Abstract
Bardoxolone methyl, a synthetic triterpenoid, improves the estimated glomerular filtration rate (GFR) in patients with chornic kidney disease and type 2 diabetes. Since the contractile activity of mesangial cells may influence glomerular filtration, we evaluated the effect of the synthetic triterpenoid RTA405 with structural similarity to bardoxolone methyl, on GFR in rats and on mesangial cell contractility in freshly isolated glomeruli. In rats, RTA 405 increased basal GFR, assessed by inulin clearance, and attenuated the angiotensin II-induced decline in GFR. RTA 405 increased the filtration fraction, but did not affect arterial blood pressure or renal plasma flow. Glomeruli from RTA 405-treated rats were resistant to angiotensin II-induced volume reduction ex vivo. In cultured mesangial cells, angiotensin II-stimulated contraction was attenuated by RTA 405, in a dose- and time-dependent fashion. Further, Nrf2 targeted gene transcription (regulates antioxidant, anti-inflammatory, and cytoprotective responses) in mesangial cells was associated with decreased basal and reduced angiotensin II-stimulated hydrogen peroxide and calcium ion levels. These mechanisms contribute to the GFR increase that occurs following treatment with RTA 405 in rats and may underlie the effect of bardoxolone methyl on the estimated GFR in patients.
INTRODUCTION
Chronic kidney disease (CKD) is characterized by progressive loss of kidney function, which may eventually result in end stage renal disease.1 There is no curative therapy currently available. Hypertension and diabetes are two major factors causing CKD.1–4 Inhibitors of the renin-angiotensin system have been shown to be modestly effective at delaying progression of CKD.5,6 Therefore, additional therapeutic agents for CKD are in need.
Increasing evidence suggests that inflammation and elevated reactive oxygen species (ROS) are associated with the development and progression of CKD.7 Agents that inhibit or modulate inflammatory responses may be therapeutic alternatives for treating CKD. Bardoxolone methyl is a synthetic triterpenoid and a potent inducer of Nrf2, a key transcription factor that regulates antioxidant, anti-inflammatory, and cytoprotective responses.8,9 Kidney function is compromised in nrf2-deficient mice.10 Nrf2 is also suppressed in models of CKD and in patients with diabetes.11–13 Increased expression and activity of Nrf2 by bardoxolone methyl has been shown to ameliorate ischemic acute kidney injury in mice.14 Importantly, in recent short-term (8 weeks) and long-term (52 weeks) clinical trials in patients with CKD and type 2 diabetes (T2D), bardoxolone methyl significantly increased estimated glomerular filtration rate (eGFR) and improved several other markers of renal function.15, 16 The precise mechanism underlying the effects of bardoxolone methyl on glomerular filtration is still being investigated.
Glomerular filtration is controlled by intra- and extra-glomerular factors. Glomerular mesangial cells play an important role in regulating GFR.17,18 MCs surround the capillary loops within the glomerulus and possess many characteristics similar to vascular smooth muscle cells, including contractile function that regulates blood flow through adjacent capillaries and the capillary surface area available for filtration.18 Dysfunction of MCs is associated with a variety of renal diseases, including diabetic CKD.19–23 The present study was conducted to test the hypothesis that RTA 405, an analog of bardoxolone methyl, affects GFR in part by acutely modulating the contractile function of MCs.
RESULTS
Effect of RTA 405 on Inulin Clearance in Rats
In Sprague Dawley rats, inulin clearance was used to measure the effect of RTA 405 on GFR. As shown in Fig. 1A, RTA 405 significantly increased resting state GFR from 0.49 ± 0.07 ml/min/100 g BW to 1.18 ± 0.23 ml/min/100 g BW (P<0.01). To assess the effect of RTA 405 on Ang II-induced changes in GFR, the same rats were infused with Ang II at 1.7 ng/min/100 g BW and GFR was measured 30 minutes later. In control rats, Ang II decreased GFR from 0.49 ± 0.07 ml/min/100 g BW to 0.28 ± 0.06 ml/min/100 g BW (P<0.001). In rats pretreated with RTA 405, Ang II decreased GFR from 1.18 ± 0.23 ml/min/100 g BW to 0.76 ± 0.13 ml/min/100 g BW (P<0.05). A statistically significant difference in Ang II-induced GFR decline was observed between control and RTA 405-treated animals (0.28 ± 0.06 ml/min/100 g BW vs. 0.76 ± 0.13 ml/min/100 g BW, P<0.01). Fig. 1B shows the average percent change in Ang II-induced GFR decline. In control rats, GFR decreased by 41.5 ± 7.7% while in RTA 405-treated rats, GFR decreased by 21.3 ± 9.8%. Therefore, animals treated with RTA 405 exhibited an attenuation of Ang II-induced GFR decline; however, the difference was not statistically significant.
Fig. 1. Effect of RTA 405 on basal GFR and Ang II-induced changes in GFR in rats.
