Abstract
Plasma hemoglobin (Hb) released during intravascular hemolysis has been associated with numerous deleterious effects that may stem from increased nitric oxide (NO) scavenging, but has also been associated with reactive oxygen species generation and platelet activation. Therapies that convert plasma oxyHb to metHb, or metHb to iron-nitrosyl Hb, could be beneficial because these species do not scavenge NO. In this study, we investigated the effects of Angeli’s Salt (AS, sodium α-oxyhyponitrite, Na2N2O3), a nitroxyl (HNO) and nitrite (NO2−) donor, on plasma Hb oxidation and formation of iron-nitrosyl Hb from metHb, and on the vasoactivity of plasma Hb. We hypothesized that AS could ameliorate hemolysis-associated pathology via its preferential reactivity with plasma Hb, as opposed to red cell encapsulated Hb, and through its intrinsic vasodilatory activity. To test this hypothesis, we infused (n=3 per group) (1) cell-free Hb and AS, (2) cell-free Hb + 0.9% NaCl, (3) AS + 3% Albumin, and (4) 3% Albumin + 0.9% NaCl (colloid controls for Hb and AS, respectively) in a canine model. Co-infusion of AS and cell-free Hb led to preferential conversion of plasma Hb to metHb, but the extent of conversion was lower than anticipated based on the in vivo concentration of AS relative to plasma Hb. This lower metHb yield was likely due to reactions of nitroxyl-derived AS with plasma components such as thiol-containing compounds. From a physiological and therapeutic standpoint, the infusion of Hb alone led to significant increases in mean arterial pressure (p=0.03) and systemic vascular resistance index (p=0.01) compared to controls. Infusion of AS alone led to significant decreases in these parameters and co-infusion of AS along with Hb had an additive effect on reversing the effects of Hb alone on the systemic circulation. Interestingly, in the pulmonary system, the decrease in pressure when AS is added to Hb was significantly less than would have been expected compared to the effects of Hb and AS alone suggesting that inactivation of scavenging with AS reduced the direct vasodilatory effects of AS on the vasculature. We also found that, AS reduced platelet activation when administered to whole blood in vitro. These data suggest that AS-like compounds could serve as a therapeutic agent to counteract the negative vasoconstrictive consequences of hemolysis that occur in hemolytic anemia’s, transfusion of stored blood, and other diseases. Increases in metHb in the red blood cell, the potential of AS for neurotoxicity, and hypotension would need to be carefully monitored in a clinical trial.
Keywords: Angeli’s Salt, cell-free hemoglobin, methemoglobin, nitroxyl
Introduction
Nitric oxide (NO) is an important signaling molecule that regulates vascular tone, and inhibits both platelet activation and expression of endothelial adhesion molecules [1–8] [9–11]. Endothelial NO synthase produces NO which diffuses to the smooth muscle where it activates soluble guanylyl cyclase. However, the diffusional radius of NO from the source of production in the endothelium to the receptor in the smooth muscle is limited by reactions with hemoglobin (Hb) in the blood stream [12]. Hb rapidly reacts with NO with a rate constant of 5 × 107 M−1s−1 via the “dioxygenation” reaction [13–15]
| (1) |
where HbFeIIO2 refers to oxygenated ferrous (iron valence state of +2) Hb (oxyHb) and HbFeIII refers to the oxidized ferric (+3) form or methemoglobin (metHb). This reaction converts NO to nitrate, which is essentially irreversible, and eliminates the NO signaling potential.
The high concentration of Hb in the blood, approximately 10 mM heme at physiological hematocrit, contained within red cells presented a paradox for how NO could signal without being scavenged [12]. Theoretical calculations and experiments with free Hb indicate that in the presence of 10 mM Hb (concentrations in terms of heme), NO concentrations are close to zero. This paradox of how NO signals in the background of so much Hb is resolved by the fact that when Hb is encapsulated in the red blood cell it scavenges much less than when it is free in plasma. This is mainly due to the fact that the reaction of NO and red blood cell encapsulated Hb is rate limited by the time it takes for NO to diffuse to the red blood cell [16–19], an effect largely determined by a cell free zone between the endothelium and red cell and by the unstirred layer around red cells, though NO diffusion across the red cell membrane may also contribute to the rate limitation of the reaction [18–20]. Through these mechanisms, red blood cell encapsulated Hb scavenges NO up to 1000 times slower than does cell-free (or plasma) Hb. Importantly, all of these diffusional barriers are disrupted by hemolysis increasing the concentration of plasma Hb. Indeed, computational simulations suggest that as little as 1 μM plasma Hb can decrease NO bioavailability [21], and experiments in vitro and in vivo show that 6 μM heme in Hb is sufficient to inhibit NO-dependent vasodilation [22,23]. While it is possible that much of the NO scavenging is occurring extravascularly, the effect of cell-free Hb on limiting NO bioavailability is increased when the Hb extravasates [21].
Efficient scavenging of NO by plasma Hb has been postulated to be largely responsible for hypertension and increased vascular resistance associated with use of Hb based oxygen carrier “blood substitutes” [24–29]. In addition, there is evidence that NO scavenging by plasma Hb also contributes to pathology in hemolytic anemia’s including sickle cell disease [30–34] as well as upon transfusion of older stored blood [22,35,36]. However, the extent of the contribution of plasma Hb to pathology in these conditions through NO scavenging has not been settled [37–39]. Some have argued that the amount of plasma Hb in sickle cell disease or similar conditions is too low to contribute substantially to pathology [37], while others have suggested that other mechanistic routes such as oxidative damage may constitute the major pathways for deleterious effects of plasma Hb [38,39].
One test of the hypothesis that NO scavenging from plasma Hb is largely responsible for vascular pathology is to inactivate or diminish the NO scavenging ability of the plasma Hb. Administration of exogenous NO preferentially converts oxyHb to metHb via Equation 1, with only a tiny fraction of red cell encapsulated Hb being affected. MetHb does not scavenge NO like oxyHb, and would thus be expected to be less vasoactive. Indeed, inhaled NO was demonstrated to decrease NO consumption by plasma Hb in patients with sickle cell disease [31]. In addition, inhaled NO normalized hemolysis-induced increases in mean arterial pressure (MAP) and systemic vascular resistance index (SVRI) in a canine model [40]. This effect of inhaled NO was associated with preferential conversion of oxyHb to metHb and a reduction in NO consumption by plasma containing Hb [40]. These data suggest that plasma and/or extravasated Hb scavenges NO, which results in vasoconstriction and contributes to the pathology of hemolytic diseases.
Nitrite has also been shown to counteract vasoconstriction and its consequences in a canine hemolysis model [41]. The action of nitrite can be attributed to both its intrinsic vasodilatory potential [42] as well as its reactivity with deoxygenated and oxygenated Hb to form metHb [43–47].
| (2) |
| (3) |
The vasodilatory action of nitrite has been proposed to derive from its reaction with deoxygenated Hb to form NO (Equation 3) [42].
In this study we explore the ability of Angeli’s Salt (AS, sodium α-oxyhyponitrite, Na2N2O3) to counter the negative hemodynamic effects of plasma and/or extravasated Hb. AS spontaneously decomposes into nitrite and nitroxyl (HNO).
| (4) |
Nitroxyl reacts rapidly with oxyHb to form metHb and NO with a rate constant on the order of 107 M−1s−1.
| [ 48,49] | (5) |
The NO formed in this reaction can then oxidize another oxyHb molecule via Equation 1 so that one HNO molecule oxidizes two oxyHb molecules to form two metHb molecules.
| (6) |
HNO also reacts with metHb to form a ferrous heme NO adduct (iron-nitrosyl Hb) with a rate constant of about 106 M−1s−1 (assuming similar kinetics as in the case of myoglobin) [49].
| (7) |
Nitrite released from AS is also expected to react with oxyHb via Equation 2.
