Abstract
Musculoskeletal pain conditions, particularly those associated with temporomandibular joint and muscle disorders (TMD) affect a large percentage of the population. Identifying mechanisms underlying hyperalgesia could contribute to the development of new treatment strategies for the management of TMD and other muscle pain conditions. In this study, we provide evidence of functional interactions between two ligand-gated channels, P2X3 and TRPV1, in trigeminal sensory neurons, and propose that the interactions serve as an underlying mechanism for the development of mechanical hyperalgesia. Mechanical sensitivity of the masseter muscle was assessed in lightly anesthetized rats via an electronic anesthesiometer (Ro et al., 2009). Direct intramuscular injection of a selective P2X3 agonist, αβmeATP, induced a dose- and time-dependent hyperalgesia. Mechanical sensitivity in the contralateral muscle was unaffected suggesting local P2X3 mediate the hyperalgesia. Anesthetizing the overlying skin had no effect on αβmeATP-induced hyperalgesia confirming the contribution of P2X3 from muscle. Importantly, the αβmeATP-induced hyperalgesia was prevented by pretreatment of the muscle with a TRPV1 antagonist, AMG9810. P2X3 was co-expressed with TRPV1 in masseter muscle afferents confirming the possibility for intracellular interactions. Additionally, in a subpopulation of P2X3/TRPV1 positive neurons, capsaicin-induced Ca2+ transients were significantly amplified following P2X3 activation. Finally, activation of P2X3 induced phosphorylation of serine, but not threonine, residues in TRPV1 in trigeminal ganglia cultures. Significant phosphorylation was observed at 15 min, the time point at which behavioral hyperalgesia was prominent. Previously, activation of either P2X3 or TRPV1 had been independently implicated in the development of mechanical hyperalgesia. Our data propose P2X3 and TRPV1 interact in a facilitatory manner, which could contribute to the peripheral sensitization known to underlie masseter hyperalgesia.
Keywords: rat, trigeminal ganglia, masseter, mechanical hyperalgesia, αβmeATP
1. Introduction
Patients with temporomandibular disorders (TMD) often suffer from mechanical hyperalgesia arising in masticatory muscles (Harness et al., 1990; Stohler, 1999). Transient Receptor Potential V1 (TRPV1) are increasingly recognized as important players underlying mechanical hyperalgesia (Pomonis et al., 2003; Walker et al., 2003; Honore et al., 2005; Fujii et al., 2008). Specifically in muscle, blocking TRPV1 attenuates mechanical hyperalgesia induced by eccentric muscle contraction (Fujii et al., 2008). Additionally, masseteric injection of the TRPV1 agonist capsaicin significantly lowers noxious mechanical thresholds in humans and rats (Arendt-Nielsen et al., 2008; Ro et al., 2009), which could result from sensitization of muscle nociceptors (Hoheisel et al., 2004).
TRPV1 function as signal integrators downstream of several pro-inflammatory G-protein coupled receptors (GPCRs) (Levine and Alessandri-Haber, 2007). In particular, TRPV1 functionally interact with the P2Y purinergic GPCRs. P2Y1 potentiates TRPV1 in a PKC-dependent manner (Tominaga et al., 2001). P2Y2 knockout (KO) mice exhibit deficits in capsaicin-induced Ca2+ transients and thermal hyperalgesia (Malin et al., 2008). Similarly, TRPV1 KO mice exhibit no ATP-induced thermal hyperalgesia. Selective activation of P2Y2 potentiates capsaicin currents in dorsal root ganglia (DRG) neurons (Moriyama et al., 2003).
Recently, TRPV1 were also linked to the ionotropic purinergic receptor P2X3 in DRG (Piper and Docherty, 2000; Stanchev et al., 2009). The channels co-immunoprecipitate when co-transfected in HEK293 cells (Stanchev et al., 2009). Alpha,beta-methyleneadenosine triphosphate (αβmeATP), a P2X3 agonist used to simulate ATP release, restores the attenuation of TRPV1 expression in a PKC dependent manner in DRG following sympathectomy (Xu et al., 2010). These data suggest P2X3 and TRPV1 interact at the cellular level.
P2X3 have been implicated in craniofacial pain. Increased face wiping behavior following tooth movement was attenuated by the P2X antagonist TNP-ATP (Yang et al., 2009). Carrageenan or αβmeATP injections into the temporomandibular joint (TMJ) evoked nociceptive responses that were significantly blocked by co-injection with the P2X antagonist PPADS (Oliveira et al., 2005). In muscle, masseteric injection of αβmeATP dose-dependently reduced pressure pain threshold (Shinoda et al., 2008). Although the involvement of peripheral P2X3 in craniofacial pain is evident the cellular mechanisms remain to be elucidated.
Recent studies in DRG have linked P2X3 activation to Ca2+-dependent kinases, which are known to sensitize TRPV1 (Bhave et al., 2003; Jung et al., 2004). Treatment of DRG cultures with ATP or αβmeATP resulted in translocation and increases in the phosphorylated form of CaMKII (Hasegawa et al., 2009). If P2X3 are also co-expressed with TRPV1 in masseter afferents similar pathways could underlie P2X3-induced hyperalgesia. Since a copious amount of ATP is released in the muscle during injury or inflammation (Reinohl et al., 2003; Hoheisel et al., 2004), we hypothesized that activation of masseteric P2X3 invokes signaling cascades that converge on TRPV1 producing mechanical hyperalgesia. We first reproduced and validated P2X3-induced mechanical hyperaglesia in our orofacial muscle pain model. We then investigated whether (1) P2X3-induced masseter mechanical hyperaglesia is TRPV1-dependent, (2) P2X3 and TRPV1 co-express in masseter afferents, (3) P2X3 activation enhances TRPV1 function in trigeminal ganglia (TG), and (4) P2X3 activation leads to TRPV1 phosphorylation in TG.
2. Experimental Procedures
2.1 Animals
Male Sprague Dawley rats (100–150 and 300–350 gm; Harlan, Indianapolis) were used in the present study. A total of 153 rats were used to complete this study. All of the animals were housed in a temperature-controlled room under a 12:12 light-dark cycle with access to food and water ad libitum. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) and under a University of Maryland approved Institutional Animal Care and Use Committee protocol.
2.2 Behavioral Studies
Noxious chemical or mechanical stimulation of the masseter muscle evokes characteristic shaking of the ipsilateral hindpaw in lightly anesthetized rats (Ro et al., 2003; Han et al., 2008; Sanchez et al., 2010). We have previously described the use of this behavior for testing mechanical sensitivity of the masseter muscle in rats (Ro and Capra, 2006; Ro et al., 2007; Ro et al., 2009; Saloman et al., 2011). The lightly anesthetized model allows standardized delivery of drugs and repeated application of mechanical stimuli in deep craniofacial tissue such as the masseter or TMJ which is much more difficult in awake-behaving rats. Briefly, rats were initially anesthetized with an intraperitoneal (i.p.) injection of sodium pentobarbital (40 mg/kg). A level of ‘light’ anesthesia was determined by providing a noxious pinch to the tail or the hindpaw with a serrated forceps. Rats typically respond to the noxious pinch on the tail with an abdominal contraction and with a withdrawal reflex to the noxious pinch of a hindpaw about 15 min after the initial anesthesia. Once the animal reached this level a metal clip calibrated to produce 600 gm of force was applied 5 consecutive times, and experiments were continued only after the animals showed a reliable reflex response to every clip application. A tail vein was connected to an infusion pump (Harvard Apparatus, Pump11) for continuous infusion of pentobarbital. The rate of infusion was adjusted to maintain a relatively light level of anesthesia throughout the duration of the experiment (3 mg/hr).