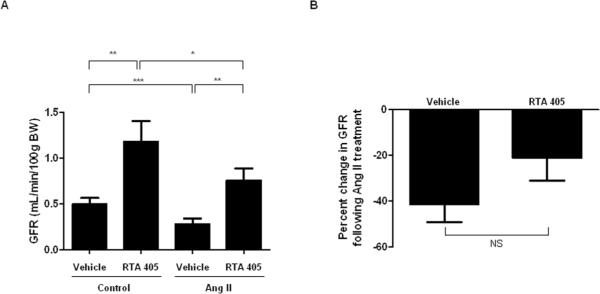
Rats were treated with RTA 405 at 100 mg/kg body weight (N=16) or the same volume of vehicle (sesame oil; N=15) by oral gavage once daily for 3 consecutive days. GFR was measured 12–16 hours after the last dose of RTA 405 was received. A: Effect of vehicle or RTA 405 on GFR in rats before and after treatment with Ang II. B: Percent change in GFR following treatment with Ang II. NS, not significant;.* P<0.05; ** P<0.01; *** P<0.001
Effect of RTA 405 on Renal Hemodynamics in Rats
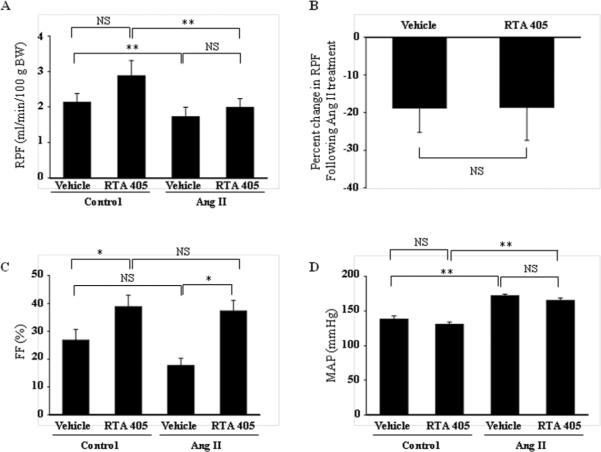
To determine whether the effect of RTA 405 on glomerular filtration was attributable to a change in renal hemodynamics, we estimated renal plasma flow (RPF) by measuring para-aminohippurate (PAH) clearance in control or RTA 405-treated rats. RTA 405 did not significantly change RPF (Fig. 2A). Ang II treatment significantly reduced RPF in both control and RTA 405-treated groups. However, the percent change in Ang II-induced reduction of RPF between control and RTA 405-treated groups was comparable (Fig. 2B, P>0.05). In agreement with a reduction of RPF, infusion of Ang II significantly increased mean arterial blood pressure in both groups. Again, the pressing effect of Ang II was not affected by RTA 405 (Fig. 2D). These results suggest that RTA 405 treatment did not change renal hemodynamics in rats.
Fig. 2. Effect of RTA 405 on basal and Ang II-induced changes in RPF (A and B), basal FF (C) and blood pressure (D) in rats.
Rats were treated with RTA 405 at 100 mg/kg body weight (N=24) or the same volume of sesame oil (N=23) by oral gavage once daily for 3 consecutive days. Measurements were conducted 12–16 hours after the last dose of RTA 405 was received. In D, mean arterial blood pressure was measured before (Control) and 30 min after infusion of Ang II in both groups of rats. The numbers of rats in each group (Vehicle vs. RTA 405) were: for RPF, 18 vs. 17; for FF, 16 vs. 15, and for mean arterial blood pressure, 6 vs. 6. NS, not significant; * P<0.05; ** P<0.01.
Effect of RTA 405 on Filtration Fraction
Filtration fraction (FF) is the ratio of GFR to RPF. To further evaluate the effect of RTA 405 on glomerular filtration function, we calculated the FF in rats with and without RTA 405 treatment before and after infusion of Ang II. As shown in Fig. 2C, RTA 405 significantly increased FF at resting state and after Ang II infusion. Because RTA 405 did not significantly alter RPF (Fig. 2 A&B), the increase in FF indicates that the RTA 405-mediated increase in GFR is not due to an increase in RPF.
Effect of RTA 405 on Basal Arterial Blood Pressure in Conscious Rats
To further assess whether blood pressure was involved in the effect of RTA 405 on GFR, arterial blood pressure was measured in control and RTA 405-treated rats under conscious state at baseline (Day 0) and Day 3 (8h post-dose). No significant difference in systolic or diastolic blood pressure was observed between control and RTA 405-treated rats (Fig. 3).
Fig. 3. Effects of RTA 405 on basal arterial blood pressure in conscious rats.
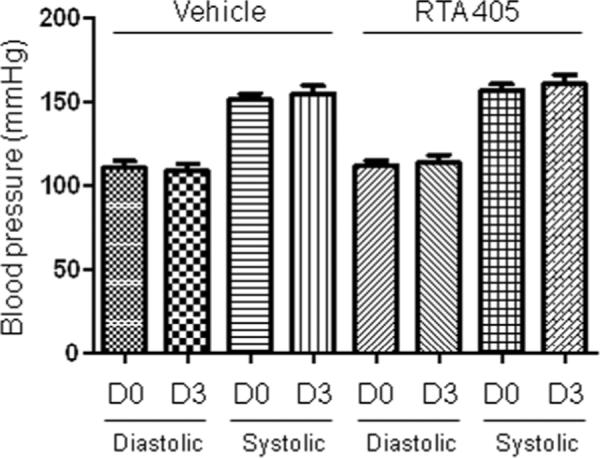
Sprague Dawley rats were administered sesame oil or RTA 405 at a dose of 100 mg/kg body weight by oral gavage once daily for three consecutive days. Diastolic and systolic blood pressure was measured using tail cuff in conscious vehicle- and RTA 405-treated rats before treatment (D0) and 8 hours post-dose on Day 3 (D3). Fifteen rats in each group. Data are expressed as mean ± SD.
Effect of RTA 405 on Contractile Response of Freshly Isolated Glomeruli
Since RTA 405 significantly increased GFR without affecting RPF (Figs. 1&2), we postulated that changes within the glomeruli might underlie the effect of RTA 405. In this regard, MCs are a good candidate because their contractile property enables them to alter glomerular filtration surface area and thereby GFR.24–26 We next determined if RTA 405 treatment affected MC contractility by measuring changes in the volume of freshly isolated glomeruli in response to Ang II. As shown in Fig. 4, application of 1 μM Ang II decreased glomerular volume in control rats by 10.5 ± 1.2% and 8.4 ± 1.2% at 1 minute and 20 minutes after addition of Ang II, respectively. However, in rats pre-treated with RTA 405 for 3 days, the Ang II-induced response was almost completely abolished (0.04 ± 0.77% at 20 min after Ang II, Fig. 4).
Fig. 4. Effect of RTA 405 on freshly isolated rat glomerular volume in response to Ang II (1 μM).
