doi:10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Simmonds DJ, Mahone EM, Mostofsky SH. Moderate variability in stimulus presentation improves motor response control. Journal of Clinical and Experimental Neuropsychology. 2009;31:483–488. doi: 10.1080/13803390802272036. [DOI] [PMC free article] [PubMed] [Google Scholar]