The baseline mechanical threshold for evoking hindpaw responses was determined 15 min prior to drug injections using an electronic VF anesthesiometer (IITC Life Science, Inc, Woodland Hills, CA). A rigid tip (diameter 2 mm) attached to the VF meter was applied to the masseter muscle until the animals responded with hindpaw shaking. The animal’s head was rested flat against the surface of the table when pressing the anesthesiometer on the masseter in order to provide a stable set-up. The threshold was defined as the lowest force necessary to evoke the hindpaw response.
Changes in masseter sensitivity were then assessed at 15, 30, 45, 60 and 90 min following drug treatments. We calculated percent changes in VF thresholds following drug treatments with respect to the baseline threshold and plotted against time. In order to assess the overall magnitude of drug-induced changes in masseter sensitivity over time, the area under the curve (AUC) was calculated for the normalized data for each rat using the trapezoid rule. All animals were kept warm throughout the experiments with thermal blankets.
Data Analysis
For behavioral studies, the time-dependent mean percent changes in mechanical thresholds were normalized to the baseline threshold and analyzed with a two-way ANOVA with repeated measures. In addition, either the student t-test or one-way ANOVA was used to evaluate the overall magnitude of mechanical hyperalgesia assessed as AUC. All multiple group comparisons were followed by the appropriate post hoc test. The significance of these and all subsequent statistical analyses was set at P < 0.05.
2.3 Experimental and control groups for behavioral studies
To assess whether activation of P2X3 induces mechanical hyperalgesia, the masseter muscle was treated with a specific P2X3 agonist, αβmeATP, (250, 500, 750 µg/20 µl) or the vehicle. Doses were adapted from Shinoda et al. (2008). To confirm we are measuring the effect of P2X3 activation on muscular sensitivity, a topical anesthetic (20% benzocaine) was applied to the skin overlying the muscle prior to injecting the masseter with 750 µg/20 µl αβmeATP. There is also a possibility that αβmeATP injected into the masseter can mediate its effects by activating centrally located P2X3. In order to evaluate possible systemic effects, in a separate group of animals, the highest dose of αβmeATP (750 µg) was administered into the masseter contralateral to the muscle being tested for mechanical sensitivity. In order to confirm the specificity of αβmeATP effects, the masseter muscle was pretreated with a selective P2X3 antagonist, A-317491. In this experiment A-317491 (7.5, 250 µg/10 µl) or the vehicle was injected 5 min prior to the αβmeATP (750 µg). Doses of A-317491 were adapted from Wu et al. (2004). In order to determine if TRPV1 are involved in P2X3-mediated effects, animals were pretreated with a TRPV1 antagonist, AMG9810 (1, 10, 100 nmol/10 µl), or vehicle prior to αβmeATP (750 µg). In groups in which animals received multiple injections, the drugs were administered 5 min apart. The number of rats per group is indicated within the figures. In general, groups consisted of 6–8 rats, totaling 106 rats used in the behavioral studies. Further experiments examining the specificity of αβmeATP and AMG9810 were conducted using the Ca2+ Imaging technique and are described within the text.
2.4 Drug Preparation and Administration for Behavior Studies
αβmeATP (pH was adjusted to 7.0 using NaOH) and A-317491 (Sigma) were dissolved in PBS. AMG9810 (Tocris; Ellisville, MO, USA) was dissolved in 5% DMSO, 10% Tween-80, and 85% PBS (Ruparel et al., 2008). In order to make sure that the drugs and vehicles were administered in the same target region of the muscle the injection site was determined by palpating the masseter muscle between the zygomatic bone and the angle of the mandible. Injections were made with a 27-gauge needle. Upon contacting the mandible the needle was slowly withdrawn into the mid-region of the masseter and injections were made for 5–10 sec.
2.5 Labeling of Masseter Afferents and Immunohistochemistry
Initially, 4 rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). Masseter muscles were exposed and 2% Fast Blue (FB; 10 µl) was injected bilaterally into multiple sites to retrogradely label muscle afferents. To avoid leakage the needle was left in place for 1–2 min before slow retraction. The injection site was then covered with petroleum jelly and sutured (4.0 silk). After 7 days, rats underwent transcardial perfusion with 4% paraformaldehyde in PBS (250 ml; pH 7.3). TG were extracted and post-fixed for 90 min, placed in 30% sucrose at 4°C overnight, and sectioned coronally at 12 µm. Alternate sections were collected and mounted on gelatin-coated slides. The sections were incubated overnight with primary antisera for TRPV1 (1:1000; Rabbit Cat# RA10110; Neuromics, Edina, MN) and P2X3 (1:3000; Guinea pig Cat# AB5896; Millipore, Billerica, MA). For immunofluorescence, sections were incubated for 1 hr in Alexa 488 conjugated goat anti-rabbit antiserum (1:250; Invitrogen West Grove, PA) and Cy3 goat anti-guinea pig antiserum (1:250; Jackson ImmunoResearch, West Grove, PA) at room temperature. The primary antibodies for TRPV1 or P2X3 were omitted from processing from select sections to control for non-specific staining. Trigeminal and facial motor nuclei were also evaluated as positive and negative controls for FB labeling, respectively.
The immunohistochemical data were analyzed using Image J (NIH) threshold analysis. Only the labeled neurons that showed a clear nucleus were included in the counting. The percentages of masseter afferents labeled with TRPV1 and/or P2X3 were calculated and presented as mean ± standard error of the mean (SE). P2X3/TRPV1/FB positive cells were also categorized according to size (small <400µm2, medium 400–1000 µm2, and large >1000 µm2)(Chun et al., 2008).
The rabbit anti-TRPV1 corresponds to residues 4–21 of rat TRPV1 (RASLDSEESESPPQENSC). The specificity of the TRPV1 antibody has previously been established (Guo et al., 1999; Kim et al., 2008). Western blots and immunohistochemistry of DRG reveal staining colocalizes with a TRPV1 antibody targeting a different an entirely separate epitope (N-terminal) and preadsorption with peptide blocks the signal. Additionally, the antibody only detects signal in HEK293 cells transfected with TRPV1 as opposed to nontransfected cells. The guinea pig anti-P2X3 (Lot# LV1518241) is a 15 amino acid peptide corresponding to amino acids 383–397 of the carboxy-terminus of rat P2X3. The specificity of the P2X3 antibody was previously confirmed in TG by preadsorption studies as well as omission of the antibody which abolished all specific staining (Guo et al., 1999; Kim et al., 2008). Antibodies against this epitope stain HEK293 cells transfected with P2X3, but fail to stain untransfected cells (Vulchanova et al., 1997). The signal was abolished with peptide preadsorption.
2.6 Primary Culture of TG Neurons
Rat TG neurons were cultured as described previously (Lee et al., 2012; Wang et al., 2012) TG were extracted from 100–150 gm rats and minced in DMEM/F12 (Sigma) containing Horse Serum (HS) and penicillin/streptomycin/glutamine (PSG) on ice, incubated in media containing 1 mg/ml collagenase type XI (Sigma) for 30 min at 37°C with agitation. Following trituration, cells were incubated for 2 min in 0.05% Trypsin/0.1% EDTA at 37°C with agitation. For Ca2+ Imaging: One rat (2 TG) was used for one culture and cells were separated via a Percoll gradient for 12 min at 900-g and plated on poly-L-ornithine and laminin-coated glass coverslips. The neurons were cultured in 2 ml DMEM (Gibco) containing HS and PSG at 37°C under 5% CO2. 16–24 hrs later, cells were loaded with Fura2- acetoxymethyl ester (Fura2-AM) and pluronic acid (Anaspec Inc) for 40 min at 37°C. For Immunoprecipitation: TG from two rats were used for one culture and the cells were plated on laminin-coated 12-well plates. In this situation cultures were used for experimentation 48 hrs later. No growth factors or other additional supplements were included.