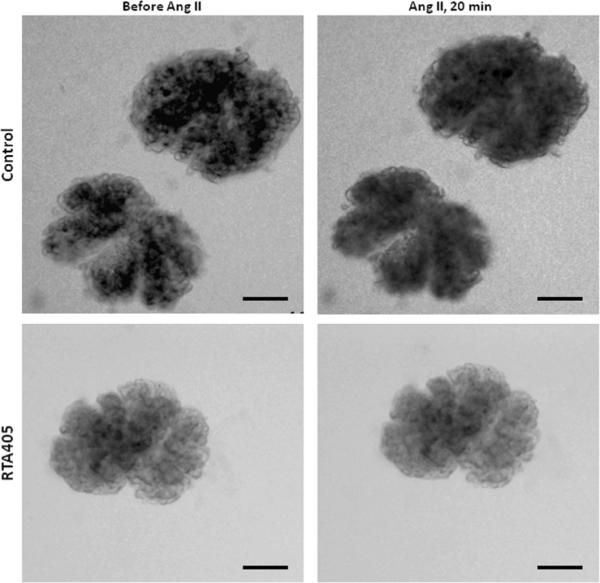
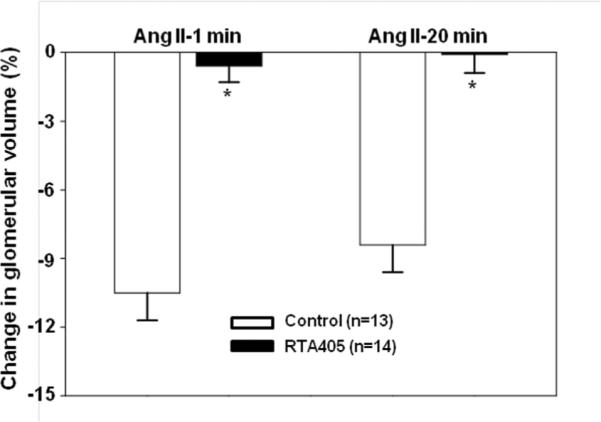
Rats were treated with RTA 405 at 100 mg/kg body weight or the same volume of sesame oil through an oral gavage for 3 consecutive days. A. Representative images, showing glomeruli before Ang II and 20 min after Ang II. Scale bars represent 50 μm. B. Summary data from 13 glomeruli isolated from 4 control rats and 14 glomeruli isolated from 4 RTA 405 treated rats. * P<0.05, RTA 405 vs. Control. n indicates the number of glomeruli analyzed in each group.
Effect of Synthetic Triterpenoids on the Contractile Response of Mesangial Cells
To further explore the role of MCs, we examined the effect of RTA 405 on Ang II-stimulated MC contraction.17 Cultured human MCs were pre-treated with vehicle (control) or RTA 405 for 16 h and then treated with Ang II for 30 minutes. As shown in Fig. 5, Ang II induced a significant reduction of the planar surface area of control MCs. This contractile response was significantly attenuated by RTA 405 in a dose-dependent manner and was observed at concentrations as low as 50 nM (Fig. 5A). The calculated IC50 was 72 nM (Fig. 5B).
Fig. 5. Inhibition of RTA 405 on Ang II-induced contraction of human MCs.
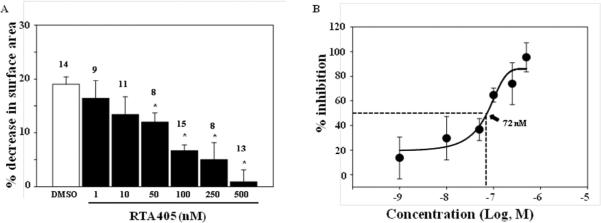
Cells were treated with either DMSO or various concentrations of RTA 405 for ~16 hours at 37°C. A: * P<0.05, compared with DMSO. The numbers on top of bars indicate the cell number for analysis in each group. B: Percent inhibition was calculated by normalizing responses in the RTA 405-treated cells to the response in DMSO-treated cells. The data was fit to a sigmoidal curve with 4 parameters and IC50 was calculated as a function of the fitting curve.
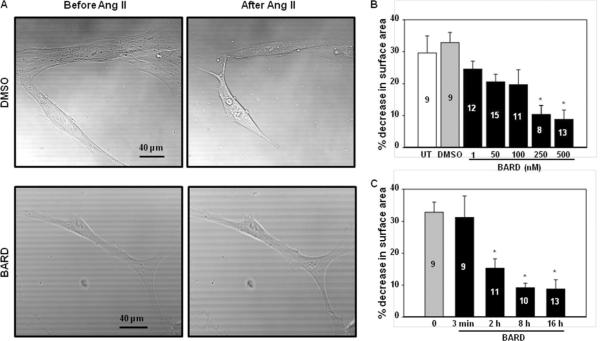
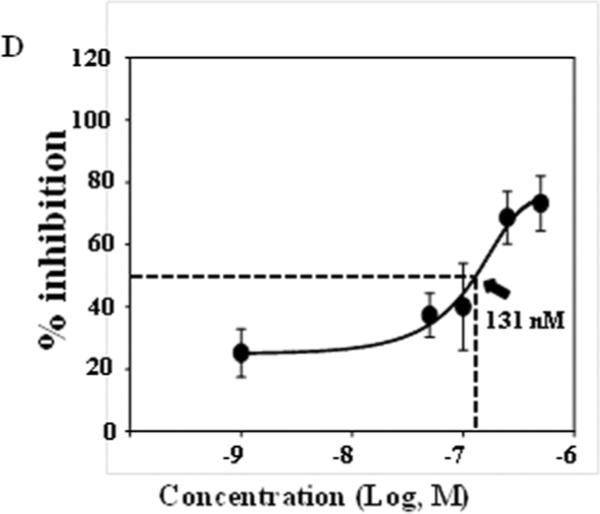
We also examined the effect of bardoxolone methyl on Ang II-induced MC contraction. As shown in Fig. 6A and B, Ang II induced a remarkable reduction of the planar surface area of control MCs. This response was attenuated by bardoxolone methyl in a dose-dependent manner. Significant inhibition was observed at 250 nM and 500 nM (Fig. 6B). Fig. 6C shows the time-course effect of bardoxolone methyl treatment at 500 nM. Pre-treatment with bardoxolone methyl for 3 minutes did not have any effect on the Ang II-evoked contraction. However, significant inhibition was observed with pre-treatment lengths of 2h, 8h and 16h. Fig. 6D shows the inhibitory efficiency of bardoxolone methyl on MC contractility. These results demonstrate that bardoxolone methyl has an inhibitory effect on Ang II-induced contraction of human MCs in a dose- and time-dependent manner.
