Abstract
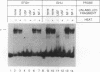
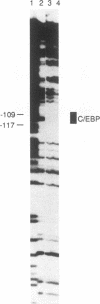
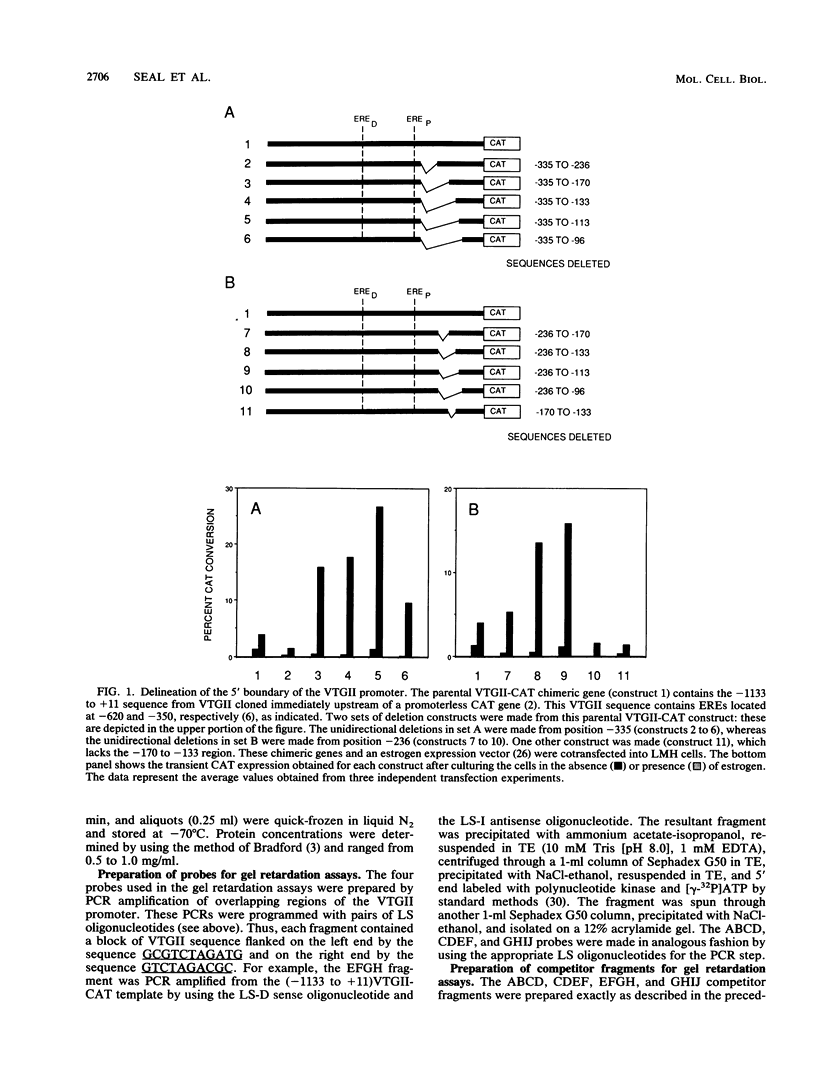
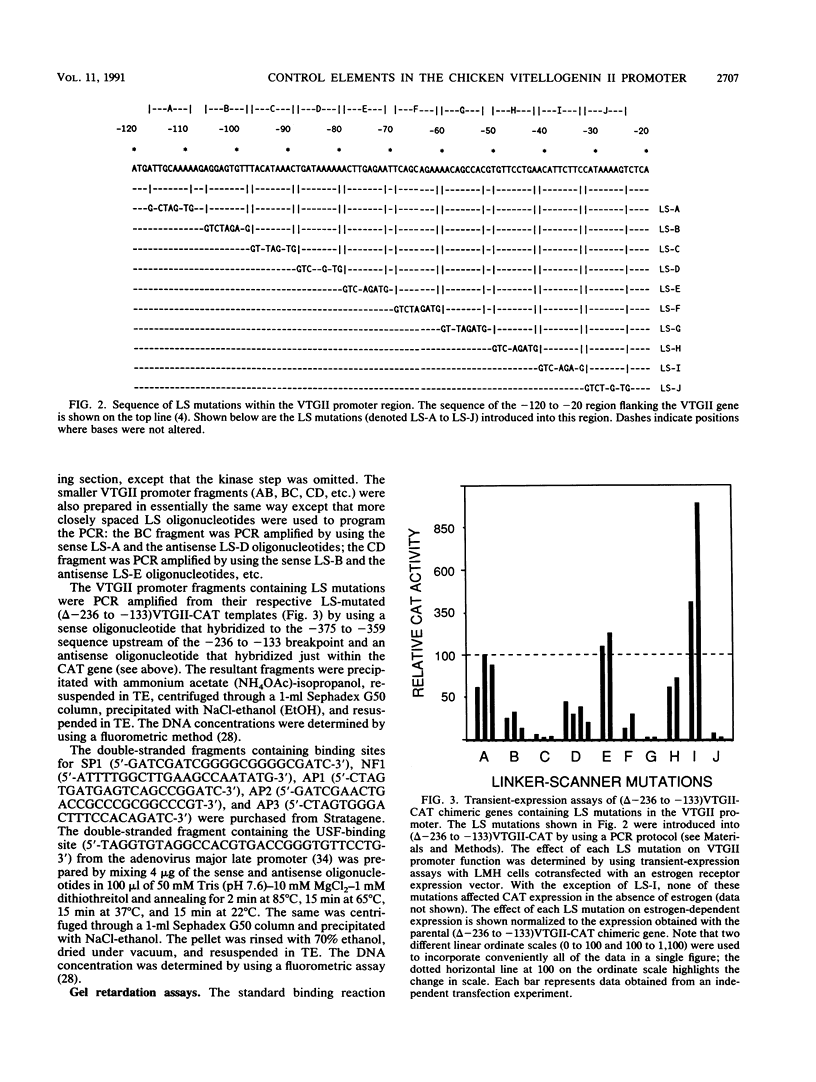
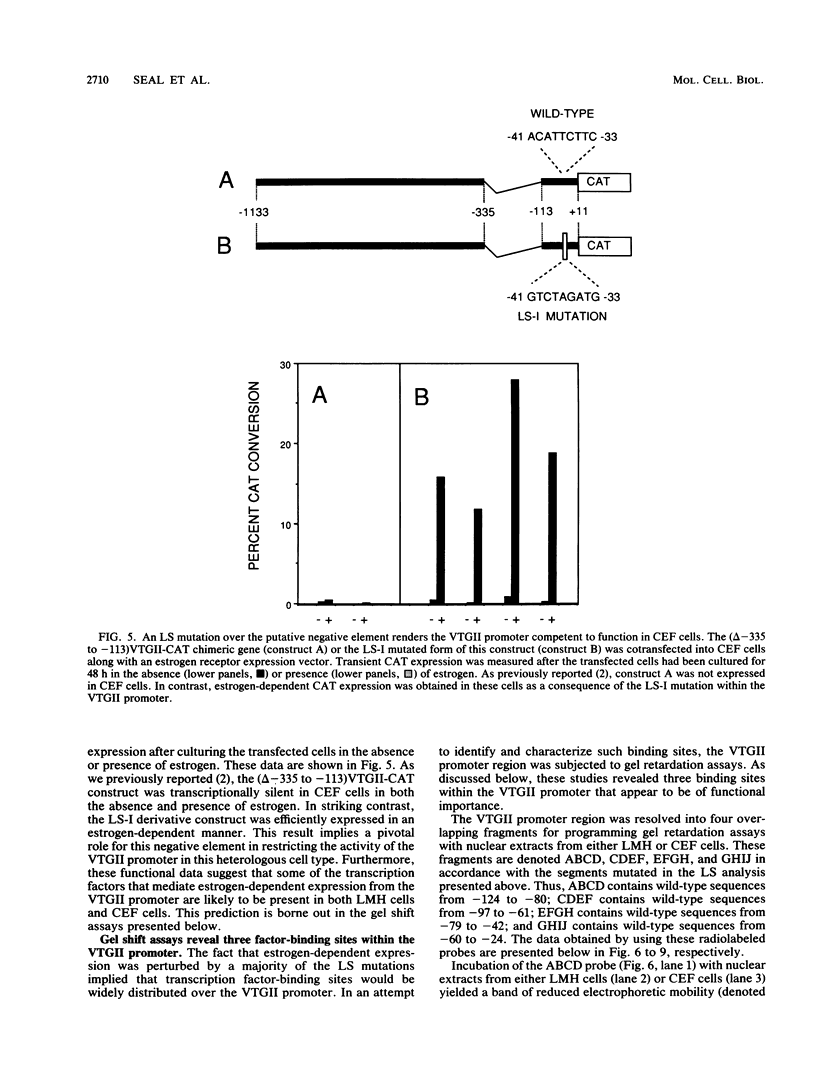
The endogenous chicken vitellogenin II (VTGII) gene is transcribed exclusively in hepatocytes in response to estrogen. We previously identified two estrogen response elements (EREs) upstream of this gene. We now present an analysis of the VTGII promoter activated by these EREs in response to estrogen. Chimeric VTGII-CAT genes were cotransfected into LMH chicken hepatoma cells along with an estrogen receptor expression vector, and transient CAT expression was assayed after culturing the cells in the absence or presence of estrogen. An analysis of constructs bearing deletions downstream of the more proximal ERE indicated that promoter elements relevant to transcription in LMH cells extend to between -113 and -96. The relative importance of sequences within the VTGII promoter was examined by using 10 contiguous linker scanner mutations spanning the region from -117 to -24. Although most of these mutations compromised VTGII promoter function, one dramatically increased expression in LMH cells and also rendered the VTGII promoter capable of being activated by cis-linked EREs in fibroblasts cotransfected with an estrogen receptor expression vector. Gel retardation and DNase I footprinting assays revealed four factor-binding sites within this promoter. We demonstrate that three of these sites bind C/EBP, SP1, and USF (or related factors), respectively; the fourth site binds a factor that we denote TF-V beta. The biological relevance of these findings is suggested by the fact that three of these binding sites map to sites previously shown to be occupied in vivo in response to estrogen.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergink E. W., Kloosterboer H. J., Gruber M., Ab G. Estrogen-induced phosphoprotein synthesis in roosters. Kinetics of induction. Biochim Biophys Acta. 1973 Feb 4;294(1):497–506. doi: 10.1016/0005-2787(73)90105-6. [DOI] [PubMed] [Google Scholar]