2.7 Ratiometric Ca2+ Imaging Study
Ratiometric Ca2+ imaging was performed as described previously (Lee et al., 2012; Wang et al., 2012). Primary TG cultures were loaded with 20 µl of 1 mM Fura2-AM and 2µl of 20% pluronic acid (Anaspec, Inc) for 40 min at 37°C in Calcium Imaging Buffer (CIB). CIB contained in mM: NaCl 130; KCl 3; CaCl2 2.5; MgCl2 0.6; 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10; Sucrose 10; NaHCO3 1.2 (pH 7.45, 320 mOsm adjusted with mannitol). Following a 15 min wash period for de-esterification, dual images (510 nm emission) were collected every 2 sec using NIS Elements (Nikon). The Fura response (FR) was defined as the ratio of background-subtracted emissions measured during excitation at 340 and 380 nm.
Drugs were diluted to final concentrations in CIB: αβmeATP (50 µM), capsaicin (10 nM, 1 µM), AMG9810 (1 µM), and A-317491 (500 nM, 1 µM, 3 µM). Stock solutions were generated as follows: αβmeATP and A-317491 10 mM in H2O, capsaicin 10 mM in ethanol, and AMG9810 10 mM in DMSO.
Data Analysis
We calculated the changes in fura response (ΔFR, FRpeak minus FRbaseline) in every cell. Baseline was defined as the average of the five data points prior to a given stimulus application. A neuron was considered a responder if the Fura response to αβmeATP or capsaicin application was above threshold. Threshold was determined following a similar method reported in Chung et al., (2003). A histogram was generated from a population of neurons to a given stimulus protocol. The ΔFR greater than two standard deviations from the peak ΔFR of non-responding neurons was classified as responsive. The data were analyzed with two-way ANOVA with repeated measures, followed by Bonferroni’s test. Non-parametric data were analyzed with a Kruskal-Wallis one-way ANOVA or Mann-Whitney test.
2.8 Immunoprecipitiation
Cultures were dissociated with Ripa lysis buffer (Cell Signaling; Danvers, Ma). Equal amounts of purified protein were incubated with 3 µl of TRPV1 antibody (Cat#PC420; Calbiochem; San Diego, CA) at 4°C overnight, and then the substrate/antibody complex was incubated with protein A/G Agarose beads (Santa Cruz Biotechnology; Santa Cruz, CA) for 2 hrs at 4°C. LDS sample buffer including SDS was added to elute proteins from the protein A/G beads. Samples were separated by 4–12% NuPage gel (Invitrogen; Carlsbad, CA) electrophoresis and subjected to immunoblotting. To assess the level of Ser and Thr phosphorylation, membranes were treated with primary antibodies anti-phosphoserine 16B4 (1:1000; Santa Cruz) or anti-phosphothreonine 14B3 (1:1000; Chemicon) followed by the secondary antibody mouse IgG HRP (1:5000). Membranes were developed with Amersham ECL Plus reagents (GE HealthCare Life Science, Piscataway, NJ), then stripped and re-probed with anti-TRPV1 (1:1000; rabbit; Calbiochem) followed by rabbit-HRP (1:5000) to verify the amount of protein. The TRPV1 antibody was a purified rabbit polyclonal antibody directed against a synthetic peptide corresponding to amino acids 824–838 of rat TRPV1. The specificity of the anti-TRPV1 antibody has been tested previously in immunoprecipitation experiments as well as TRPV1 knockout tissue (Pabbidi et al., 2008).
Chemoluminscence was applied to visualize antigen-antibody complexes on blots that were exposed to X-ray film. Films were scanned and quantified using Image J Software (NIH). Phosphorylation signals were normalized to that of pan-TRPV1, which served as the control and confirmation of the TRPV1 band. The data was subjected to a one-way ANOVA. All multiple group comparisons were followed by a post-hoc test (Duncan’s). Data are shown as mean ± SE obtained from 6 repeated experiments. TRPV1 phosphorylation at serine and threonine residues was assessed using 6 cultures each of which were generated from 2 rats. A total of 24 rats were used to complete these immunoprecipitation experiments.
3. Results
3.1 P2X3-induced masseter hyperalgesia
Intramuscular injection of αβmeATP induced a dose- and time-dependent mechanical hyperalgesia (Fig. 1). Specifically, the two highest doses of αβmeATP, 500 and 750 µg, induced significant mechanical hyperalgesia (Drug: F = 33.251, P < 0.001; Fig. 1A). Hyperalgesia was observed at 15, 30 and 45 min post injection, with mechanical thresholds returning to baseline by 60 min (Time: F = 66.046, P < 0.001, Fig. 1A). In order to examine the overall magnitude of drug effect irrespective of time, AUC was calculated for the normalized data for each rat. Both 500 and 750 µg of αβmeATP induced significant mechanical hyperalgesia compared to PBS (F = 34.701, P < 0.001, Fig. 1B). The dose of 750 µg of αβmeATP was used throughout the remainder of this study.
Figure 1.
αβmeATP induces mechanical hyperalgesia in the masseter muscle. (A) The line graphs depict P2X3 agonist αβmeATP-induced changes in mechanical thresholds in the rat masseter muscle. (B) The bar graphs show the overall effects of different doses of αβmeATP on mechanical sensitivity, as shown by Area Under the Curve. (C) The overall effect of intramuscular αβmeATP on masseter sensitivity was examined with topical application of Benzocaine (20%). (D) The overall effect of αβmeATP (750 µg) was compared between the injected and the contralateral muscles. (E) The specificity of αβmeATP was examined with A-317491, a selective P2X3-receptor antagonist. A-317491 was injected into the masseter 5 min prior to αβmeATP. BL indicates baseline 15 min prior to αβmeATP injection. Black arrow indicates αβmeATP injection at 0 min. +, * indicates significant (P < 0.05) group and time effects, respectively.
The Von Frey (VF) anesthesiometer was applied to the masseter through the overlying skin. Therefore, similar to experiments performed previously (Ro et al., 2009), the overlying skin was anesthetized with a 20% benzocaine gel to confirm changes in mechanical sensitivity resulted from activation of muscular afferents. Intramuscular injections of αβmeATP induced a mechanical hyperalgesia that was not significantly different between animals with and without anesthetized skin, as shown by the AUC (t = 0.274, P = 0.790, Fig. 1C). The efficacy of the anesthetic was confirmed with a known algesic, capsaicin. The same concentration of benzocaine completely prevented intradermal capsaicin-induced nocifensive hindpaw shaking behavior, a behavior that lasted 45 min in the absence of benzocaine. A separate group of animals was tested to rule out the possibility that a high dose of αβmeATP (750 µg) could induce systemic effects. Mechanical sensitivity was tested in the muscle contralateral to the αβmeATP injection. The AUC was significantly different from the ipsilateral masseter which suggests the αβmeATP effect was mediated by local P2X3 receptors (t = −11.977, P < 0.001, Fig. 1D). In order to confirm the specificity of αβmeATP, we pretreated the masseter with the selective P2X3 antagonist A-317491 (Jarvis et al., 2002; Wu et al., 2004). A-317491 dose-dependently blocked the effects of αβmeATP (F = 46.690, P < 0.001, Fig. 1E). In conclusion, αβmeATP induces a local P2X3 mediated masseter mechanical hyperaglesia.