We hypothesized that AS would alleviate hemolysis-induced vasoconstriction based on these three potential mechanisms of action: (1) The AS-derived HNO preferentially converts plasma oxyHb to metHb, [50] (2) HNO can then further react with metHb to form nitrosyl Hb via Equation 7, [49] and (3) HNO and nitrite have inherent vasodilatory properties [42,51]. Any molecule that reacts quickly with Hb (with a rate constant of 107 M−1s−1 or greater) is expected to react preferentially with plasma Hb as opposed to red cell encapsulated Hb, and we have previously demonstrated this to be the case for AS-derived HNO in mixtures of plasma and red cell encapsulated Hb [50]. Conversion of metHb to nitrosyl Hb could be beneficial by attenuating down-stream oxidative redox reactions of metHb. Finally, in addition to hypoxia-induced vasorelaxation from nitrite, HNO (from AS and other sources) has been shown to act as a vasodilator [51]. Thus, we tested the biochemical and physiological effects of AS infusion during cell-free Hb infusions in instrumented dogs, allowing for frequent blood sample collection for reaction product analysis and full hemodynamic assessments at multiple time points.
MATERIALS AND METHODS
Experimental Design
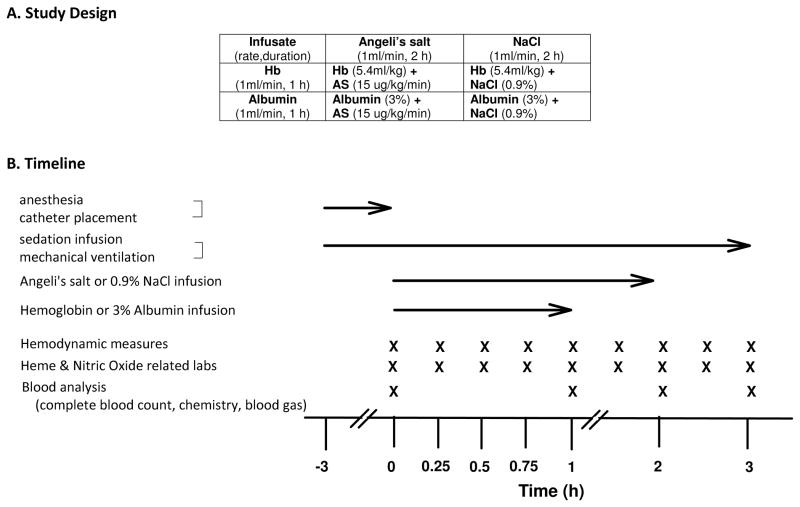
All experiments were approved by the Animal Care and Use Committee of the Clinical Center at the National Institutes of Health. Twelve purpose-bred beagles (1–2 yr, 6.65–8.3 kg) were studied over 3 h.
Animals were randomized to one of four experimental groups (n=3 per group): (1) cell-free Hb and AS, (2) cell-free Hb + NaCl, (3) AS + Albumin, and (4) albumin + NaCl (controls for Hb and AS, respectively)(Figure 1A). Hemoglobin (canine), prepared as described previously[40], was infused at 0.266 mM/kg/min (in heme) for one hour or an equivalent volume infusion of 3% Human Albumin (Alb) given their similar molecular weights (64kd vs. 67kd, respectively). AS was infused at 15μg/kg/min for 2 hours or a molar equivalent volume of 0.9% NaCl (Hospira, Lake Forest, IL)(Figure 1B). To ensure the integrity of the AS, the solution was prepared in an alkaline (10 mM NaOH, pH = 11–12) and confirmed that no peak was present at 212 nm (no significant decomposition of AS to nitrite). Assuming a blood volume of 80 mL/Kg, the total Hb infused was targeted to reach (0.266 μmoles/kg/min)(60 minutes)/(0.08L/kg) = 200 μM assuming no clearance. The amount of AS infused, if none were consumed, was targeted to be (15 μg/kg/min)(120 min)/(122 μg/μmole) (0.08 L/kg) = 184 μM, using a molecular weight of 122.
Figure 1.
Protocol timeline
If one HNO molecule results in two metHb molecules (that is two ferric hemes), one would need 100 μM AS to convert the 200 μM oxygenated Hb (measured in heme concentration and not including any converted by nitrite) and this would be achieved 65 minutes after the start of the infusion.
Two control groups (3% Albumin and 0.9% NaCl) were used to account for colloid osmotic effects of Hb and AS, respectively. Measurements during the 3 h experiment were obtained before infusions started at time 0 h, during the Hb (Alb) infusions from 0 to 1 h, and for 2 h after the infusion was completed.
On the day of the study, anesthesia was induced via mask inhalation using isoflurane (1–5%) and the animals were then intubated (6 mm, Rusch, Deluth, GA) and mechanically ventilated (Servo-I, Maquet, Wayne, NJ) (fractional inspired oxygen = 50%, positive end expiratory pressure = 5 cm H20, ventilation rate = 15 breaths/minute, tidal volume = 20ml/kg) for the duration of the study. Femoral arterial (20-gauge), external jugular venous (8-French) and radial venous (18-gauge) catheters (Maxxim Medical, Athens, TX) were placed percutaneously using aseptic techniques. Foley urinary catheters (Cook, Foley 8 Fr, 55 cm) were also placed in all animals using aseptic techniques. After catheter placement, the anesthetic gas was discontinued and continuous infusions of midazolam (2.5–5 μg/kg/min) and fentanyl (0.16 μg/kg/min) were initiated and maintained for the duration of the study.
Sedation Protocol
The level of sedation was evaluated continuously after initiation of the midazolam and fentanyl infusion. Criteria for adequacy of sedation: 1) breathing comfortably but without voluntary limb movement. 2) eyes remain central in the orbit. 3) unresponsive to light tactile stimuli, i.e. leg movement. Any animals not meeting these minimal criteria were given a bolus of midazolam (15 mg) and an increase in midazolam infusion rate (50 μg/min). Criteria were also used for reducing midazolam sedation infusion rate by 50 μg/min: 1) Palpebral reflexes not present. 2) The animal not responsive to painful stimuli (toe squeeze).
Data Collection
MAP and heart rate (HR) were obtained from the femoral artery catheter. A pulmonary artery thermodilution catheter (7-French, Abbott Critical Care, Chicago, IL) was introduced through the external jugular vein catheter to measure central venous pressure (CVP), pulmonary artery occlusion pressure (PAOP), and determine cardiac output (CO). SVRI was calculated (MAP − CVP)/CI where CI = CO/weight (kg) as was pulmonary vascular resistance index (PVRI) calculated (PAP − PAOP)/CI. Stoke volume index (SVI) = CI/HR.
The amount of total plasma Hb and percent of oxygenated Hb were obtained by spectral deconvolution of absorption spectra as described previously [40]. In some cases an integrating sphere detector (Varian Cary 100 or Perkin Elmer Lambda 9 spectrometer) was used to minimize effects of light scattering. The amount of metHb in both whole blood and plasma fractions was determined by electron paramagnetic resonance (EPR) spectroscopy (Bruker EMX 10/12) operating at 9.4 GHz using 15-G modulation, 10.1-milliwatt power, 81.92-ms time constant, and 41.94-s scan over 700 G at 5 K using liquid helium. Measurements of nitrosyl Hb were also made using EPR. The spectra were collected at 110 K operating at 9.4 GHz, 5-G modulation, 10.1-milliwattpower, with a 81.92-ms time constant, and 83.8-s scans over 1000 G.
Concentrations of Hb in plasma were obtained after sedimentation of red blood cells. The concentration of plasma Hb in the whole blood volume is related to that obtained in the supernatant by a factor of 1-Hct, where Hct is the fractional hematocrit. The average hematocrit was 39% in our experiments. Red cell metHb is reported as the average intra-erythrocytic concentration. It is calculated as METRBC = (METWB − (1-Hct)*METP)/Hct, where, METWB = MetHb concentration in whole blood, METP = MetHb concentration in plasma, and METRBC = MetHb concentration in red blood cells.
Hemodynamic measurements, blood draws, and spectrophotometric-based quantification of plasma Hb concentration were obtained at 0-, 0.25-, 0.5-, 0.75-, 1.0-, 1.5-, 2.0-, 2.5-, and 3.0-h time points. Laboratory measurements (complete blood count, serum chemistries, and arterial blood gas analysis) were obtained at 0, 1, 2, 3 h. After the study was completed, while still sedated, all animals were euthanized (Beuthanol, 75 mg/kg IV).