Fig. 6. Effect of Bardoxolone methyl on Ang II-induced contraction of human MCs.
A. Representative morphology of MCs before and 30 min after 1 μM Ang II stimulation. Cells were treated with DMSO (vehicle) or bardoxolone methyl (BARD) at 500 nM for ~16 h at 37°C. B. Dose dependent effect of bardoxolone methyl on Ang II-induced MC contraction. Cells were treated with DMSO or various concentrations of bardoxolone methyl for ~16 h at 37°C. UT, untreated; * P<0.05, compared with DMSO group. The numbers in bars indicate the number of cells analyzed. C. Time course effect of bardoxolone methyl on Ang II-induced MC contraction. MCs were treated with either DMSO or bardoxolone methyl at 500 nM for different time periods at 37°C. * P<0.05, compared with both 0 and 3 min groups. The numbers inside bars indicate the number of cells analyzed. D: Percent inhibition was calculated by normalizing responses in bardoxolone methyl-treated cells to the response in DMSO-treated cells. The data was fit to a sigmoidal curve with 3 parameters and IC50 was calculated as a function of the fitting curve.
Effect of RTA 405 on Ang II-induced Ca2+ Elevation in Mesangial Cells
Ang II-induced contraction of MCs is mediated by an increase in [Ca2+]i.18,19 We postulated that RTA 405 would suppress the Ang II-induced increase in [Ca2+]i resulting in suppression of MC contraction. To test this hypothesis, we assessed fura-2 fluorescence-indicated [Ca2+]i in response to Ang II in MCs with and without RTA 405 treatment. Consistent with previous studies,17 Ang II evoked a rapid and transient increase in cytosolic Ca2+ in the presence of extracellular 1 mM Ca2+. Removal of extracellular Ca2+ immediately reduced the [Ca2+]i to lower than baseline levels (Fig. 7A, UT). The Ang II response was inhibited by losartan, indicating an AT1 receptor-mediated effect (Fig. 7A). Treatment with PEG-catalase, a potent ROS scavenger, also markedly reduced the Ca2+ response, suggesting an involvement of ROS in the Ang II signaling in MCs (Fig. 7A). The Ang II response had the same pattern in control and RTA 405-treated cells, but the response was significantly attenuated in RTA 405-treated cells (Fig. 7A). Interestingly, RTA 405 also lowered basal [Ca2+]i. Calculated increases in [Ca2+]i in response to Ang II in MCs with different treatments are presented in Figure 7B.
Fig. 7. Effect of RTA 405 on Ang II-induced Ca2+ response in human MCs.
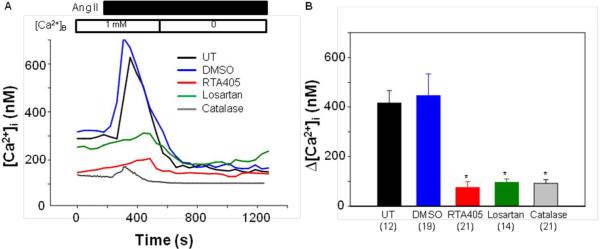
A. Representative traces, showing [Ca2+]i in response to 1 μM Ang II in MCs without treatment (UT) or treated with DMSO at 1:1000 dilution or RTA 405 at 100 nM for ~16 h, or with losartan at 5 μM for 5 min, or with PEG-catalase at 300 unit/ml for 30 min. [Ca2+]B indicates the Ca2+ concentration in bathing solution. B. Summarized Ca2+ increases in response to Ang II from experiments presented in A. Δ[Ca2+]i was calculated by subtracting [Ca2+]i before application of Ang II from the peak [Ca2+]i after application of Ang II. The numbers inside the parentheses represent the number of cells analyzed. * P<0.01, compared with both UT and DMSO groups.
Effect of RTA 405 on ROS Production in Mesangial Cells
The activity of synthetic triterpenoids is primarily mediated through activation of the Keap1-Nrf2 pathway which regulates the expression of a host of antioxidant genes.8,9 Intracellular ROS are produced in the signaling pathways of many vasoconstrictors, such as Ang II.27–31 Thus, the inhibitory effect of RTA 405 on Ang II-induced MC contraction and [Ca2+]i might be through modulation of intracellular redox status. Measurement of peroxide-sensitive fluorescent probe 2',7'-dichlorofluorescin was conducted to examine intracellular ROS levels. As shown in Fig. 8A, RTA 405 treatment significantly reduced the basal level of peroxide as compared to control cells. More importantly, Ang II significantly increased intracellular peroxide levels (Fig. 8B) and this response was blocked by losartan (Fig 8B). RTA 405 significantly suppressed the Ang II-evoked increase in peroxide levels (Fig. 8B). These results demonstrate that RTA 405 not only reduces the basal intracellular level of ROS, but also inhibits agonist-stimulated ROS production in MCs.
Fig. 8. Effect of RTA 405 on intracellular ROS production in human MCs.
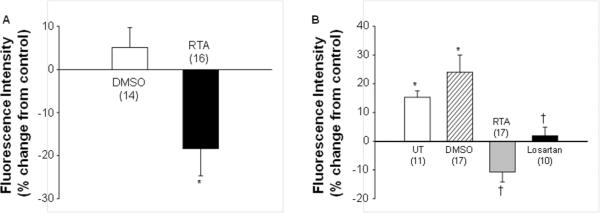
A. Basal intracellular ROS levels in MCs treated with either DMSO at 1:1000 dilution or RTA 405 (RTA) at100 nM for ~16 h. DCF-indicated intracellular ROS levels were measured with a fluorescence plate reader and were expressed as percent changes from the fluorescence intensity in MCs without treatment. * P<0.05, compared with DMSO group. B. Production of intracellular ROS in response to Ang II (1 μM) in MCs without treatment (UT) or treated with DMSO at 1:1000 dilution or RTA 405 at 100 nM for ~16 h at 37°C, or treated with losartan at 5 μM for 5 min at room temperature. The intracellular ROS levels in all groups were expressed as percent changes in fluorescence intensity from the level in MCs without any treatment. * P<0.05, compared with baseline; †: * P<0.05, compared with both UT and DMSO groups. The numbers inside parentheses represent the number of experiments repeated in each group.