3.2 TRPV1 mediates P2X3- induced masseter hyperalgesia
To determine if TRPV1 are involved in P2X3-induced masseter hyperalgesia, animals were treated with a TRPV1 antagonist, AMG9810, prior to αβmeATP in the same muscle. AMG9810 dose-dependently prevented P2X3-induced masseter hyperalgesia (Drug: F = 43.106, P < 0.001, Fig. 2A). Mechanical hyperalgesia was observed at 15, 30, and 45 min (Time: F = 170.503, P < 0.001, Fig. 2A). In order to assess the overall magnitude of drug effect irrespective of time a one-way ANOVA was performed on the AUC. AMG9810 significantly prevented αβmeATP-induced mechanical hyperalgesia compared to vehicle (F = 54.797, P < 0.001, Fig. 2B). When the highest dose of AMG9810 (100 nmol) or DMSO was given alone there was no significant drug effect (F = 2.619, P = 0.137, Fig. 2C). While there was a slight but significant elevation of the mechanical sensitivity over time neither AMG9810 nor DMSO altered mechanical thresholds at earlier time points during which αβmeATP effects are prominent (Fig. 2C). Mechanical hyperalgesia was generally resolved by these later time points (Fig. 1A) and 10 nmol AMG9810, a 10-fold lower dose, significantly prevented αβmeATP effects (Fig. 2A).
Figure 2.
αβmeATP-induced masseter hyperalgesia involves TRPV1. (A) Line graph shows changes in masseter mechanical thresholds induced by αβmeATP in the presence of the TRPV1 antagonist, AMG9810. (B) Bar graph shows the overall magnitude of drug effect as measured by Area Under Curve (AUC). BL: baseline 15 min prior to αβmeATP injection. Gray arrow: vehicle or antagonist injection 5 min prior to αβmeATP injection. Black arrow: αβmeATP injection at 0 min. (C) Mechanical thresholds were measured at the indicated time points following the injection of AMG9810 (100 nmol) or vehicle. (n = 6/group). +, * indicates significant (P < 0.05) group and time effects, respectively.
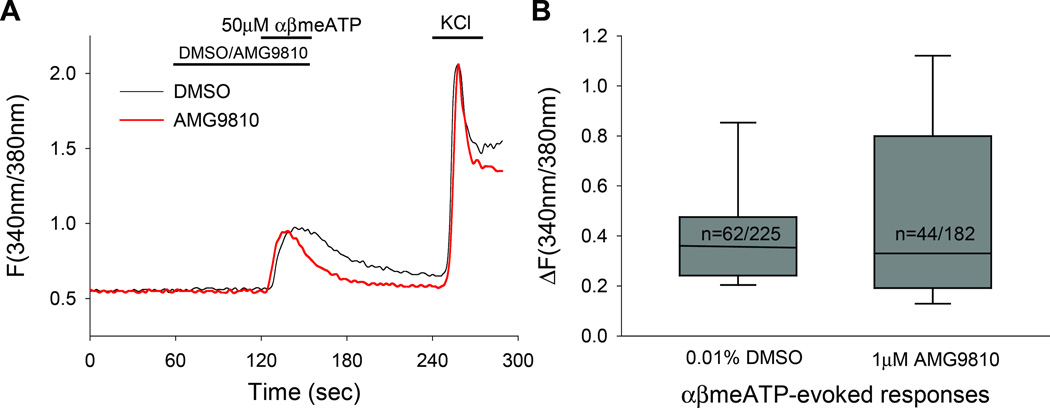
To exclude the possibility that AMG9810 directly suppresses P2X3, we examined the effects of AMG9810 on αβmeATP responses. Since it is difficult to assess the nonspecific effects of AMG9810 on P2X3 in vivo, we examined Ca2+ responses in TG cultures by performing ratiometric Ca2+ imaging. We evaluated the neuronal responses evoked by αβmeATP (50 µM) following pretreatment and co-application with either AMG9810 (1 µM) or vehicle (Fig. 3A). This protocol was tested on 2 cultures generated from 2 rats. There was no significant difference in the amplitude of αβmeATP–evoked responses between AMG9810 and vehicle treated neurons (AMG9810 = 0.51 ± 0.06, vehicle = 0.42 ± 0.03, P = 0.96, Mann-Whitney test, Fig. 3B). The proportions of the responding neurons were not significantly different either [vehicle, 27% (62/225); AMG9810, 24% (44/182); P= 0.58, Fisher’s exact test]. These results suggest that AMG9810 did not directly suppress P2X3 in the behavioral experiments.
Figure 3.
The effect of AMG9810 on αβmeATP-evoked Ca2+ responses. (A) Example traces showing αβmeATP-evoked responses in the presence of vehicle or AMG9810. (B) Box-and-whisker plot demonstrating the magnitude of αβmeATP-evoked responses in the presence or absence of AMG9810 (1 µM). The number of neurons that responded to αβmeATP as a proportion of KCl responders is displayed within the boxes. There were no statistical differences in either the amplitudes or proportion of αβmeATP-evoked responses.
3.3 Co-expression of TRPV1 and P2X3 in masseter afferents
Although co-expression of P2X3 and TRPV1 has been demonstrated in TG neurons (Eriksson et al., 1998; Ichikawa et al., 2004; Ichikawa and Sugimoto, 2004; Kim et al., 2011), to our knowledge co-expression in masseter afferents has not been reported. To establish a cellular basis for the functional interaction observed between P2X3 and TRPV1 we sought to confirm co-expression of these channels in masseter afferents. The expression profile of TRPV1 and P2X3 was assessed in 400 masseter afferents. The retrograde labeling of masseter afferents by Fast Blue (FB) was limited to the mandibular division of TG. TRPV1 were observed in predominantly small to medium sized neurons (Fig. 4A). While P2X3 were expressed in all sized cells they were predominant in medium sized neurons (Fig. 4A). Specifically, TRPV1 were expressed in 34.6 ± 5.3% in masseter afferents and P2X3 29.5 ± 7.2% (Fig 4B). TRPV1 and P2X3 were co-expressed in 7.2 ± 3.1% of masseter afferents, with a mean soma size of 646.2 ± 59.7µm2 (Fig. 4B).
Figure 4.
The somata of masseter afferents labeled by retrograde transport of Fast Blue (FB) were assessed for TRPV1 and P2X3 immunoreactivity. (A) Micrographs illustrating TRPV1 and P2X3 expression in TG neurons, an arrow indicates the FB positive neuron. (B) Soma size distribution of TRPV1 and P2X3 positive masseter afferents in TG based on cell body area (µm2), small (<400µm2), medium (400–1000 µm2), and large (>1000 µm2). The expression profile of TRPV1 and P2X3 was assessed in 400 masseter afferents. Scale bar=20µm
3.4 P2X3 sensitizes TRPV1 Ca2+ transients
To assess the P2X3-TRPV1 interaction at the cellular level we utilized the following protocol in conjunction with the Ca2+ imaging technique. Depicted in Fig. 5A and 5B, an initial sub-maximal concentration of capsaicin (10 nM) was applied to assess the basal responses across individual cells and TG culture preparations. Ten minutes later, vehicle in the control group or αβmeATP in the experimental group was applied for 1 min. Following the vehicle or αβmeATP application, a second application of 10 nM capsaicin was made. A sub-maximal concentration of capsaicin was used to minimize the desensitizing effects of repeated capsaicin applications, and also to ensure that sensitization can be clearly observed while ceiling effects are avoided. In the control condition, after the second 10 nM capsaicin application αβmeATP was applied to identify P2X3 receptor positive cells. At the end of the experiment, a supramaximal concentration of capsaicin (1 µM) was applied to confirm the identification of all TRPV1 neurons in both experimental and control conditions.
Figure 5.