In Vitro Experiments
Chemicals were obtained from Sigma Chemicals unless otherwise noted. AS (50 μM, Cayman Chemical, Ann Arbor, MI) was added to 1 mM Hb (in heme) (Interstate Blood Bank, Memphis, TN) and incubated at 37°C for 2 hours. Aliquots of the samples were collected after two hours and frozen in liquid nitrogen for determination of metHb using EPR as described above, and some samples were analyzed for metHb concentration using absorption spectroscopy. The Hb (human) was either purified and diluted in PBS, purified and diluted in plasma, encapsulated in red blood cells suspended in PBS, or encapsulated in red blood cells contained in whole blood and diluted in plasma. Samples were also made using Hb in PBS with the addition of 10mM glutathione (GSH) or 5g/dL bovine serum Alb (~750 μM), and Hb in plasma that was incubated with 10mM N-Ethylmaleimide (NEM) at 37°C for 1 hour prior to use with Hb. MetHb levels are reported after subtraction of that formed by control experiments where 50 μM nitrite was added to a similar set of samples. Thus, the reported levels are those due to reaction with nitroxyl alone.
Platelet activation and cytokine production from whole blood samples
Blood obtained from healthy volunteers with IRB approval was collected into 0.109M sodium citrate at a ratio of 9:1. Blood used in a single trial came from a single donor; however, different donors were used for the multiple repeats of these experiments. Under sterile conditions, 4.3 mM Angeli’s salt solution was infused into the blood. A 4.3mM solution of NaCl was infused into a control sample of blood. Infusion occurred over two hours, to a final concentration of 184μM AS or NaCl. Samples were held at 20°C and placed on a rocking plate. After two hours, aliquots were removed to assess platelet activation. Some aliquots of the remainder were combined with lipopolysaccharide (LPS-EB ultrapure isolated from E. coli 0111:B4, InvivoGen) at a final concentration of 200 ng/mL. Samples containing AS and LPS, AS alone, NaCl and LPS, or NaCl alone were incubated at 37°C for six hours. Samples were under air containing 5% CO2 and were mixed occasionally. Following incubation, samples were centrifuged and plasma was removed and frozen on dry ice to assess cytokine production. Cytokine levels in plasma samples were determined on a Bio-plex Protein Array System (Bio-Rad Laboratories Inc., Hercules, CA) using Bio-Plex Precision Pro human cytokine 3-plex assay panel. Interleukin-1 beta (IL-1b), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) were simultaneously determined according to the manufacturer’s instructions.
For measurements of platelet activation, 1 ml of whole blood was centrifuged at 100 g for 10 minutes, and platelet rich plasma (PRP) was removed and diluted 1:10 in PBS, pH 7.4. ADP concentrations of 10 nM, 100 nM, 1000 nM and 2500 nM were added to the diluted PRP. Samples were incubated for 10 minutes at room temperature followed by transfer into FITC-PAC-1 and PerCp-CD61 antibodies for 15 minutes in the dark, then diluted 1:25 in 1% formaldehyde. A BD FACS Calibur flow cytometer and Cell Quest Pro software were used for data collection and analysis. The activation threshold was set so 99% of the baseline platelets were beneath the threshold.
Statistical Analysis
SAS version 9.2 (Cary, NC) was used for all analyses. The rates of in vivo hemoglobin concentration changes were analyzed using linear mixed models (SAS PROC MIXED) to account for repeated measures. The slopes were allowed to be different after one hour for OxyHb (due to stopping OxyHb infusion) and after two hours for MetHb (due to stopping AS infusion). In vitro MetHb concentrations were analyzed using one-way analysis of variance. Log-transformation was used to satisfy model assumptions (e.g. normality, equal variance). Changes from baseline values for systemic blood pressures (MAP, SVRI, CVP, PAOP), cardiac parameters (CI, HR, SVI), and pulmonary parameters (PAP, PVRI) were analyzed with linear mixed models to assess the effects of AS, cell-free Hb and their interaction. Contrasts were constructed to evaluate the effects of AS in the presence and absence of cell-free Hb, and the effects of cell-free Hb treatment in the presence and absence of AS. Platelet activation and cytokine production from white blood cells were analyzed similarly using linear mixed models. Random effects were incorporated to account for repeated measures. Standard diagnostics were performed to assess the assumptions of the model (e.g. normality, homoskedasticity). All two-sided p-values ≤ 0.05 are considered significant.
Results
Levels and redox status of plasma Hb with and without AS co-infusions
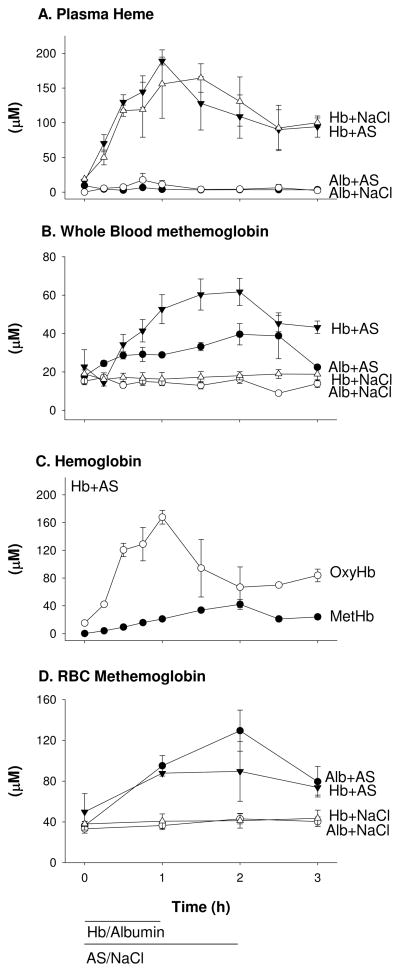
The two groups receiving similar cell-free Hb infusions with either AS or NaCl nearly reached the targeted level of 200 μM (in heme) after 1 h and then declined over the next 2 h to 100 μM (Figure 2A). In control animals where Alb was infused instead of Hb, an insignificant amount (generally less than 5 μM) of plasma Hb was measured. To determine if metHb was being produced in the intravascular space, whole blood metHb was measured (Figure 2B). Infusion of NaCl with either Hb or Alb resulted in no increase in metHb from baseline (P=0.6 for differences in slope). Infusion of Hb with AS led to an increase in whole blood metHb reaching a maximum of 62 μM after 2 h (P<0.0001 for differences in slope).
Figure 2.
Hemoglobin concentrations and species. (A) Total plasma heme levels in animals co-infused with cell-free Hb and AS, cell-free Hb and NaCl, Alb and AS, Alb and NaCl determined by absorption spectroscopy and spectral deconvolution fitting. (# of data points analyzed at a given treatment time point; n=3 for 23, n=2 for 9, and n=1 for 4). (B) Whole blood metHb levels in animals receiving the above infusions determined by EPR spectroscopy. (# of data points analyzed at a given treatment time point; n=3 for 26, n=2 for 8, and n=1 for 2) (C) Plasma Hb specie levels in animals co-infused with AS and cell-free Hb. MetHb was determined by EPR spectroscopy. (# of data points analyzed at a given treatment time point; n=3 for 24, n=2 for 10, and n=1 for 2). OxyHb was determined as the difference between total plasma Hb and metHb, confirmed by spectral deconvolution. Some samples were lost due to contamination or EPR tube breakage. (D) Average intra-erythrocytic (RBC) metHb levels in animals receiving the above 4 infusions. The error bars report the standard error of the mean.