- Binder R., MacDonald C. C., Burch J. B., Lazier C. B., Williams D. L. Expression of endogenous and transfected apolipoprotein II and vitellogenin II genes in an estrogen responsive chicken liver cell line. Mol Endocrinol. 1990 Feb;4(2):201–208. doi: 10.1210/mend-4-2-201. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burch J. B., Evans M. I. Chromatin structural transitions and the phenomenon of vitellogenin gene memory in chickens. Mol Cell Biol. 1986 Jun;6(6):1886–1893. doi: 10.1128/mcb.6.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Evans M. I., Friedman T. M., O'Malley P. J. Two functional estrogen response elements are located upstream of the major chicken vitellogenin gene. Mol Cell Biol. 1988 Mar;8(3):1123–1131. doi: 10.1128/mcb.8.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Fischer A. H. Chromatin studies reveal that an ERE is located far upstream of a vitellogenin gene and that a distal tissue-specific hypersensitive site is conserved for two coordinately regulated vitellogenin genes. Nucleic Acids Res. 1990 Jul 25;18(14):4157–4165. doi: 10.1093/nar/18.14.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B. Identification and sequence analysis of the 5' end of the major chicken vitellogenin gene. Nucleic Acids Res. 1984 Jan 25;12(2):1117–1135. doi: 10.1093/nar/12.2.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. C., Shapiro D. J. An NF1-related vitellogenin activator element mediates transcription from the estrogen-regulated Xenopus laevis vitellogenin promoter. J Biol Chem. 1990 May 15;265(14):8176–8182. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Corthésy B., Cardinaux J. R., Claret F. X., Wahli W. A nuclear factor I-like activity and a liver-specific repressor govern estrogen-regulated in vitro transcription from the Xenopus laevis vitellogenin B1 promoter. Mol Cell Biol. 1989 Dec;9(12):5548–5562. doi: 10.1128/mcb.9.12.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthésy B., Léonnard P., Wahli W. Transcriptional potentiation of the vitellogenin B1 promoter by a combination of both nucleosome assembly and transcription factors: an in vitro dissection. Mol Cell Biol. 1990 Aug;10(8):3926–3933. doi: 10.1128/mcb.10.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst S., Embretson J. E., Anderson D. C., Mullins J. I., Fultz P. N. Sequence analysis and acute pathogenicity of molecularly cloned SIVSMM-PBj14. Nature. 1990 Jun 14;345(6276):636–640. doi: 10.1038/345636a0. [DOI] [PubMed] [Google Scholar]