Effect of Bardoxolone Methyl on Nrf2 Target Gene Expression in Mesangial Cells
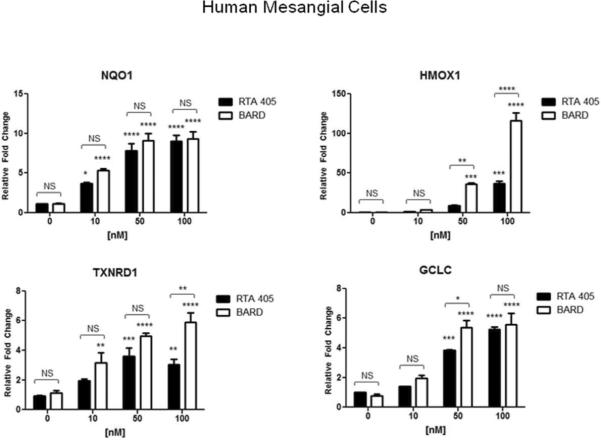
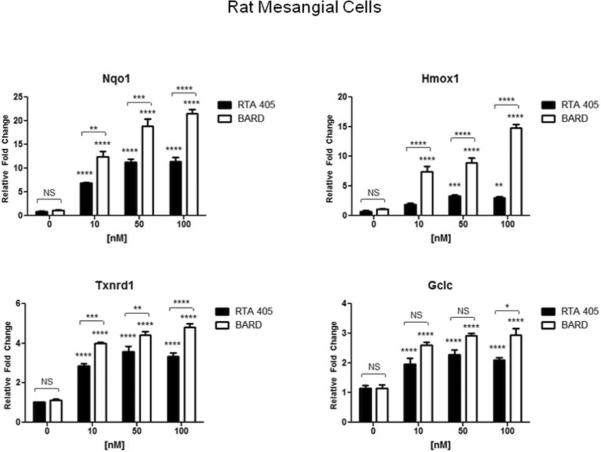
Nrf2 regulates the transcription of a wide array of antioxidant and cytoprotective genes. The synthetic triterpenoids have previously been shown to inhibit ROS production in a Nrf2-dependent manner.8,33 Therefore, it is possible that suppression of Ang II-induced H2O2 by RTA 405 in MCs is mediated through activation of Nrf2. To determine whether the Keap1-Nrf2 pathway is functional and responsive to synthetic triterpenoids in MCs, human and rat MCs were treated with increasing concentrations of bardoxolone methyl or RTA 405, and expression of Nrf2 target genes heme oxygenase (decycling) 1 (HMOX1), NAD(P)H dehydrogenase, quinone 1 (NQO1), glutamate-cysteine ligase, catalytic subunit (GCLC), and thioredoxin reductase 1 (TXNRD1), was monitored by quantitative RT-PCR (Fig. 9). In both cell lines, bardoxolone methyl and RTA 405 significantly increased transcript levels of Nrf2 target genes in a dose-dependent manner, with bardoxolone methyl being generally more potent than RTA 405. (Fig. 9). Acute (30 min) or prolonged (16.5 h) treatment of MCs with Ang II did not affect the RTA 405-mediated increase in expression of these Nrf2 target genes (Supplementary Information, Fig. 1S). Furthermore, RTA 405 treatment also increased the activity of several Nrf2- related enzymes in rat kidneys (Supplementary Information, Fig. 2S).
Fig. 9. Bardoxolone methyl and RTA 405 induced expression of Nrf2 target genes in cultured MCs.
Human MCs (A) and rat mesangial cells (B) were treated with the indicated concentrations of bardoxolone methyl or RTA 405 for 16 hours. Cells were harvested for RNA isolation and cDNA synthesis, and Nrf2 target gene expression was evaluated by real-time PCR. Fold-change values (relative to DMSO-treated samples) are the average of three independent experiments. Nqo1: NAD(P)H dehydrogenase quinone 1; Hmox1: heme oxygenase 1; Gclc: glutamate-cysteine ligase, catalytic subunit; Txnrd1: thioredoxin reductase 1. Error bars represent standard deviation.error of the mean. * P<0.05; ** P<0.01; *** P<0.001; **** P<.<0.0001 vs. 0 nM. NS; not significant.
DISCUSSION
Recently completed phase 2 clinical trials with bardoxolone methyl have demonstrated a significant improvement in eGFR in patients with CKD and T2D.15,16 However, the precise mechanism responsible for this effect is still being investigated. Consistent with the clinical data for bardoxolone methyl, the present study demonstrated that RTA 405 increased basal GFR and blunted Ang II-induced GFR decline in Sprague Dawley rats. RTA 405 was used in these animal studies as it possesses pharmacologic properties that are similar to bardoxolone methyl, but is better tolerated by Sprague Dawley rats. Based on the similar effect of RTA 405 and bardoxolone methyl in rats and humans, we conducted additional ex vivo and in vitro experiments to investigate the mechanism underlying the observed increase in GFR.
Blood pressure, renal blood flow, and mesangial tone are regulators of GFR. Increased blood pressure could lead to increased GFR by raising net filtration pressure. This study shows that treatment of rats with RTA 405 does not increase basal or Ang II-increased blood pressure. Consistent with this result, Pergola et al. demonstrated that the change in eGFR in CKD patients did not correlate with a change in blood pressure after 52 weeks of treatment with bardoxolone methyl.16 Therefore, RTA 405-induced increase in GFR is unlikely to be due to increase in systemic blood pressure.
The increase in GFR seen with RTA 405 could also result from an increase in RPF. In the present study, despite a significant increase in basal GFR, there was no significant change in basal RPF in Sprague Dawley rats treated with RTA 405, as measured by PAH clearance (Fig. 2C). Furthermore, rats treated with RTA 405 maintained a significantly high level of GFR after administration of Ang II without significant changes in RPF. Taken together, these observations do not support changes in renal hemodynamics as a mechanism for the effect of RTA 405 on GFR.