Of concern, co-infusion of AS and Alb resulted in an increase in whole blood metHb to about 40 μM (from 18μM) indicating the oxidation reaction with AS was also occurring in the red cells (the only source of Hb without the infusion of cell-free Hb) as well as the plasma. In breaking down the plasma Hb species produced during co-infusion of Hb and AS, we found at 1 h, metHb accounts for 11% of total plasma Hb increasing to 39% by 2 h and declining (with a similar increase in oxyHb) after AS was terminated (Figure 2C). To determine the amount of oxidation of Hb in RBC’s, we co-infused AS with either Hb or Alb and calculated the metHb in RBC’s (Figure 2D). The average intra-erythrocytic metHb formed with AS co-infused with Alb increased by 93 μM from baseline and reached a maximum of 129 μM (P<0.0001 for slope) or about 0.6% of intra-erythrocytic Hb (assuming average 20 mM Hb, all concentrations on a heme basis) in the absence of infused Hb. When Hb was infused, the average intra-erythrocytic MetHb concentration reached 90 μM (an increase of 40 μM from baseline, P=0.0002 for slope). Although AS produced increases in metHb when co-infused with Hb or Alb, an analysis of the concentrations of metHb formed in the red cell and in the plasma compartment indicated a preferential reactivity of AS with plasma Hb to form metHb. Red cell metHb of 16 μM and plasma metHb of 26 μM formed (when calculating concentrations in the whole blood volume using the simultaneously measured hematocrit values) despite 100-fold more Hb in the red cell. However, the reactions had not formed iron-nitrosyl-Hb as expected by Equation 7 (none was detected by EPR, data not shown).
Mechanism for reduced metHb and iron-nitrosyl-Hb yields during AS infusion
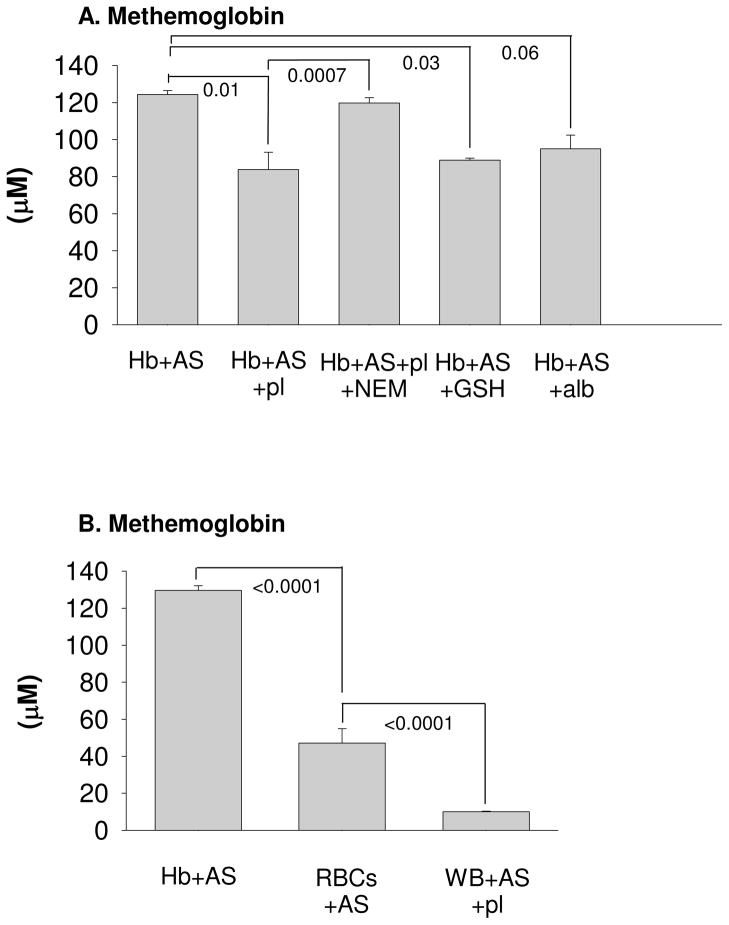
The metHb yield from AS infusion was less than what would be expected given the amount of AS infused (about 180 μM total) and Equation 6 indicating that one nitroxyl molecule leads to oxidation of two oxyHb molecules. However, only about 39% of the plasma Hb was converted to metHb by the end of the AS infusion, while the rest remained in the ferrous oxygenated form (Figure 2C). Once the AS infusion stopped, metHb levels fell and oxyHb levels began to increase suggesting reductive mechanisms in the plasma (Wang et al., unpublished). To understand the low metHb yield, in vitro experiments were performed (Figure 3A). AS (50 μM) was added to excess (1 mM) Hb (cell-free or red cell encapsulated) and the nitroxyl-dependent metHb yield was measured after 2 h. When plasma was added, the yields were significantly lower (P=0.01, compared to Hb alone), indicating that plasma components (likely thiol-containing compounds) can compete with the reaction of oxygenated Hb and nitroxyl. To determine the role of thiols in decreasing the yield of metHb in addition to the reducing components of plasma, NEM was added to the samples. NEM binds to thiol residues preventing reactivity with HNO. Thus, Hb diluted in plasma that had been treated with NEM significantly reduced the effects of plasma on Hb oxidation (P=0.0007). When specific thiols such as reduced glutathione and Alb were added to Hb, yields were significantly reduced (P=0.03 for glutathione, P=0.06 for Alb) similar to the effect observed with the addition of plasma. To examine if this reaction occurred in the red cell, AS was added to RBC’s resulting in a significantly lower metHb yield compared to cell-free Hb (P<0.0001) and suggesting nitroxyl is reacting with intra-erythrocytic thiol compounds (Figure 3B). When AS was added to whole blood and plasma, metHb yields were further reduced (p<0.0001) compared to RBC’s and AS. These data suggest that the lower than expected metHb yields from AS infusions in vivo were due to both competing reactions with thiol-containing compounds and reducing components of the plasma and RBC.
Figure 3.
In vitro experiments designed to examine low metHb yields in vivo. MetHb formation, determined by EPR spectroscopy or absorption spectroscopy, was due to infusion of AS (50 μM) added to: (A) oxyHb (1mM) diluted in phosphate buffered saline, oxyHb (1mM) diluted in plasma, oxyHb (1 mM) diluted in plasma that was incubated with NEM (10 mM) for 1 hour at 37°C prior to mixing with oxyHb, oxyHb (1mM) + GSH (10 mM), and oxyHb (1 mM) + bovine serum albumin (5g/dL, ~750 μM). Error bars represent one standard deviation, n=3. (B) AS (50 μM) added to: oxyHb (1 mM), red blood cells (1mM) diluted in phosphate buffered saline, and whole blood (1mM) diluted in plasma. Concentrations were determined by EPR spectroscopy after incubation for 2 hours at 37°C. Error bars report the standard error of the mean, n=3.
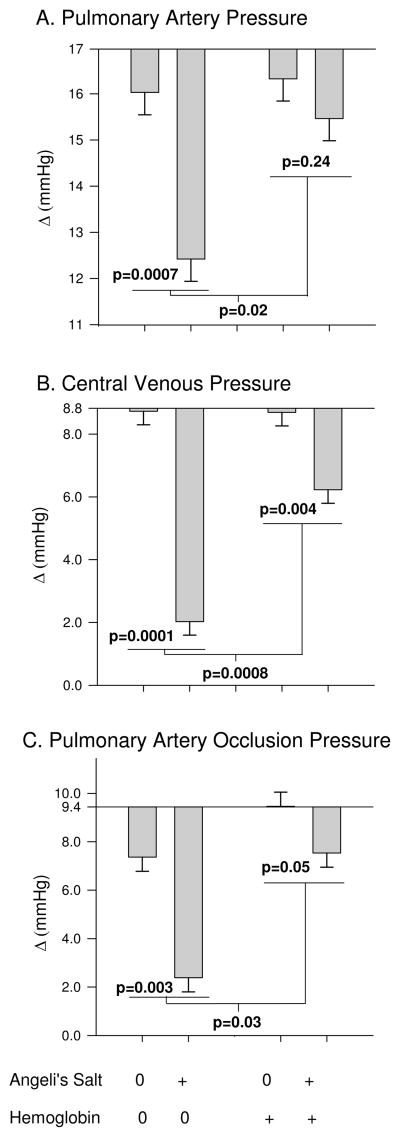
Pulmonary artery pressures and Cardiac Filling Pressures (combined effects of AS and plasma Hb that are significantly less than would have been expected if individual effects were added together, interaction)
AS (plus Alb) infused animals over the course of the 3h experiment had a decrease from baseline in mean PAP (P=0.0007), CVP (P<0.0001) and PAOP (P=0.0003) compared toNaCl (plus Alb) infused controls (Figure 4). AS co-infused with Hb over this time period had a decrease in mean CVP (P=0.004) and PAOP (P=0.05), but no significant change in PAP (P=0.24) compared to Hb infusion alone. However, the changes produced with co-infusion of AS and cell-free Hb were significantly less than would be expected based on the additive effects of AS and cell-free Hb given alone (P=0.02, P=0.0008 and P=0.03, respectively for an interaction). This suggests the two treatments are working, at least in part, through the same mechanism where cell-free Hb’s known effect of NO scavenging resulted in increasing vascular pressures while this scavenging also blocked the AS vasodilatory effect through donating NO activity.