- Döbbeling U., Ross K., Klein-Hitpass L., Morley C., Wagner U., Ryffel G. U. A cell-specific activator in the Xenopus A2 vitellogenin gene: promoter elements functioning with rat liver nuclear extracts. EMBO J. 1988 Aug;7(8):2495–2501. doi: 10.1002/j.1460-2075.1988.tb03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbrecht A., Lazier C. B., Protter A. A., Williams D. L. Independent developmental programs for two estrogen-regulated genes. Science. 1984 Aug 10;225(4662):639–641. doi: 10.1126/science.6740331. [DOI] [PubMed] [Google Scholar]
- Evans M. I., O'Malley P. J., Krust A., Burch J. B. Developmental regulation of the estrogen receptor and the estrogen responsiveness of five yolk protein genes in the avian liver. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8493–8497. doi: 10.1073/pnas.84.23.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. I., Silva R., Burch J. B. Isolation of chicken vitellogenin I and III cDNAs and the developmental regulation of five estrogen-responsive genes in the embryonic liver. Genes Dev. 1988 Jan;2(1):116–124. doi: 10.1101/gad.2.1.116. [DOI] [PubMed] [Google Scholar]
- Gordon D. A., Shelness G. S., Nicosia M., Williams D. L. Estrogen-induced destabilization of yolk precursor protein mRNAs in avian liver. J Biol Chem. 1988 Feb 25;263(6):2625–2631. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Moncharmont B., Jiricny J., Saluz H., Hertner T. In vitro secondary activation (memory effect) of avian vitellogenin II gene in isolated liver nuclei. Proc Natl Acad Sci U S A. 1986 Jan;83(1):43–47. doi: 10.1073/pnas.83.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Ohno T., Panyim S., Schuerch A. R. Appearance of vitellogenin mRNA sequences and rate of vitellogenin synthesis in chicken liver following primary and secondary stimulation by 17 beta-estradiol. Eur J Biochem. 1978 Mar 15;84(2):355–361. doi: 10.1111/j.1432-1033.1978.tb12175.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Nomura K., Hirayama Y., Kitagawa T. Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 1987 Aug 15;47(16):4460–4464. [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Philipsen J. N., Hennis B. C., Ab G. In vivo footprinting of the estrogen-inducible vitellogenin II gene from chicken. Nucleic Acids Res. 1988 Oct 25;16(20):9663–9676. doi: 10.1093/nar/16.20.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud J. P., Bouton M. M., Gallet-Bourquin D., Philibert D., Tournemine C., Azadian-Baulanger G. Comparative study of estrogen action. Mol Pharmacol. 1973 Jul;9(4):520–533. [PubMed] [Google Scholar]