The effects of αβmeATP on capsaicin-induced Ca2+ responses in TG neurons. Representative traces show Fura ratio (FR) from TG neurons in (A) αβmeATP and (B) vehicle treated groups. Each color trace represents responses from one neuron. (C) Averaged changes in Fura response (ΔFR) of the first and second 10 nM capsaicin application in αβmeATP and vehicle-treated groups. This protocol was tested on 12 cultures generated from 12 rats. + denotes significant effect at P < 0.05 and * indicates P < 0.05 following post-hoc analysis.
The proportion of capsaicin responsive neurons that responded to αβmeATP was the same across conditions (Table 1). The proportion of αβmeATP responsive neurons that responded to capsaicin was also similar across conditions (Table 1). In our analysis we included only neurons that responded to both capsaicin and αβmeATP (experimental = 217 and control = 180). In order to determine the effect of P2X3 activation on TRPV1 activity, we compared capsaicin-induced Ca2+ transients following treatment with either vehicle or 50 µM αβmeATP (Fig. 5A,B). We observed two types of responses; some neurons exhibited decreased responses to the second capsaicin treatment (desensitization) while others displayed increased responses (sensitization). The proportion of neurons showing desensitizing and sensitizing responses was similar in control and experimental groups (Table 1).
Table 1.
Response Profile of Neurons in Ca2+ Imaging Study
| Vehicle/Control | Experimental | |
|---|---|---|
| % of capsaicin responders that respond to αβmeATP | 31% (180/588) | 31% (317/703) |
| % of αβmeATP responders that respond to capsaicin | 90% (180/199) | 92% (217/235) |
| Proportion of αβmeATP/capsaicin responders exhibit sensitization | 33% (67/180) | 37% (71/217) |
| Proportion of αβmeATP/capsaicin responders exhibit desensitization | 62% (111/180) | 64% (139/217) |
In the desensitizing subset of neurons, the magnitude of the second capsaicin-evoked response was significantly smaller compared to the first (Time: F = 72.573, P < 0.001). This desensitization occurred to the same extent in both control and αβmeATP treated neurons (Drug: F = 1.193, P = 0.276; ΔFRexperimental, 1St = 0.28 ± 0.04, 2nd = 0.08 ± 0.02; ΔFRcontrol, 1St = 0.21 ± 0.30, 2nd = 0.07 ± 0.01). In the sensitizing subset of neurons, however, αβmeATP treatment evoked a significantly greater increase in Ca2+ influx compared to vehicle (Drug: F = 8.672, P = 0.004; Time: F = 18.632, P < 0.001, Fig. 5C). Furthermore, cells exhibiting desensitizing behavior typically showed a larger Ca2+ influx during the initial capsaicin application whereas the cells exhibiting sensitizing responses tended to have initially smaller Ca2+ responses regardless of treatment group (Desensitizing: ΔFRexperimental 1St = 0.28 ± 0.04, ΔFRcontrol 1S = 0.21 ± 0.30; Sensitizing: ΔFRexperimental 1St = 0.06 ± 0.01, ΔFRcontrol 1St = 0.04 ± 0.01). This suggests that P2X3 activation potentiates the sensitization of TRPV1 responses in this subset of TG neurons.
Since αβmeATP can activate P2X3 as well as P2X1 receptors (Torres et al., 1998; North and Surprenant, 2000), it is possible that a portion of the αβmeATP effects in our experiments could be mediated by P2X1. To exclude this possibility, we tested the effects of the selective P2X3 antagonist A-317491 ((Jarvis et al., 2002), 500 nM, 1 µM, 3 µM) on the effects of αβmeATP (50 µM). We saw a significant dose-dependent reduction in the percentage of responsive neurons (P < 0.001, Fig. 6A) and the amplitude of αβmeATP-evoked Ca2+ transients (P < 0.001, Fig. 6B). These data suggest that the αβmeATP effects we observed are mediated primarily by P2X3.
Figure 6.
The αβmeATP effect is mediated by P2X3. (A) The number of responses and (B) the magnitude of responses evoked by 50 µM αβmeATP in the presence of the selective P2X3 antagonist, A-317491. This protocol was tested on 5 cultures generated from 5 rats. The data were analyzed with Kruskal-Wallis One-Way Anova. + indicates significant effect at P < 0.05.
3.5 P2X3 induces phosphorylation of TRPV1 at specific residues
As another measure of P2X3-TRPV1 interactions we investigated whether activation of P2X3 induces phosphorylation of TRPV1. It is not feasible to test phosphorylation at the primary afferent terminal level since the neural elements in the muscle tissue are too low to detect meaningful biochemical changes. Therefore, we assessed the relative level of TRPV1 phosphorylation in TG cultures 15, 30, and 45 min following the application of αβmeATP (50 µM). The application of αβmeATP caused a time-dependent increase in serine phosphorylation (p-Ser) of TRPV1 (Fig. 7A and B). This increase peaks 15 to 30 min after the application, however, it only reached significance at 15 min. Interestingly, the mechanical hyperalgesia which develops following αβmeATP injection in the in vivo condition was most prominent during the same time period. The same concentration of αβmeATP did not induce significant changes in the phosphorylation of TRPV1 at threonine residues (Fig. 7C and D). As a control for phosphorylation, we have treated TG cultures with either the PKC activator PMA or vehicle (0 or 15 min). PKC is known to phosphorylate serine residues on TRPV1 (Numazaki et al., 2002; Bhave et al., 2003; Mandadi et al., 2006). PMA (1µmol) produced increased phosphorylated serine compared to vehicle and no treatment (Fig. 8). Additional experiments were also completed using the western blot technique to assess whether there were any changes in the levels of TRPV1 expression 15, 30, and 45 min following the application of αβmeATP (50µM). There were no significant differences in TRPV1 expression (Fig. 9). These data provide support for P2X3-TRPV1 interactions and that P2X3 activation leads to TRPV1 phosphorylation at specific sites.
Figure 7.
The effect of αβmeATP on TRPV1 phosphorylation in TG cultures. (A,C) Immunoblots for p-Ser and p-Thr following IP using an anti-TRPV1 antibody (upper). Immunoblots using anti-TRPV1 antibody from the same sample in the upper panel (lower). The samples were collected at the indicated time points following αβmeATP (50 µM) treatment. (B,D) Averaged relative optical densities (p-Ser/TRPV1 and p-Thr/TRPV1) are also shown. * denotes significant effect at P < 0.05. n = 6 for each time point.
Figure 8.
The effect of PMA on serine phosphorylation, and the effect of αβmeATP on TRPV1 expression in TG cultures. (A) (upper) Immunoblots for p-Ser following IP using an anti-TRPV1 antibody. Samples were either naïve, treated with 1µM PMA or vehicle for 15 min. (lower) Immunoblots of the same sample using an anti-TRPV1 antibody. (B) Representative western blot for TRPV1 (upper) and GAPDH (lower) from the same sample. The samples were collected at the indicated time points following αβmeATP (50 µM) treatment. (C) Averaged relative optical density (TRPV1/GAPDH). n = 3 for each time point.
4. Discussion
This study demonstrated a functional interaction between P2X3 and TRPV1, which contributes to the development of masseter mechanical hyperalgesia. First, we validated that muscular P2X3 activation induces mechanical hyperaglesia with our own orofacial model. Second, we demonstrated that P2X3-induced mechanical hyperaglesia is mediated by TRPV1. Next, we determined that TRPV1 and P2X3 are expressed in a subpopulation of masseter afferents. Fourth, we illustrated that P2X3 activation enhances capsaicin-induced responses in a subset of TG neurons. Finally, we reported P2X3 activation induces residue specific phosphorylation of TRPV1 in sensory neurons at time points in which mechanical hyperalgesia was observed. These data provide novel information suggesting the interaction between two ligand-gated channels, P2X3 and TRPV1, contributes to the development of muscle mechanical hyperalgesia.