Figure 4.
Pulmonary artery pressures and Cardiac Filling Pressures. A) mean pulmonary artery pressure, B) central venous pressure C) pulmonary artery occlusion pressure (+/− standard error). The p value over each bar pair shows the effect of AS, the average difference from baseline over the course of the 3h experiment, compared to NaCl (AS control). The bar pair on the left shows the effects of AS in the absence of Hb (Alb as control) and on the right shows the effects of AS in the presence of plasma Hb. The p-value in the middle represents if there is a significant difference between the effect of AS in the presence of Hb vs. in the absence of plasma Hb (interaction effect).
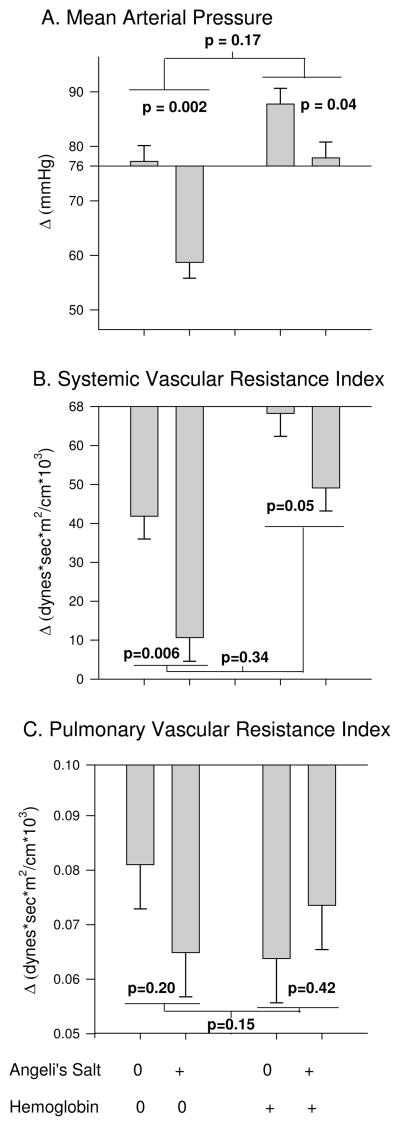
Systemic and Pulmonary Pressures (combined effects of AS and plasma Hb similar to that expected if individual effects were added together)
AS (plus Alb) infused animals over the course of the 3h experiment had a decrease from baseline in mean MAP (P=0.002), SVRI (P=0.006) and no significant effect on PVRI (P=0.2) compared to NaCl (plus Alb) controls (Figure 5). Of note, cell-free Hb given alone significantly increased MAP (P=0.03) and produced less of a decrease (an increase) in SVRI (P=0.01) compared to NaCl (plus Alb) controls. Co-infusion of AS with cell-free Hb over this same time period had a mean decrease in MAP (P=0.04), SVRI (P=0.05), yet no significant effect on PVRI (P=0.42) compared to cell-free Hb infusion alone. However, the changes produced with co-infusion of AS and cell-free Hb were smaller but not significantly less than would be expected based on the additive effects of AS and cell-free Hb given alone (P=0.17, P=0.34 and P=0.15, respectively for an interaction). Though a significant interaction in these effects was not found, the changes in these parameters trended in the same direction as the significant interactions found with other parameters (PAP, CVP and PAOP). The lack of significance could be explained by a lack of power given the relatively small sample size (n=3 per group).
Figure 5.
Systemic and Pulmonary Pressures. A) mean arterial pressure, B) systemic vascular resistance index, C) pulmonary vascular resistance index (+/− standard error). The p value over each bar pair shows the effect of AS, the average difference from baseline over the course of the 3h experiment, compared to NaCl (AS control). The bar pair on the left shows the effects of AS in the absence of Hb (Alb as control) and on the right shows the effects of AS in the presence of plasma Hb. The p-value in the middle represents if there is a significant difference between the effect of AS in the presence of Hb vs. in the absence of plasma Hb (interaction effect).
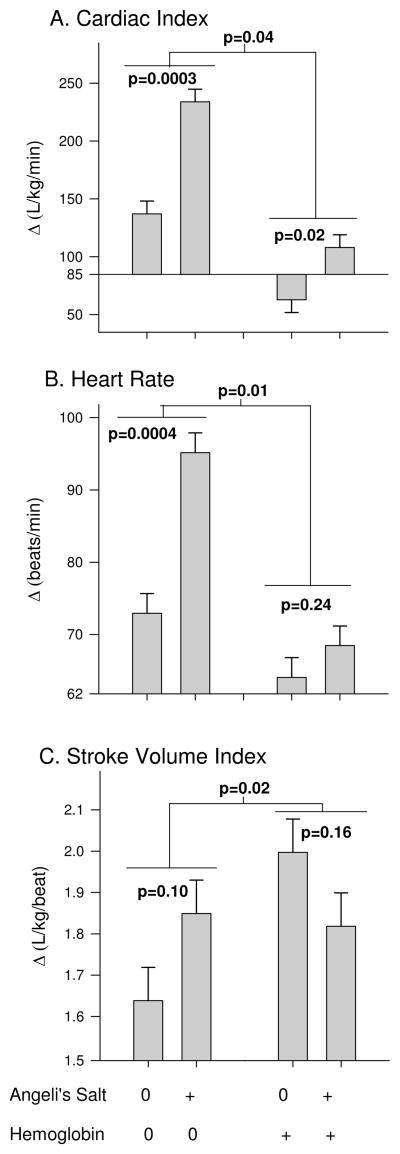
Cardiac Performance (indirect effects of AS and plasma Hb that are significantly less than would have been expected if individual effects were added together, interaction)
These effects of co-infusion of AS and cell-free Hb on cardiac performance is, in part, a function of changes in preload and afterload. Nonetheless, AS (plus Alb) infused animals over the course of the 3h experiment had an increase from baseline in mean CI (P=0.0003), HR (P=0.0004) and no significant effect on SVI (P=0.1) compared to NaCl infused controls (Figure 6). Co-infusion of AS and cell-free Hb over this time period still had a mean increase in CI (P=0.02) but no significant effect on HR (P=0.24) or SVI (P=0.16) compared to cell-free Hb (plus NaCl) infusion alone. However, the changes produced with co-infusion of AS and cell-free Hb were significantly less than would be expected based on the additive effects of AS and cell-free Hb given alone (P=0.04, P=0.01 and P=0.04, respectively for an interaction).
Figure 6.
Cardiac Performance. A) cardiac index, B) heart rate, C) stroke volume index (+/− standard error). The p value over each bar pair shows the effect of AS, the average difference from baseline over the course of the 3h experiment, compared to NaCl (AS control). The bar pair on the left shows the effects of AS in the absence of Hb (Alb as control) and on the right shows the effects of AS in the presence of plasma Hb. The p-value in the middle represents if there is a significant difference between the effect of AS in the presence of Hb vs. in the absence of plasma Hb (interaction effect).