- Ryden T. A., Beemon K. Avian retroviral long terminal repeats bind CCAAT/enhancer-binding protein. Mol Cell Biol. 1989 Mar;9(3):1155–1164. doi: 10.1128/mcb.9.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Walker P., Martinez E., Mérillat A. M., Givel F., Wahli W. Identification of estrogen-responsive DNA sequences by transient expression experiments in a human breast cancer cell line. Nucleic Acids Res. 1986 Nov 25;14(22):8755–8770. doi: 10.1093/nar/14.22.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R., Fischer A. H., Burch J. B. The major and minor chicken vitellogenin genes are each adjacent to partially deleted pseudogene copies of the other. Mol Cell Biol. 1989 Aug;9(8):3557–3562. doi: 10.1128/mcb.9.8.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro M., Pawlak A., Jost J. P. Positive and negative regulatory elements of chicken vitellogenin II gene characterized by in vitro transcription competition assays in a homologous system. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3047–3051. doi: 10.1073/pnas.87.8.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P., Germond J. E., Brown-Luedi M., Givel F., Wahli W. Sequence homologies in the region preceding the transcription initiation site of the liver estrogen-responsive vitellogenin and apo-VLDLII genes. Nucleic Acids Res. 1984 Nov 26;12(22):8611–8626. doi: 10.1093/nar/12.22.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J., Philipsen J. N., Ab G. Tissue-specific and steroid-dependent interaction of transcription factors with the oestrogen-inducible apoVLDL II promoter in vivo. EMBO J. 1988 Sep;7(9):2757–2763. doi: 10.1002/j.1460-2075.1988.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A., Seldran M., Jost J. P. An estrogen-dependent demethylation at the 5' end of the chicken vitellogenin gene is independent of DNA synthesis. Nucleic Acids Res. 1984 Jan 25;12(2):1163–1177. doi: 10.1093/nar/12.2.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S., Liu J. K., DiPersio C. M. Site-directed mutagenesis reveals a liver transcription factor essential for the albumin transcriptional enhancer. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5469–5473. doi: 10.1073/pnas.87.14.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]