The P2X3 type ATP receptors are unique nociceptive targets because they are expressed almost exclusively in peripheral sensory neurons (Chen et al., 1995; Bradbury et al., 1998; Dunn et al., 2001). P2X3 were previously implicated in masseter hyperalgesia. αβmeATP injected into the masseter induced a mechanical hyperalgesia that was blocked by the non-specific antagonist PPADS (Shinoda et al., 2008). Here, we replicated αβmeATP-induced masseter hyperalgesia in another orofacial muscle pain model. In both models, no hyperalgesia was observed in the masseter contralateral to the αβmeATP injection suggesting a peripheral mechanism of action. We validated the effect with a specific P2X3 antagonist confirming it is P2X3-mediated. Our benzocaine control experiments confirmed the αβmeATP effect is mediated by P2X3 innervating muscle. Together, our data suggest local P2X3 are mediating masseter mechanical hyperalgesia. In humans, ATP infusion during capsaicin application significantly enhances touch-evoked hyperalgesia (Hamilton et al., 2000) suggesting a facilitatory interaction between purinoceptors and TRPV1. In our data, P2X3-induced masseter hyperalgesia was prevented by TRPV1 blockade supporting the notion that P2X3 and TRPV1 interact in a facilitatory manner.
Previously, there was little to no information available regarding the extent and pattern of co-expression in muscle afferents between P2X3 and TRPV1. Immunohistochemistry studies reported approximately 33–58% of all TG neurons co-express P2X3 and TRPV1 (Eriksson et al., 1998; Ichikawa et al., 2004; Ichikawa and Sugimoto, 2004). More recently, the expression pattern of P2X3 and TRPV1 in masseter afferents have been looked at independently of each other. Specifically, 22% and 24–38% of masseter afferents express P2X3 and TRPV1, respectively (Ambalavanar et al., 2005; Takeda et al., 2005; Simonetti et al., 2006; Staikopoulos et al., 2007; Ro et al., 2009). We observed percentages of P2X3 (29.5 ± 7.2%) and TRPV1 (34.6 ± 5.3%) immunoreactive masseter afferents consistent with the current literature, and that 7.2 ± 3.1% medium-sized afferents co-express P2X3 and TRPV1. To our knowledge this is the first report of the co-expression pattern of these two channels in masseter afferents.
There is an apparent discrepancy between the small proportion of masseter afferents co-expressing TRPV1 and P2X3 and the robust behavioral phenotype. This disparity could be attributed to a variety of factors. There is precedence for a specific but small population of receptor-specific neurons producing robust physiological effects. For instance, TRPM8, which is expressed in only 5–10% of DRG neurons (Reid and Flonta, 2001; McKemy et al., 2002; Peier et al., 2002) mediate cold sensation in the range of 22–27°C (Peier et al., 2002; Bautista et al., 2007). TRPM8 null mice exhibit an array of deficits including a reduction in avoidance of cold zones, increased response latencies to the cold plate test, and reduced responses to acetone (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007). Similarly, MrgprA3 which is the major receptor responsible for chloroquine (CQ)-induced itch, is expressed in only 4–5% of DRG neurons (Liu et al., 2009). Mrgpr-cluster Δ−/− mice have ~30 Mrgpr genes deleted and they exhibit a significant reduction in CQ-induced scratching behavior (Liu et al., 2009). Mrgpr-cluster Δ−/− DRG display a considerably reduced Ca2+ response to CQ, which is rescued by MrgprA3 expression.
Furthermore, pathophysiological manipulations such as masseter or TMJ inflammation, masseter stretching or eccentric contraction, as well as tooth movement significantly increase P2X3 expression in TG for several days (Ambalavanar et al., 2005; Shinoda et al., 2005; Yang et al., 2009; Dessem et al., 2010). Thus, it is likely that under clinical or injurious conditions the extent of co-expression between these channels increases, providing a greater opportunity for these channels to interact.
Moreover, during an inflammatory event, mediators are released that can modulate TRPV1, further enhancing the observed behavioral effects. Thus, as opposed to a reduced in vitro preparation, other signaling sources could be present in vivo. In the case of this study, it is possible that the mechanical stimulation or cation channel activation, particularly TRPV1, induced the activation of mast cells (Turner et al., 2007). Degranulation of mast cells induces release of a protease, tryptase, which is known to sensitize TRPV1 (Amadesi, 2004). Although additional sensitization of TRPV1 by resident modulators could enhance the effects of P2X3 in vivo, our behavioral and cellular data still support the notion that P2X3 contributes to a facilitatory interaction with TRPV1.
When we applied a submaximal concentration of capsaicin (10 nM) repeatedly in the Ca2+ imaging experiments, we observed desensitizing effects in ~65% of neurons. αβmeATP treatment did not alter the extent of desensitization in this population. When we focused our analysis on the 35% of neurons that do not undergo desensitization, we discovered the sensitizing effects of αβmeATP.
We believe the desensitizing effect is reflective of the classical Ca2+-dependent desensitization that typically occurs following repeated application of capsaicin (Cholewinski et al., 1993; Docherty et al., 1996; Koplas et al., 1997). It is unclear whether the P2X3-TRPV1 interaction also exists in this population. It is possible that the intracellular machineries necessary for functional interactions between the two channels are absent or not operational in this subset of neurons. Alternatively, it is possible that capsaicin-induced desensitization is too strong to reveal the modest sensitizing effect of αβmeATP. It has been shown that desensitization/sensitization of TRPV1 is dependent on the initial concentration of Ca2+ influx in DRG (Zhang et al., 2011). Thus, within the heterogeneous population co-expressing TRPV1 and P2X3, there is a subset of neurons that have the capability of being sensitized by αβmeATP. Differences in initial TRPV1 responses may confer the identity of neurons as sensitizers or desensitizers. The sources of heterogeneity of initial TRPV1 responses within the general TRPV1/P2X3 population could result from basal differences in expression level or phosphorylation state of TRPV1, which requires further investigation.
Our data demonstrates phosphorylation and sensitization of TRPV1 following pretreatment with αβmeATP. However, activation of P2X3 can lead to Ca2+ influx, which in turn facilitates desensitization of TRPV1. Such Ca2+-dependent inhibitory interactions between P2X3 and TRPV1 were reported in a heterologous system as well as in sensory neurons (Piper and Docherty, 2000; Stanchev et al., 2009). In our experiments, we applied a low concentration of capsaicin (10 nM) in which approximately 35% of neurons did not undergo desensitization upon repeated application. However, in the case of applying higher capsaicin concentrations (e.g., 10 µM), as adopted in previous studies (Piper and Docherty, 2000; Stanchev et al., 2009), the population of neurons displaying desensitization would be predominant and hence sensitizing interactions would hardly be observed. Thus, physiologically we presume that both potentiating and inhibiting interactions between P2X3 and TRPV1 could occur in nociceptors, and that the consequence of the interactions could be dependent on multiple factors such as the extent of initial activation and kinase availability. Further examination of cellular mechanisms underlying dynamic interactions between P2X3 and TRPV1 is warranted.
