Abstract
Enamel formation involves highly orchestrated intracellular and extracellular events; following development, the tissue is unable to regenerate, making it a challenging target for tissue engineering. We previously demonstrated the ability to trigger enamel differentiation and regeneration in the embryonic mouse incisor using a self-assembling matrix that displayed the integrin-binding epitope RGDS (Arg-Gly-Asp-Ser). To further elucidate the intracellular signaling pathways responsible for this phenomenon, we explore here the coupling response of integrin receptors to the biomaterial and subsequent downstream gene expression profiles. We demonstrate that the artificial matrix activates focal adhesion kinase (FAK) to increase phosphorylation of both c-Jun N-terminal kinase (JNK) and its downstream transcription factor c-Jun (c-Jun). Inhibition of FAK blocked activation of the identified matrix-mediated pathways, while independent inhibition of JNK nearly abolished phosphorylated-c-Jun (p-c-Jun) and attenuated the pathways identified to promote enamel regeneration. Cognate binding sites in the amelogenin promoter were identified to be transcriptionally up-regulated in response to p-c-Jun. Furthermore, the artificial matrix induced gene expression to specifically increase the abundance of amelogenin, the main protein expressed during enamel formation, and the CCAAT enhancer binding protein alpha (C/EBPα), which is the known activator of amelogenin expression. Elucidating these cues not only provides guidelines for the design of synthetic regenerative strategies and opportunities to manipulate pathways to regulate enamel regeneration, but can provide insight into the molecular mechanisms involved in tissue formation.
1. Introduction
Tooth enamel does not remodel or regenerate and is the only tissue of ectodermal origin in mammals that undergoes biomineralization [1–4]. During enamel formation, ameloblast cells synthesize and secrete a complex mixture of tissue specific matrix proteins into the extracellular space, composed largely of amelogenin, ameloblastin and enamelin, which facilitate the organization and architecture of the mineral phase, hydroxyapatite (HAP) [2, 4, 5]. Over 90% of the developing enamel extracellular matrix (ECM) is made up of amelogenin protein. Amelogenin is considered to be an intrinsically disorganized protein yet it self-assembles to form nanospheres. These nanosphere assemblies subsequently control and pattern the growth of HAP crystallites, which almost entirely replaces the protein matrix, leaving only trace amounts of occluded protein that improve the mechanical properties of the enamel tissue [6–12].
During tooth development, the differentiation of enamel-secreting ameloblasts is regulated and achieved by epithelial-mesenchymal interactions through the exchange of signals with the underlying neural crest-derived ectomesenchyme across a basement membrane [13, 14]. These signals are transduced by either taking a direct route, through the regulatory mechanisms of specific growth factors, or an indirect route, through ECM ligands that reciprocally communicate with cell surface receptors, such as integrins. Indirect signaling cascades that are triggered by integrin activation are complex, however, initial integrin engagement involves recruitment of proteins to stabilize the cytoskeleton and produce focal adhesions, protein complexes that link the cell interior to its external environment. Focal adhesion kinase (FAK), a component of the focal adhesion, acts as a signaling scaffold that exhibits phosphorylation-regulated kinase activity to a number of downstream effector molecules [15, 16]. These signaling mechanisms amplify the initial response and result in changes to gene expression by activating or inhibiting specific transcription factors or cell division control factors that serve to regulate cell fate in the dental epithelium [4, 17, 18]. The expression of the major enamel matrix protein, amelogenin, is correlated with increased cell differentiation and is regulated by members of the CCAAT enhancer binding protein family (C/EBP), notably C/EBPα [19, 20] and C/EBPΔ [21]. In the absence of such signals, ameloblast differentiation and subsequent formation of the enamel tissue does not occur.
The ECM provides a physical microenvironment for cells and is capable of initiating intracellular signaling through cell adhesion events. Activation of cell response through the external environment provides ECM mimetic materials the opportunity to guide cell fate, tissue repair and tissue regeneration. Cell-to-matrix interactions provide signaling capacity that is essential for proper differentiation and maturation of enamel tissue. Although cell adhesions can be heterogeneous in nature, they are all mediated through integrins, transmembrane receptors that couple the intracellular actin to the extracellular milieu. A common integrin-binding motif, RGDS, has been incorporated into the design of several biomimetic materials and explored extensively [22, 23]. However, RGDS is much less studied in the context of enamel formation where it may provide signaling that is necessary for ameloblast differentiation during embryonic development and enamel regeneration. For example, RGDS has been identified in the second most abundant enamel matrix protein, ameloblastin (Ambn), which is involved in cell adhesion to the enamel ECM [24, 25] and disrupts ameloblast adhesion and differentiation during tissue development when truncated in genetically engineered animals [25]. Furthermore, we have previously demonstrated the capacity of an artificial matrix composed of self-assembling peptide amphiphile (PA) nanofibers to act as an ECM mimetic by displaying a high density of RGDS epitopes for improved cell adhesion, cell delivery and nucleation of a crystallographically organized HAP [26–31]. Naïve dental epithelial cells up-regulated their integrin receptors, resulting in cell proliferation and ameloblast differentiation in response to a branched RGDS PA biomaterial (bRGDS PA) [32]. These ameloblasts synthesize, secrete and mineralize a regenerated enamel nodule in vivo at the site of injection of the bRGDS PA matrix [32]. In this study, we explore the regulatory pathways involved in transducing signals between integrin receptors and the nucleus, leading to the establishment of the ameloblast phenotype and to enamel regeneration.