Effects of AS infusion on platelets and white blood cells (WBC)
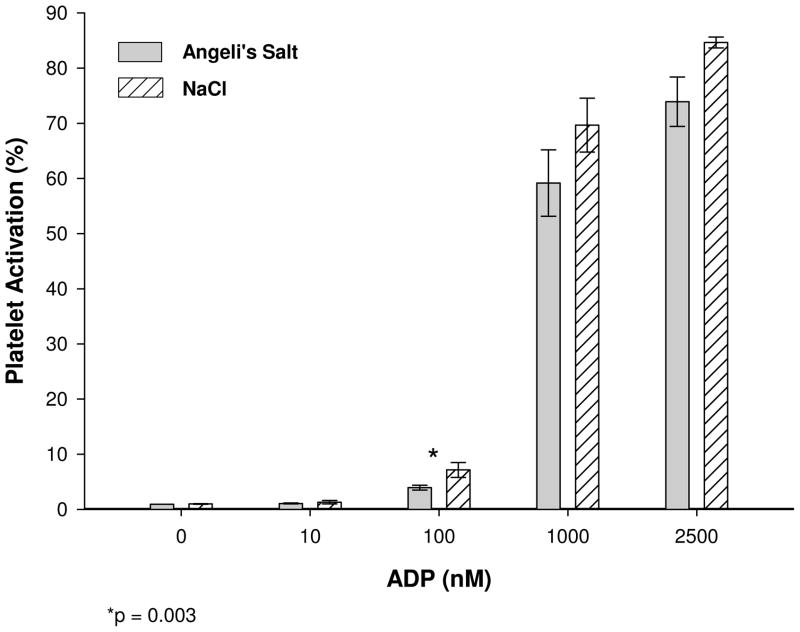
In order to assess effects of AS infusions we conducted in vitro experiments where we infused AS under conditions that mimicked our canine infusions in vivo. We found that AS reduced ADP-dependent platelet activation at the steepest part of the dose response curve (100 nM ADP) compared to control infusions using NaCl (p=0.003, Figure 7). Infusion of nitrite alone (final concentration of 184 μM) instead of AS had no effect on platelet activation (data not shown), indicating the action of AS on platelets can be attributed to HNO. Cytokine production from WBC’s (IL-1b, IL-6, TNFα) in response to exposure to LPS was not affected by the presence or absence of AS (p=0.72, p=0.98, p=0.37, respectively)(data not shown).
Figure 7.
Plaletet Activation. AS was slowly infused into whole blood as described in the methods section. Platelet activation was determined by FITC conjugated PAC-1 fluorescence. PRP was obtained from whole blood previously infused with AS or NaCl. Platelet agonist ADP was added at concentrations of 10 nM, 100 nM, 1000 nM and 2500 nM and platelet activation was measured by flow cytometry. Platelet activation is ploted as the average ± the standard error. Platelet activation was reduced at all concentrations of ADP above 10 nM and was significantly reduced at 100 nM. (n = 5, * p < 0.003).
Discussion
Infusions of cell-free Hb in our canine model led to vasoconstriction and associated hemodynamic changes similar to those reported previously [40,41]. Infusion of AS led to vasorelaxation and decreased pulmonary and systemic hemodynamic parameters. These effects may be due to the actions of either or both nitrite and nitroxyl (HNO). Together, these effects added synergistically in the pulmonary circulation with a similar trend observed in the systemic circulation, but not reaching significance. The synergy of these observed effects could be explained by the action of AS to neutralize NO scavenging by cell-free Hb in the plasma via conversion to metHb as well as by the inherent vasodilatory properties of AS.
We have previously found that when AS is added to a mixture of red blood cells and cell-free Hb in phosphate buffered saline, the cell-free Hb is preferentially converted to metHb compared to that encapsulated in the red blood cells [50]. We attributed this preferential reactivity to the fact that, like NO, HNO reacts extremely rapidly with Hb so that its reaction with red cell encapsulated Hb is rate-limited by the time it takes for HNO to diffuse to the red cell. The preferential reactivity can be quantified by the relation
| (8) |
where HbO2 is the oxygenated form of metHb, and the subscripts r and f refer to the RBC encapsulated and plasma Hb respectively. This equation states that the amount of metHb made in the red cell or plasma fraction depends on the intrinsic, bimolecular (normalized by the concentration of Hb) rate constant and the amount of reacting material in each fraction. The concentrations (indicated by brackets) represent the moles of the species in the total volume. Thus,
| (9) |
where [metHb]s represents the concentration of metHb in the supernatant after sedimentation of the red blood cells. A similar equation is used to determine [HbO2]ffrom the concentration of oxyHb in the supernatant ([HbO2]f = (1-Hct)* [HbO2]s where the subscript “s” refers to the supernatant). In our previous experiments on red cells and plasma Hb in saline, we found the preferential reactivity at 42% hematocrit to be 57. Using the data in Figure 2, we calculate
Thus, we find that in our in vivo experiments (where the average hematocrit was 39%) the preferential reactivity was 114. That the preferential reactivity was higher in vivo than in vitro may be due to the possibility of nitroxyl reacting with Hb in the plasma zone while red cells are excluded from this volume.
Although the measured preferential reactivity is most likely due to rate limitations of diffusion of HNO to the RBCs, as previously demonstrated examining the hematocrit dependence of preferential reactivity [50], it may also be due to reactions within the RBC or RBC membrane. Like NO and oxygen, HNO partitions more in lipid than aqueous phases and thus, as suggested before in the case of NO [52], HNO might react with oxygen in the membrane. However, given how slow that reaction is compared to that with Hb, it is most likely negligible.
Although the preferential reactivity of metHb formation was similar to, or even greater than, what we have found previously, [50] the absolute metHb yield was much lower. Our data in Figure 3 suggest that this lower than expected yield could be explained by reactions between nitroxyl and plasma components. We estimated the rate at which plasma components react with nitroxyl by comparing the quantities of metHb formed by reacting oxyHb with AS in the presence or absence of plasma. The rate constant for the plasma reaction was found using the relation
where [HNO+plasma] is the concentration of nitroxyl that reacted with plasma, [HNO+Hb] is the concentration of nitroxyl that reacted with Hb, is the rate constant for the reaction of plasma with nitroxyl, is the molar rate constant for the reaction of oxyHb and nitroxyl (107M−1s−1), and [Hb] is the initial concentration of oxyHb (1mM). Any nitroxyl that did not react with the oxyHb to form metHb was assumed to have reacted with plasma components. Using this approach, we calculated the reaction rate of plasma with nitroxyl to be in the range of with an average value of . This first order rate constant compares to that of 100 μM cell-free Hb with nitroxyl (about 103 s−1), so that reaction of HNO with plasma components could explain our lower than expected yield of metHb during AS infusion. The first order rate constant for the reaction of plasma and HNO obtained by the above method is also consistent with published values of plasma thiol levels (about 0.5 mM) [53] and rate constants (about 2 × 106 M−1s−1), [49] predicting an observed rate constant of 103 s−1. These observed rate constants would predict that thiols can compete with plasma Hb for HNO and that the lifetime of HNO in plasma would be on the order of milliseconds.
Under certain in vitro conditions, we have previously found that when AS is added to oxyHb, some nitrosyl Hb is formed, probably via the reaction of nitroxyl and metHb (Equation 7) [50]. Like metHb, nitrosyl Hb does not scavenge NO and would potentially be a product of interest, however, we did not observe any nitrosyl Hb formation via the nitroxyl/metHb reaction or any other pathway in our in vivo measurements. The lack of any measureable nitrosyl Hb can be explained by the low metHb yield, since the reaction of nitroxyl and metHb must compete with the reaction of nitroxyl and oxyHb. Inspection of Figure 2, and using published rate constants [49], we find that one would expect (107/106)(10) = 100 times more metHb to be made than nitrosyl Hb so that only a few hundred nanomolar nitrosyl Hb would be made, below our detection limit of 500nM.
AS has been shown in this study to act as a direct vasodilator and to inactivate the NO scavenging observed in the presence of plasma Hb. Both mechanisms appear equally active in the systemic vasculature where the combined effects of Hb and AS were found to be additive when compared to the effects of Hb and AS alone. Whereas in the pulmonary system, the decrease in pressure when AS is added to Hb was significantly less than would have been expected compared to the effects of Hb and AS alone suggesting that inactivation of scavenging with AS reduced the direct vasodilatory effects of AS on the vasculature. Previous studies have shown the role these properties play using other NO donors [40].