Increased TRPV1 function, sensitization, is well documented. One of the mechanisms through which such potentiation of TRPV1 function occurs is phosphorylation events (Bhave et al., 2002; Bhave et al., 2003; Jung et al., 2004; Mandadi et al., 2006; Jeske et al., 2008; Woo et al., 2008; Jeske et al., 2009). For example, PKC targets Ser-800, Ser-502, Thr-704, and Thr-144 on TRPV1 (Numazaki et al., 2002; Bhave et al., 2003; Mandadi et al., 2006). CaMKII, on the other hand, targets Ser-502 and Thr-704 (Jung et al., 2004). Our data shows P2X3-induced TRPV1 serine phosphorylation at the time point in which we observed peak hyperaglesia in vivo. Our data suggests distinct kinases may be involved in the P2X3-TRPV1 interaction.
It is well established that GPCRs form ‘functional units’ with TRPV1 (Levine and Alessandri-Haber, 2007), but now there are data emerging to suggest ligand-gated channels form ‘functional units’ as well. Both glutamate and ATP are mediators released in high concentrations during injury and inflammation. Their receptors, P2X3 and NMDA receptor (NMDAR), are nonselective cation channels co-expressed with TRPV1 in masseter afferents. Similar to our data, direct activation of NMDARs induces a masseter hyperalgesia that is prevented by treatment with a TRPV1 antagonist (Lee et al., 2012). Akin to P2X3 activation, NMDAR activation induces phosphorylation of only serine residues on TRPV1. Recently, we also demonstrated that NMDARs functionally interact with TRPV1 in a CaMKII- and PKC-dependent manner (Lee et al., 2011; Lee et al., 2012). TRPV1 is known to be phosphorylated and sensitized by CaMKII and PKC (Premkumar and Ahern, 2000; Bhave et al., 2003; Jung et al., 2004; Woo et al., 2008). ATP receptors, including P2X3, have been linked to CaMKII and PKC kinase activation (Tominaga et al., 2001; Han et al., 2008). It will be interesting to determine whether these two cation channels interact via these Ca2+-dependent kinases.
5. Conclusions
This study identifies a new interaction, P2X3-TRPV1 in trigeminal sensory neurons, which could contribute to the development of mechanical hyperalgesia. In light of a recent study that showed the role of P2X5-ASIC3 interaction in muscle afferents under muscle ischemia condition (Birdsong et al., 2010) our data reinforces the notion that channel-channel interactions in sensory neurons provide mechanisms by which nociceptor function is dynamically modulated. TMD patients often suffer from mechanical hyperalgesia of masticatory musculature (Harness et al., 1990; Stohler, 1999). Unfortunately, currently available pharmacological interventions are often ineffective and produce undesirable side effects (Cowan et al., 1988; Sheldon et al., 1990; Cheng et al., 1993). Further elucidating these peripheral mechanisms could lead to the advent of more targeted and effective therapeutics for pain management that are unaccompanied by such unwanted side effects.
Highlights.
P2X3 induced mechanical hyperalgesia is attenuated by a TRPV1 antagonist
A P2X3 agonist potentiated capsaicin-induced Ca2+ transients in a subset of neurons
P2X3 activation induced serine phosphorylation of TRPV1
Acknowledgements
The authors thank Gregory Haynes for technical assistance performing behavioral experiments. This study was supported by NIH grants F31 DE20966 to JLS, DE020866 to MKC, and RO1 DE16062 to JYR.
Abbreviations
- αβmeATP
alpha,beta-methylene adenosine triphosphate
- AUC
area under the curve
- CIB
calcium imaging buffer
- CQ
chloroquine
- DRG
dorsal root ganglia
- GPCR
G-protein coupled receptor
- FR
Fura Response
- FB
fast blue
- KO
knockout
- LDS
lithium dodecyl sulfate
- NMDAR
NMDA receptor
- TMJ
temporomandibular joint
- TMD
temporomandibular joint and muscle disorder
- TRPV1
transient receptor potential V1
- TG
trigminal ganglia
- SDS
Sodium dodecyl sulfate
- p-Ser
serine phosphorylation
- SE
standard error of the mean
- VF
von frey
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain. 2005;117:280–291. doi: 10.1016/j.pain.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Svensson P, Sessle BJ, Cairns BE, Wang K. Interactions between glutamate and capsaicin in inducing muscle pain and sensitization in humans. European Journal of Pain: Ejp. 2008;12:661–670. doi: 10.1016/j.ejpain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) . Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing Muscle Ischemia: Coincident Detection of Acid and ATP via Interplay of Two Ion Channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Molecular & Cellular Neurosciences. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Wu D, Decena J, Soong Y, McCabe S, Szeto HH. Opioid-induced stimulation of fetal respiratory activity by [D-Ala2]deltorphin I. European journal of pharmacology. 1993;230:85–88. doi: 10.1016/0014-2999(93)90413-c. [DOI] [PubMed] [Google Scholar]
- Cholewinski A, Burgess GM, Bevan S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience. 1993;55:1015–1023. doi: 10.1016/0306-4522(93)90315-7. [DOI] [PubMed] [Google Scholar]
- Chun YH, Frank D, Lee JS, Zhang Y, Auh QS, Ro JY. Peripheral AMPA receptors contribute to muscle nociception and c-fos activation. Neuroscience research. 2008;62:97–104. doi: 10.1016/j.neures.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea Michael R, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated Cold Sensitivity in TRPM8 Null Mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Cowan A, Zhu XZ, Mosberg HI, Omnaas JR, Porreca F. Direct dependence studies in rats with agents selective for different types of opioid receptor. Journal of Pharmacology & Experimental Therapeutics. 1988;246:950–955. [PubMed] [Google Scholar]
- Dessem D, Ambalavanar R, Evancho M, Moutanni A, Yallampalli C, Bai G. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X3 expression in a functionally relevant manner. PAIN. 2010;149:284–295. doi: 10.1016/j.pain.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 Is Required for Cold Sensation in Mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Docherty R, Yeats J, Bevan S, Boddeke H. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Archiv European Journal of Physiology. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Progress in neurobiology. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Bongenhielm U, Kidd E, Matthews B, Fried K. Distribution of P2X3 receptors in the rat trigeminal ganglion after inferior alveolar nerve injury. Neuroscience Letters. 1998;254:37–40. doi: 10.1016/s0304-3940(98)00656-9. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140:292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. European Journal of Neuroscience. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgesia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- Han SR, Lee MK, Lim KH, Yang GY, Jeon HJ, Ju JS, Yoon YW, Kim SK, Ahn DK. Intramuscular administration of morphine reduces mustard-oil-induced craniofacial-muscle pain behavior in lightly anesthetized rats. European Journal of Pain: Ejp. 2008;12:361–370. doi: 10.1016/j.ejpain.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Harness DM, Donlon WC, Eversole LR. Comparison of clinical characteristics in myogenic, TMJ internal derangement and atypical facial pain patients. Clinical Journal of Pain. 1990;6:4–17. doi: 10.1097/00002508-199003000-00003. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Kohro Y, Tsuda M, Inoue K. Activation of cytosolic phospholipase A2 in dorsal root ganglion neurons by Ca2+/calmodulin-dependent protein kinase II after peripheral nerve injury. Molecular pain. 2009;5:22. doi: 10.1186/1744-8069-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, El Kouhen R, Lee CH, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. Journal of Pharmacology & Experimental Therapeutics. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. The co-expression of P2X3 receptor with VR1 and VRL-1 in the rat trigeminal ganglion. Brain research. 2004;998:130–135. doi: 10.1016/j.brainres.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Fukunaga T, Jin HW, Fujita M, Takano-Yamamoto T, Sugimoto T. VR1-, VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain research. 2004;1008:131–136. doi: 10.1016/j.brainres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. PAIN. 2009;146:301–307. doi: 10.1016/j.pain.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. Journal of Biological Chemistry. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- Kim HY, Chung G, Jo HJ, Kim YS, Bae YC, Jung SJ, Kim JS, Oh SB. Characterization of Dental Nociceptive Neurons. Journal of Dental Research. 2011;90:771–776. doi: 10.1177/0022034511399906. [DOI] [PubMed] [Google Scholar]