In addition to renal hemodynamics, changes in the surface area and permeability of the glomerular filtration membrane can also alter GFR. A major finding of this study was that RTA 405 significantly inhibits MC contraction. MCs are located within glomerular capillary loops and their contractile properties enable them to alter intraglomerular capillary flow and filtration surface area and thereby GFR. Previous studies demonstrate that MCs are a target for Ang II-mediated regulation of GFR.17,24,25 In the present study, RTA 405 dose-dependently suppressed Ang II-stimulated contraction of human MCs and Ang II-induced decrease in the volume of rat glomeruli. Taken together, these data suggest that inhibition of Ang II-induced MC contraction results in preservation of filtration surface area in whole glomeruli. These in vitro and ex vivo findings are consistent with the effect of RTA 405 on GFR observed in intact animals, and further suggest potential contribution from a MC-mediated mechanism for improvement of eGFR in CKD patients treated with bardoxolone methyl.
Based on the conclusion that a MC-mediated mechanism plays a role in the effect of RTA 405 on GFR, we set out to further elucidate the mechanism of action of this class of drugs in cultured MCs. As in vascular smooth muscle cells, the contractility of MCs is tightly controlled by [Ca2+]i. Ang II-induced elevation of intracellular Ca2+ is attributed to release from the sarcoplasmic/endoplasmic reticulum, as well as influx from the extracellular compartment.17,33,34 It has recently been demonstrated by our group and others that voltage-operated, receptor-operated and store-operated Ca2+ channels are involved in Ang II-mediated Ca2+ entry and MC contraction.17,35,36 In particular, we have demonstrated the important role of the canonical transient receptor potential 1 (TRPC1) and 6 (TRPC6) in this process in human MCs.17,20 These receptors function as store-operated and/or receptor-operated Ca2+ channels and are structurally and functionally distinct from the L-type voltage-gated calcium channels that are inhibited by calcium channel blockers such as amlodipine.37–39 Although a direct effect of RTA 405 on the TRPC1 and TRPC6 calcium channels can not be formally excluded at this time, it is likely that the inhibition of Ang II-induced Ca2+ influx is related to the potent antioxidant effects of this class.
Numerous reports from studies of vascular smooth muscle cells have demonstrated that ROS activate diverse pathways to increase [Ca2+]i and subsequently trigger smooth muscle contraction.40 Likewise, reducing ROS levels in vascular smooth muscle cells has been shown to inhibit Ang II-induced increases in [Ca2+]i and cell contraction.29,30 MCs and vascular smooth muscle cells have similar properties and we hypothesized that the mechanisms that occur in smooth muscle cells involving modulation of Ca2+ influx by ROS may also apply to MCs. In this study, we have demonstrated that PEG-catalase abolishes the Ang II-mediated increase in [Ca2+]i in mesangial cells, supporting a role for ROS in Ang II-mediated MC contraction. As antioxidants and modulators of inflammation, synthetic triterpenoids upregulate a variety of antioxidant genes through activation of the Keap1/Nrf2 pathway.8,14,32 The present study showed that RTA 405 treatment significantly reduced intracellular ROS levels in cultured MCs, which correlates well with induction of antioxidant Nrf2 target genes in MCs treated with bardoxolone methyl and RTA 405. Thus, similar to the mechanism established in vascular smooth muscle cells, induction of Nrf2 and the corresponding decrease in intracellular ROS levels by RTA 405 in MCs may contribute to inhibition of the Ca2+ response and MC contraction that occurs with RTA 405 treatment.
We recently found that H2O2 has dual effects on TRPC6 channels.19,20,30,31 On the one hand, H2O2 acutely activated TRPC6 channels.30,31 On the other hand, chronic exposure to ROS suppressed the synthesis of TRPC6 protein19,20 and thus, decreased the number of functional channels. In the present experimental settings, RTA 405 suppressed Ang II-induced responses in a range of minutes (Ca2+ response) or tens of minutes (contraction). Therefore, the observed RTA 405 effects are consistent with inhibition of acute ROS effects.
In summary, our findings integrated from in vivo, ex vivo and in vitro studies demonstrate that RTA 405 increased GFR at resting state and during Ang II stimulation in Sprague Dawley rats. Our results implicate induction of Nrf2, reduction of intracellular ROS levels, suppression of the glomerular MC Ca2+ response, and suppression of MC contraction as mechanisms contributing to the underlying effect. Inhibition of MC contraction may lead to a relative increase in glomerular capillary surface area resulting in improved GFR. This conclusion is schematically depicted in Fig. 10. Since the activity of the Ang II system and the intracellular levels of ROS in kidneys are substantially elevated in CKD,1 our study provides a cellular and molecular mechanism that likely contributes to the improvement of renal function by bardoxolone methyl in patients with CKD.
Fig. 10. The mechanistic pathway for inhibition of Ang II-induced MC contraction by synthetic triterpenoids.
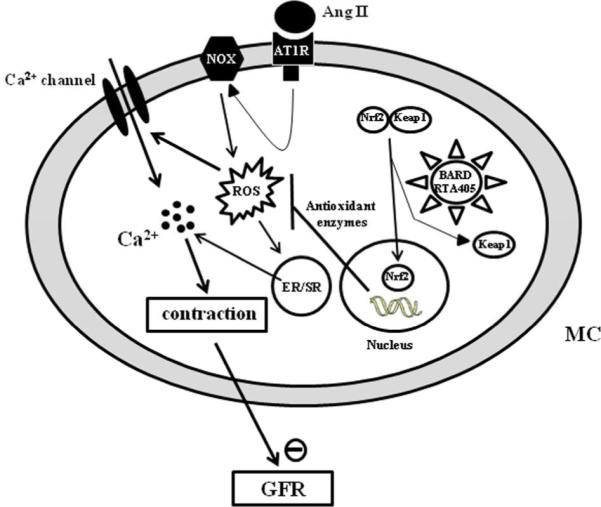
In MCs, Ang II generates ROS by activating NADPH oxdiases (NOX) through AT1 receptor signaling pathway. ROS subsequently raises [Ca2+]i either by stimulating Ca2+ release from endoplastic reticulum (ER)/sarcoplastic reticulum (SR) or by acutely activating Ca2+ permeable channels in the plasma membrane or both. An elevation of intracellular Ca2+ level triggers MC contraction and eventually results in a decrease in GFR. Synthetic triterpenoids bind to Keap1 in the cytoplasm of MC, which activates the Nrf2 transcription factor. In the nucleus of MCs, activated Nrf2 then promotes transcription of a variety of genes encoding antioxidant enzymes. The increased abundance of antioxidant enzymes reduces intracellular ROS produced by the Ang II signaling pathway, and consequently maintains an intracellular redox balance during Ang II stimulation (for simplicity, a classical Ang II-AT1 receptor signaling pathway was omitted).