Comparing the effects of AS infusion on mediating hemolysis to those using inhaled NO published previously also merits consideration [40]. In both cases, administration of the nitrogen oxide (NO or HNO/nitrite) acted to counter effects associated with hemolysis-dependent vasoconstriction. However, in the case of NO, these results were most likely due predominantly to inactivation of NO scavenging by plasma Hb through conversion to metHb. On the other hand, the action of AS seems to have been mainly, or at least largely, due to its ability to vasodilate. Indeed, infusion of AS in the absence of hemolysis in this study led to substantial vasodilation whereas NO administration in the absence of hemolysis did not have this effect [40]. In addition, while AS only resulted in conversion of about 39% of plasma Hb to metHb, NO inhalation led to about 80% conversion [40]. Thus, both approaches could alleviate hemolysis-dependent vasoconstriction through both inactivation of vasoconstricting oxyHb and by compensatory vasodilating effects. The degree to which each of these approaches contributes is dependent on the nitrogen oxide donor.
It is also worth comparing the effects of AS infusion to infusion of nitrite [41]. The rate of AS infusion in millimoles per minute in the current study was about 4.5 times slower than the previous study [41] where nitrite was infused. In addition, the previous study infused nitrite for 6 h, compared to the 2 h infusion of AS used here. Thus, total millimoles infused was about 13.5 times greater in the nitrite infusion study than in the AS infusion study. Nevertheless, only about 15–20% of plasma oxyHb was converted to metHb with nitrite infusions alone [41] and we observed 39% conversion with AS. This increased efficiency was also reflected in a greater reduction of hemodynamic parameters (MAP, SVRI, CVP, PAOP) in response to AS infusion when compared to the reduction of the same parameters in the nitrite study when in the presence of cell-free Hb. The more efficient conversion compared to nitrite is most likely due to greater preferential reactivity of HNO with plasma Hb compared to nitrite. In the absence of cell-free Hb, a greater reduction of hemodynamic parameters (PAP, MAP, SVRI, CVP, PAOP) during AS infusion compared to nitrite suggests a greater direct vasodilatory effect. Comparison of results using AS to those using nitrite, taking into account that the nitrite infusions were performed at a much higher molar rate and total dose, suggests that the effects of AS infusion observed here are mainly due to AS-derived HNO rather than HNO-derived nitrite.
While the main focus of this study was to examine oxidation of plasma and erythrocytic hemoglobin while monitoring the hemodynamic effects of a relatively short duration, we also considered the response of other blood cells, i.e. platelets and white blood cells to AS. Interestingly, previous work has shown that HNO decreases platelet aggregation [54] which could be beneficial when treating hemolysis. However, these previous experiments were conducted in platelet rich plasma and the effects could, in principle, have been muted by competitive reaction with Hb when red cells were present. Importantly, here we have shown that AS can reduce platelet activation even when administered to whole blood (Figure 7). In addition, AS did not appear to alter cytokine production in this study. Vanuffelen et al found AS increased neutrophil migration [55], Further work is required to establish effects of AS administration on white blood cells and how these effects would influence treatment of hemolysis or other conditions.
Conclusion
This study provides important information that would be relevant for use of AS or AS-like compounds to treat hemolysis, whether present due to hemolytic anemia, from infusion of stored blood or otherwise. AS was found to counter the negative hemodynamic effects of plasma Hb. This effect could be attributed partially to inactivation of NO scavenging through conversion to metHb, but also, and perhaps more so, due to an overriding vasodilatory effect of AS opposing the vasoconstrictive effect of cell-free Hb. The ability of AS to maintain its action in the presence of large amounts of plasma Hb, without oxidizing the plasma Hb, could be an asset to the development of Hb based oxygen carriers.
In consideration of employing AS as a therapeutic, important limitations should be kept in mind. Firstly, AS was observed to decrease MAP so that this effect would have to be closely monitored. In addition, although reactivity is preferential with cell-free Hb, red cell metHb was formed and this would also have to be monitored closely. Use of HNO donors has shown substantial DNA toxicity in cell cultures [56] and neurotoxicity [55]. Unlike NO, it has also been observed to exacerbate ischemic reperfusion injury under some conditions [57,58]. These studies also suggest underlying conditions present in disease in addition to the presence of cell free Hb such as immunologic compromise may have an effect on how AS is handled. Though, AS has properties that may be of interest as a therapeutic in the presence of hemolysis, close monitoring of hemodynamic effects and toxicity are warranted when applying this therapy in any study or clinical trial.
AS is a source of both HNO and nitrite. Previous work has shown potentially beneficial effects of nitrite alone in treating hemolysis [41], although at higher concentrations than those from this study. As the nitrite and HNO work through different mechanisms, it might be interesting to conduct future experiments examining the effects of AS with additional nitrite and compare those results to using an HNO donor that does not release nitrite.
Highlights.
Intravascular hemolysis has been associated with numerous deleterious effects due to NO scavenging
We investigated the effects of Angeli’s Salt (Na2N2O3), a nitroxyl (HNO) and nitrite (NO2−) donor
AS was found to counter the negative hemodynamic effects of plasma Hb due to inactivation of NO scavenging and direct vasodilating properties
Acknowledgments
This work was supported by NIH grants HL058091 and HL098032
The authors would like to thank the assistance of Ms. Jing Feng, Mr. Alan Hilton, and Dr. Melinda Fernandez in the performance of this study and data handling and graphing
Footnotes
Disclosures: SBK, DS, DK-S and MTG are listed as coauthors on a patent application on methods of treating hemolysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furchgott RF, Zawadzki JV. The Obligatory Role of Endothelial-Cells in the Relaxation of Arterial Smooth-Muscle by Acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-Derived Relaxing Factor from Pulmonary-Artery and Vein Possesses Pharmacological and Chemical-Properties Identical to Those of Nitric-Oxide Radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 3.Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of Guanylate Cyclase by Sodium Nitroprusside, Nitroglycerin and Nitric-Oxide in Various Tissue Preparations and Comparison to Effects of Sodium Azide and Hydroxylamine. J Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 4.Palmer RMJ, Ferrige AG, Moncada S. Nitric-Oxide Release Accounts for the Biological-Activity of Endothelium-Derived Relaxing Factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 5.Ignarro LJ. Nitric Oxide Biology and Pathobiology. San Diego: Academic press; 2000. [Google Scholar]
- 6.Battinelli EM, Loscalzo J. Nitric oxide and platelet-mediated hemostasis. In: Loscalzo J, Vita JA, editors. Nitric oxide and the cardiovascular system. Humana; Totowa, N.J: 2000. pp. 123–138. [Google Scholar]
- 7.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–62. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima S, Tohmatsu T, Hattori H, Okano Y, Nozawa Y. Inhibitory action of cyclic GMP on secretion, polyphosphoinositide hydrolysis and calcium mobilization in thrombin-stimulated human platelets. Biochem Biophys Res Commun. 1986;135:1099–104. doi: 10.1016/0006-291x(86)91041-7. [DOI] [PubMed] [Google Scholar]
- 9.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tailor A, Granger DN. Role of adhesion molecules in vascular regulation and damage. Current hypertension reports. 2000;2:78–83. doi: 10.1007/s11906-000-0063-6. [DOI] [PubMed] [Google Scholar]
- 11.Hickey MJ, Kubes P. Role of nitric oxide in regulation of leucocyte-endothelial cell interactions. Experimental physiology. 1997;82:339–48. doi: 10.1113/expphysiol.1997.sp004029. [DOI] [PubMed] [Google Scholar]