- Kim YS, Paik SK, Cho YS, Shin HS, Bae JY, Moritani M, Yoshida A, Ahn DK, Valtschanoff J, Hwang SJ, Moon C, Bae YC. Expression of P2X3 receptor in the trigeminal sensory nuclei of the rat. The Journal of Comparative Neurology. 2008;506:627–639. doi: 10.1002/cne.21544. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The Role of Calcium in the Desensitization of Capsaicin Responses in Rat Dorsal Root Ganglion Neurons. The Journal of Neuroscience. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Saloman JL, Weiland G, Auh QS, Chung M-K, Ro JY. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. PAIN. 2012;153:1514–1524. doi: 10.1016/j.pain.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Zhang Y, Chung MK, Ro JY. Society for Neuroscience. Washington, D.C.: 2011. Protein Kinase C and AKAP150 are involved in functional interactions between NMDA receptors and TRPV1 in trigeminal ganglia. [Google Scholar]
- Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochimica et biophysica acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng H-J, Geng Y, Undem BJ, Kollarik M, Chen Z-F, Anderson DJ, Dong X. Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138:484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. Journal of Neuroscience. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annual Review of Pharmacology & Toxicology. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct Phosphorylation of Capsaicin Receptor VR1 by Protein Kinase CÎμ and Identification of Two Target Serine Residues. Journal of Biological Chemistry. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Oliveira MC, Parada CA, Veiga MC, Rodrigues LR, Barros SP, Tambeli CH. Evidence for the involvement of endogenous ATP and P2X receptors in TMJ pain. European Journal of Pain: Ejp. 2005;9:87–93. doi: 10.1016/j.ejpain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Pabbidi R, Yu S-Q, Peng S, Khardori R, Pauza M, Premkumar L. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Molecular Pain. 2008;4:9. doi: 10.1186/1744-8069-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP Channel that Senses Cold Stimuli and Menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Piper AS, Docherty RJ. One-way cross-desensitization between P2X purinoceptors and vanilloid receptors in adult rat dorsal root ganglion neurones. Journal of Physiology. 2000;523:685–696. doi: 10.1111/j.1469-7793.2000.t01-1-00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. in vivo characterization in rat models of inflammatory and neuropathic pain. Journal of Pharmacology & Experimental Therapeutics. 2003;306:387–393. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Reid G, Flonta M-L. Physiology: Cold current in thermoreceptive neurons. Nature. 2001;413:480–480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- Reinohl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neuroscience letters. 2003;338:25–28. doi: 10.1016/s0304-3940(02)01360-5. [DOI] [PubMed] [Google Scholar]
- Ro JY, Capra NF. Assessing mechanical sensitivity of masseter muscle in lightly anesthetized rats: a model for craniofacial muscle hyperalgesia. Neuroscience research. 2006;56:119–123. doi: 10.1016/j.neures.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Ro JY, Capra N, Masri R. Development of a behavioral assessment of craniofacial muscle pain in lightly anesthetized rats. Pain. 2003;104:179–185. doi: 10.1016/s0304-3959(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro JY, Lee J, Capra NF, Zhang Y. Role of soluble guanylate cyclase in the trigeminal subnucleus caudalis in capsaicin-induced muscle hypersensitivity. Brain research. 2007;1184:141–148. doi: 10.1016/j.brainres.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135:271–279. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloman JL, Niu KY, Ro JY. Activation of peripheral delta-opioid receptors leads to anti-hyperalgesic responses in the masseter muscle of male and female rats. Neuroscience. 2011;190:379–385. doi: 10.1016/j.neuroscience.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez EM, Bagues A, Martin MI. Contributions of peripheral and central opioid receptors to antinociception in rat muscle pain models. Pharmacology Biochemistry and Behavior. 2010;96:488–495. doi: 10.1016/j.pbb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Sheldon RJ, Riviere PJ, Malarchik ME, Moseberg HI, Burks TF, Porreca F. Opioid regulation of mucosal ion transport in the mouse isolated jejunum. Journal of Pharmacology & Experimental Therapeutics. 1990;253:144–151. [PubMed] [Google Scholar]
- Shinoda M, Ozaki N, Sugiura Y. Involvement of ATP and its receptors on nociception in rat model of masseter muscle pain. Pain. 2008;134:148–157. doi: 10.1016/j.pain.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y. Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain. 2005;116:42–51. doi: 10.1016/j.pain.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Simonetti M, Fabbro A, D'Arco M, Zweyer M, Nistri A, Giniatullin R, Fabbretti E. Comparison of P2X and TRPV1 receptors in ganglia or primary culture of trigeminal neurons and their modulation by NGF or serotonin. Molecular pain. 2006;2:11. doi: 10.1186/1744-8069-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staikopoulos V, Sessle BJ, Furness JB, Jennings EA. Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience. 2007;144:208–216. doi: 10.1016/j.neuroscience.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanchev D, Blosa M, Milius D, Gerevich Z, Rubini P, Schmalzing G, Eschrich K, Schaefer M, Wirkner K, Illes P. Cross-inhibition between native and recombinant TRPV1 and P2X(3) receptors. Pain. 2009;143:26–36. doi: 10.1016/j.pain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Stohler CS. Muscle-related temporomandibular disorders. Journal of orofacial pain. 1999;13:273–284. [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ito M, Nasu M, Matsumoto S. Role of capsaicin-sensitive primary afferent inputs from the masseter muscle in the C1 spinal neurons responding to tooth-pulp stimulation in rats. Experimental Brain Research. 2005;160:107–117. doi: 10.1007/s00221-004-1990-2. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Haines WR, Egan TM, Voigt MM. Co-Expression of P2X1 and P2X5 Receptor Subunits Reveals a Novel ATP-Gated Ion Channel. Molecular Pharmacology. 1998;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. Journal of Pharmacology & Experimental Therapeutics. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- Wang S, Lee J, Ro JY, Chung MK. Warmth suppresses and desensitizes damage-sensing ion channel TRPA1. Molecular Pain. 2012;8 doi: 10.1186/1744-8069-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo DH, Jung SJ, Zhu MH, Park CK, Kim YH, Oh SB, Lee CJ. Direct activation of transient receptor potential vanilloid 1(TRPV1) by diacylglycerol (DAG) Molecular Pain. 2008;4:42. doi: 10.1186/1744-8069-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Whiteside GT, Lee G, Nolan S, Niosi M, Pearson MS, Ilyin VI. A-317491, a selective P2X3/P2X2/3 receptor antagonist, reverses inflammatory mechanical hyperalgesia through action at peripheral receptors in rats. European journal of pharmacology. 2004;504:45–53. doi: 10.1016/j.ejphar.2004.09.056. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang P, Zou X, Li D, Fang L, Gong K, Lin Q. The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV1 in primary afferent neurons evoked by intradermal capsaicin. Experimental Neurology. 2010;222:93–107. doi: 10.1016/j.expneurol.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Cao Y, Wang Y, Luo W, Hua X, Lu Y, Liao Z, Lai W, Zhao Z. Behavioural responses and expression of P2X3 receptor in trigeminal ganglion after experimental tooth movement in rats. Archives of Oral Biology. 2009;54:63–70. doi: 10.1016/j.archoralbio.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Daugherty SL, de Groat WC. Activation of CaMKII and ERK1/2 contributes to the time-dependent potentiation of Ca2+ response elicited by repeated application of capsaicin in rat DRG neurons. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;300:R644–R654. doi: 10.1152/ajpregu.00672.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]