METHODS
Animals and drug delivery
A total of eighty-one male Sprague Dawley rats, between two and three months of age, were used in this study. All rats were purchased from Harlan (Indianapolis, IN). The rats were randomly distributed into the sesame-oil-treated (vehicle control) and RTA 405-treated groups. The rats in RTA 405 group and vehicle control group were administered RTA 405 at 100 mg/kg body weight or the same volume of vehicle (sesame oil), respectively via oral gavage once daily for 3 consecutive days. Care and use of all animals in this study were in strict agreement with the guidelines set forth by the University of North Texas Health Science Center.
Measurement of GFR and RPF
As described in our previous publication17 with slight modification. In brief, all rats were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg). Physiological saline solution (PSS) containing 10 mg/ml FITC-inulin and para-aminohippurate (PAH) was infused into the left jugular vein at a rate of 1 ml/h/100 g body weight. After a 1 hour equilibration period, a blood sample (~100 μl) was taken and urine was collected during the next 30-min period. Then, the inulin and PAH-PSS solution containing 1 μM Ang II was infused into the rats. After 30 min equilibration period, a 30-min urine sample was collected again. At the end of the period, a larger plasma sample was taken. Concentrations of both inulin and PAH were measured using a PerkinElmer 2030 Multilabel Reader (VictorTM X3, PerkinElmer Singapore Pte Ltd). FITC-inulin was measured with excitation at 485 nm and emission at 538 nm while PAH concentration was evaluated by measuring absorbance at 450 nm.
Measurement of arterial blood pressure
Rats were administered sesame oil or RTA 405 (100 mg/kg body weight) by oral gavage once daily for three consecutive days. Blood pressure measurement in conscious rats was conducted at baseline and 8 hours post-dose on Day 3 via tail cuff using the KENT CODA apparatus. Blood pressure response to acute infusion of Ang II was measured in anaesthetized rats (intraperitoneal injection of pentobarbital at 50 mg/kg). The left carotid artery was cannulated with PE-tubing which was connected to the PL3508/P PowerLab 8/35 system (AD Instruments Inc., Colorado Springs, CO, USA) for blood pressure recording and analysis.
Glomerulus isolation and contraction assay
Isolation of glomeruli was described in our previous study.19 The glomeruli were transferred to coverslips in 35-mm petri dishes, incubated at 37°C for >30 min, and visualized under an inverted microscope (Nikon, TE-2000S, Nikon, Melville, NY). Images were captured before, 1 min after, and 20 min after application of 1 μM Ang II. The perimeter of each glomerulus was measured and the spherical surface area was calculated using ImageJ software (NIH). Glomerular volume was estimated using the following formula: V=(4A/3)(A/π)½, where A is spherical surface area and V is volume. Contractile response of a glomerulus was estimated by a difference in glomerular volume before and after Ang II.
MC contractility assay
Ang II-induced MC contraction was measured by changes in planar surface area as described in our previous publication.17
Cell culture
Human MCs were from Lonza (Basel, Switzerland) and rat MCs were from ATCC (Manassas, VA). MCs were cultured by standard methods.39
Fluorescence measurement of [Ca2+]i
Measurements of [Ca2+]i in MCs using fura-2 were performed using dual excitation wavelength fluorescence microscopy. MCs were loaded with fura-2 for 50 min at room temperature in the dark in PSS containing 2 μM acetoxymethyl ester of fura-2 (fura-2/AM), 0.09 g/dl DMSO, and 0.018 g/dl Pluronic F-127 (Molecular Probes, Eugene, OR) followed by washing 3 times. The cells were then incubated with fura-2 free PSS for an additional 20 min. Fura-2 fluorescence was monitored by a ratio technique (excitation at 340 and 380 nm, emission at 510 nm) using NIS Elements ARTM software (Nikon Instruments Inc., Melville, NY) at room temperature. [Ca2+]i was calculated using the software following the manufacturer's instructions. Calibrations were performed in vivo at the end of each experiment, and conditions of high [Ca2+]i were achieved by addition of 5 μM ionomycin, whereas conditions of low [Ca2+]i were obtained by addition of 5 mM EGTA.
Measurement of Intracellular H2O2 Level
Intracellular H2O2 level was estimated by measuring 2',7'-dichlorodihydrofluorescein (DCF) fluorescence as described in our previous study.30
Expression of Nrf2 target genes
Sub-confluent human (NHMC) or rat (RMC) mesangial cells were treated with DMSO or bardoxolone methyl for 16 hours. RNA was isolated using RNeasy® columns (Qiagen), cDNA was synthesized using iScript™ (Bio-Rad), and quantitative PCR performed in the presence of SYBR green using the Bio-Rad CFX96 Real-Time PCR System. The 2-ΔΔCt method was used to calculate changes in abundance of mRNA transcripts relative to DMSO-treated controls.
Materials
RTA 405 and bardoxolone methyl were synthesized by Reata Pharmaceutical Inc. (Irving, TX). All other chemicals were purchased from Sigma-Aldrich (Sigma, St. Louis, MO).