- 12.Lancaster JR. Simulation of the Diffusion and Reaction of Endogenously Produced Nitric-Oxide. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang KT, Huang Z, Kim-Shapiro DB. Nitric Oxide Red Blood Cell Membrane Permeability at high and low Oxygen Tension. Nitric Oxide. 2007;16:209–216. doi: 10.1016/j.niox.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nature Biotechnology. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 15.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 16.Butler AR, Megson IL, Wright PG. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta-Gen Subj. 1998;1425:168–176. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu XP, Samouilov A, Lancaster JR, Zweier JL. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 18.Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280:39024–38032. doi: 10.1074/jbc.M509045200. [DOI] [PubMed] [Google Scholar]
- 19.Azarov I, Liu C, Reynolds H, Tsekouras Z, Lee JS, Gladwin MT, Kim-Shapiro DB. Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vesicles. J Biol Chem. 2011 Jul 30; doi: 10.1074/jbc.M111.2286502011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 21.Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–1565. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donadee C, Raat NJH, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric Oxide Scavenging by Red Blood Cell Microparticles and Cell-Free Hemoglobin as a Mechanism for the Red Cell Storage Lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohl U, Lamontagne D. Impaired Tissue Perfusion after Inhibition of Endothelium-Derived Nitric-Oxide. Basic Research in Cardiology. 1991;86:97–105. doi: 10.1007/978-3-642-72461-9_11. [DOI] [PubMed] [Google Scholar]
- 24.Hess JR, Macdonald VW, Brinkley WW. Systemic and Pulmonary-Hypertension after Resuscitation with Cell-Free Hemoglobin. J Appl Physiol. 1993;74:1769–1778. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 25.Lee R, Neya K, Svizzero TA, Vlahakes GJ. Limitations of the Efficacy of Hemoglobin-Based Oxygen-Carrying Solutions. J Appl Physiol. 1995;79:236–242. doi: 10.1152/jappl.1995.79.1.236. [DOI] [PubMed] [Google Scholar]
- 26.Dou Y, Maillett DH, Eich RF, Olson JS. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys Chem. 2002;98:127–148. doi: 10.1016/s0301-4622(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 27.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radical Biology and Medicine. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Patel RP. Biochemical aspects of the reaction of hemoglobin and NO: Implications for Hb-based blood substitutes. Free Radic Biol Med. 2000;28:1518–1525. doi: 10.1016/s0891-5849(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 29.Thompson A, McGarry AE, Valeri CR, Lieberthal W. Stroma-Free Hemoglobin Increases Blood-Pressure and Gfr in the Hypotensive Rat - Role of Nitric-Oxide. J Appl Physiol. 1994;77:2348–2354. doi: 10.1152/jappl.1994.77.5.2348. [DOI] [PubMed] [Google Scholar]
- 30.Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Current Opinion in Hematology. 2003;10:99–107. doi: 10.1097/00062752-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Reiter CD, Wang XD, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 32.Gladwin MT. Unraveling the hemolytic subphenotype of sickle cell disease. Blood. 2005;106:2925–2926. [Google Scholar]
- 33.Kato GJ, McGowan VR, Machado RF, Little JA, Taylor J, VI, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris J, Sidney M, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gladwin MT, Barst RJ, Castro OL, Gordeuk VR, Hillery CA, Kato GJ, Kim-Shapiro DB, Machado R, Morris CR, Steinberg MH, Vichinsky EP. Pulmonary hypertension and NO in sickle cell. Blood. 2010;116:852–854. doi: 10.1182/blood-2010-04-282095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Current Opinion in Hematology. 2009;16:515–523. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 36.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–851. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116:687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 38.Buehler PW, Karnaukhova E, Gelderman MP, Alayash AI. Blood Aging, Safety, and Transfusion: Capturing the “Radical” Menace. Antioxid Redox Sign. 2011;14:1713–1728. doi: 10.1089/ars.2010.3447. [DOI] [PubMed] [Google Scholar]
- 39.Alayash AI. Setbacks in Blood Substitutes Research and Development: A Biochemical Perspective. Clin Lab Med. 2010;30:381. doi: 10.1016/j.cll.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Minneci PC, Deans KJ, Zhi H, Yuen PST, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minneci PC, Deans KJ, Shiva S, Zhi H, Banks SM, Kern S, Natanson C, Solomon SB, Gladwin MT. Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis. Am J Physiol-Heart Circul Physiol. 2008;295:H743–H754. doi: 10.1152/ajpheart.00151.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu XL, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 43.Brooks J. The action of nitrite on Haemoglobin in the absence of oxygen. Proc Royal Soc London - Series B, Biol Sci. 1937;123:368–382. [Google Scholar]
- 44.Doyle MP, Pickering RA, Deweert TM, Hoekstra JW, Pater D. Kinetics and Mechanism of the Oxidation of Human Deoxyhemoglobin by Nitrites. J Biol Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 45.Gamgee A. Researches on the Blood. On the Action of Nitrites on Blood. Philosophical Transactions of the Royal Society of London. 1868;158:589–625. [Google Scholar]
- 46.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin. J Biol Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piknova B, Keszler A, Hogg N, Schechter AN. The reaction of cell-free oxyhemoglobin with nitrite under physiologically relevant conditions: Implications for nitrite-based therapies. Nitric Oxide-Biol Ch. 2009;20:88–94. doi: 10.1016/j.niox.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doyle MP, Mahapatro SN, Broene RD, Guy JK. Oxidation and Reduction of Hemoproteins by Trioxodinitrate(Ii) - the Role of Nitrosyl Hydride and Nitrite. J Am Chem Soc. 1988;110:593–599. [Google Scholar]
- 49.Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He X, Azarov I, Jeffers A, Presley T, Richardson J, King SB, Gladwin MT, Kim-Shapiro DB. The potential of Angeli’s salt to decrease nitric oxide scavenging by plasma hemoglobin. Free Radical Biol Med. 2008;44:14201432. doi: 10.1016/j.freeradbiomed.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miranda KM. The chemistry of nitroxyl (HNO) and implications in biology. Coordination Chemistry Reviews. 2005;249:433–455. [Google Scholar]
- 52.Liu XP, Miller MJS, Joshi MS, Thomas DD, Lancaster JR. Accelerated reaction of nitric oxide with O-2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci USA. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lobo G-AM, Chitre SA, Rathod SM, Smith RB, Leslie R, Livingstone C, Davis J. Determination of total reduced thiol levels in plasma using a bromide substituted quinone. Electroanalysis. 2007;19:2523–2528. [Google Scholar]
- 54.Bermejo E, Saenz DA, Alberto F, Rosenstein RE, Bari SE, Lazzari MA. Effect of nitroxyl on human platelets function. Thromb Haemost. 2005;94:578–584. doi: 10.1160/TH05-01-0062. [DOI] [PubMed] [Google Scholar]
- 55.VanUffelen BE, Van der Zee J, de Koster BM, VanSteveninck J, Elferink JGR. Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochem J. 1998;330:719–722. doi: 10.1042/bj3300719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wink DA, Feelisch M, Fukuto J, Chistodoulou D, Jourd’heuil D, Grisham MB, Vodovotz Y, Cook JA, Krishna M, DeGraff WG, Kim S, Gamson J, Mitchell JB. The cytotoxicity of nitroxyl: Possible implications for the pathophysiological role of NO. Arch Biochem Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 57.Choe C-u, Lewerenz J, Fischer G, Uliasz TF, Espey MG, Hummel FC, King SB, Schwedhelm E, Boeger RH, Gerloff C, Hewett SJ, Magnus T, Donzelli S. Nitroxyl exacerbates ischemic cerebral injury and oxidative neurotoxicity. J Neurochem. 2009;110:1766–1773. doi: 10.1111/j.1471-4159.2009.06266.x. [DOI] [PubMed] [Google Scholar]
- 58.Ma XL, Cao F, Liu GL, Lopez BL, Christopher TA, Fukuto JM, Wink DA, Feelisch M. Opposite effects of nitric oxide and nitroxyl on postischemic myocardial injury. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14617–14622. doi: 10.1073/pnas.96.25.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang Z, Louderback JG, Goyal M, Azizi F, King SB. Kim-Shapiro D. B. Nitric oxide binding to oxygenated hemoglobin under physiological conditions. Biochim Biophys Acta. 2001;1568:252–260. doi: 10.1016/s0304-4165(01)00227-6. [DOI] [PubMed] [Google Scholar]