Statistical Analysis
Data were reported as means ± SE except Fig. 3 in which data were expressed as means ± SD. The one-way ANOVA plus Student-Newman-Keuls test, Student unpaired t-test, and Student paired t-test were used to analyze the differences among multiple groups, between two groups, and before and after treatment in the same group, respectively. GraphPad Prism Software was used to assess differences between RTA 405 and Bardoxolone methyl effects on Nrf2 target gene induction using a two-way ANOVA Bonferroni Multiple Comparison Test. P<0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
The work was supported by National Institutes of Health (NIH) of USA (5RO1DK079968-01A2, R. Ma). This work was also supported in part by Reata Pharmaceuticals, Inc. The authors wish to thank Dr. Scott Reisman and Dr. Justin Rains for performing experiments to assess the enzymatic activity of Nrf2 target enzymes. We are also grateful to Drs. Keith Ward and Jim McKay for their critical review and editing of the manuscript.
Source of supports
National Institutes of Health (NIH) of USA
Reata Pharmaceuticals, Inc., Irving, Texas, USA
Footnotes
Supplemental information is available at Kidney International's website.
DISCLOSURE Rhesa Stidham, Ron Bumeister, Isaac Trevino, Deborah A. Ferguson, Colin J. Meyer, and W. Christian Wigley are employed by Reata Pharmaceuticals, Inc. All other authors declared no competing interests.
REFRENCES
- 1.Townsend RR, Feldman HI. Chronic kidney disease progression. NephSAP. 2009;8:271–288. [Google Scholar]
- 2.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 3.Levin A, Djurdjev O, Beaulieu M, et al. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis. 2008;52:661–671. doi: 10.1053/j.ajkd.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Townsend RR, Feldman HI. Chronic kidney disease: past problems, current challenges, and future facets. NephSAP. 2009;8:239–243. [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patient with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.Cachofeiro V, Goicochea M, de Vinuesa SG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 8.Dinkova-Kostova AT, Liby KT, Stephenson KK, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. PNAS. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 10.Yoh K, Itoh K, Enomoto A, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 11.Jiang T, Huang Z, Lin Y, et al. The protective role of Nrf2 in STZ-induced diabetic nephropathy. Diabetes. 2010;59:850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HJ, Vaziri HD. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298:F662–671. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 13.Tan Y, Ichikawa T, Li J, et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–633. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu QQ, Wang Y, Senitko M, et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol Renal Physiol. 2011;300:F1180–F1192. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pergola PE, Krauth M, Huff JW, et al. Effect of bardoxolone methyl on kidney function in patients with T2D and stage 3b-4 CKD. Am J Nephrol. 2011;33:469–476. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 16.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 17.Du J, Sours-Brothers S, Coleman R, et al. Canonical transient receptor potential 1 channel is involved in contractile function of glomerular mesangial cells. J Am Soc Nephrol. 2007;18:1437–1445. doi: 10.1681/ASN.2006091067. [DOI] [PubMed] [Google Scholar]
- 18.Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- 19.Graham S, Ding M, Sours-Brothers S, et al. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells. Am J Physiol Renal Physiol. 2007;293:F1381–F1390. doi: 10.1152/ajprenal.00185.2007. [DOI] [PubMed] [Google Scholar]
- 20.Graham S, Gorin Y, Abboud HE, et al. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am J Physiol Cell Physiol. 2011;301:C304–C315. doi: 10.1152/ajpcell.00014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 22.Whiteside CI, Hurst RD, Stevanovic ZS. Calcium signaling and contractile response of diabetic glomerular mesangial cells. Kidney Int Suppl. 1995;48:S28–S33. [PubMed] [Google Scholar]
- 23.Striker LJ, Doi T, Elliot S, et al. The contribution of glomerular mesangial cells to progressive glomerulosclerosis. Semin Nephrol. 1989;9:318–328. [PubMed] [Google Scholar]
- 24.Navar LG, Rosivall L. Contribution of the renin-angiotensin system to the control of intrarenal hemodynamics. Kidney Int. 1984;25:857–868. doi: 10.1038/ki.1984.102. [DOI] [PubMed] [Google Scholar]
- 25.Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 26.Schlöndorff D, Bana B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20:1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 27.Seshiah PN, Weber DS, Rocic P, et al. Angiotensin II stimulation of NAD(P)H oxidase activity upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 28.Griendling KK, Sorescu D, Lassègue B, et al. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 29.Lyle A, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology. 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 30.Graham S, Ding M, Ding Y, et al. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem. 2010;285:23466–23476. doi: 10.1074/jbc.M109.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Y, Winters A, Ding M, et al. Reactive oxygen species-mediated TRPC6 activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem. 2011;286:31799–31809. doi: 10.1074/jbc.M111.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sporn MB, Liby KT, Yore MM, et al. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74:537–545. doi: 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda K, Meyer-Lehnert H, Kim JK, et al. Effect of angiotensin II on Ca2+ kinetics and contraction in cultured rat glomerular mesangial cells. Am J Physiol. 1988;254:F254–F266. doi: 10.1152/ajprenal.1988.254.2.F254. [DOI] [PubMed] [Google Scholar]
- 34.Stockand JD, Sansom SC. Large Ca2+-activated K+ channels responsive to angiotensin II in cultured human mesangial cells. Am J Physiol. 1994;267:C1080–C1086. doi: 10.1152/ajpcell.1994.267.4.C1080. [DOI] [PubMed] [Google Scholar]
- 35.Menè P, Teti A, Pugliese F, et al. Calcium release-activated calcium influx in cultured human mesangial cells. Kidney Int. 1994;46:122–128. doi: 10.1038/ki.1994.251. [DOI] [PubMed] [Google Scholar]
- 36.Kim MS, Zeng W, Yuan J, et al. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem. 2009;284:9733–9741. doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worley PF, Zeng W, Huang GN, et al. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietrich A, Gudermann T. TRPC6. Handb Exp Pharmacol. 2007;179:125–141. doi: 10.1007/978-3-540-34891-7_7. [DOI] [PubMed] [Google Scholar]
- 39.Ma R, Smith S, Child A, et al. Store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol. 2000;278:F954–F961. doi: 10.1152/ajprenal.2000.278.6.F954. [DOI] [PubMed] [Google Scholar]
- 40.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med. 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.