2. Materials and Methods
2.1. Peptide amphiphiles
The branched RGDS peptide amphiphile (bRGDS PA) and the scrambled RGDS peptide amphiphile (ScrRGDS PA) control were synthesized using standard 9-fluorenyl methoxycarbonyl (Fmoc) solid phase peptide synthesis, as previously described [33]. Briefly, using a Rink Amide MBHA resin (low loading), the protected amino acids were coupled with 4 equivalents of protected amino acid, 3.95 equivalents of 2-(1H-benzotriazole-1-yl)-1,1,2,3- tetramethyluronium hexafluorophosphate, and 6 equivalents of N,N-diisopropylethylamine in a solvent mixture of 1:1:1 dimethylformamide:dichloromethane:N-methyl-2-pyrrolidone (DMF:DCM:NMP). 4-Methyl trityl (Mtt) groups were deprotected using 2–3% trifluoroacetic acid (TFA), 5% triisopropylsilane (TIPS) in DCM and the Fmoc groups were deprotected using 30% piperidine in DMF. The molecules were cleaved from the resin using 92.5% TFA, 2.5% TIPS, 2.5% 1,2-Ethanedithiol and 2.5% water for 3 hours and precipitated using cold diethyl ether. The crude product was dried over a frit, collected, and purified using reverse phase high performance liquid chromatography on a Varian Prostar model 210 preparative scale HPLC equipped with a Phenomenex Jupiter Proteo column (C12 stationary phase, 10 μm, 90 Å pore size, 150 × 30 mm). A mobile gradient of water and acetonitrile with 0.1% TFA was used at a flow rate of 25 ml/min and elution of the molecules were monitored at 220 nm and 276 nm. Pure fractions were collected and combined, and the excess acetonitrile was removed using rotary evaporation prior to lyophilizing. Pure product was stored at −20°C until use.
2.2. Organ culture
The USC Institutional Animal Care and Use Committee Procedures approved all procedures involving vertebrate animals. Timed pregnant Swiss Webster mice at E18.5 were euthanized (vaginal plug = day 0) and tooth organs were harvested and cultured overnight at the interface of air and BGJb medium (Invitrogen, Carlsbad, CA) containing 100 μg/ml ascorbic acid (Sigma-Aldrich, St. Louis, MO), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich) in an atmosphere of 5% CO2/95% air at 37°C. The incisor primordia were injected with approximately 5–10 nanoliters BGJb culture medium (sham treatment) or 1% (w/v) bRGDS PA or ScrRGDS PA into the enamel organ epithelia along the rostral-caudal gradient of development, as previously described [32]. The PA-injected incisors were then transplanted under the kidney capsules of host mice for long-term culture of 8 to 10 weeks, harvested, and fixed overnight in freshly prepared 4% paraformaldehyde in phosphate buffered saline (PBS), pH 7.4, at 4°C. The injected organs were partly demineralized using isotonic 100 mM ethylenediaminetetraacetic acid (EDTA; Sigma-Aldrich) for histological sectioning.
2.3. Cell culture
A mouse ameloblast-like cell line, LS8, was maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Gibco/Invitrogen) [20]. Primary enamel organ epithelial (EOE) cells were isolated and recovered from newborn mouse mandibular incisors [32, 34, 35]. The incisors were dissected aseptically and incubated with 1 mg/ml dispase (Gibco/Invitrogen) at 37°C for 1 hr. The enamel organ epithelial sheets were separated from the underlying extracellular matrix and mesenchyme and digested with 0.05% trypsin/EDTA (Gibco/Invitrogen) at 37°C for 10 min. Cells were collected by centrifugation for 5 min at 500 × g and cultured in DMEM containing 20% FBS overnight, then maintained in supplemented medium keratinocyte growth medium (KGM-2) (Lonza, Walkersville, MD) without serum. The primary EOE cells were maintained in basal KGM-2 medium overnight before treatment with the selected PAs.
Inhibition studies of phosphorylation of integrin-associated pathways were performed with the FAK specific inhibitor, PF 573228 (Sigma-Aldrich, St. Louis, MO, USA) or the selective JNK inhibitor SP 600125 (Tocris, R&D, Minneapolis, MN, USA). Inhibitor was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) at an appropriate concentration, stored at −20°C and diluted to the desired concentration for delivery to the cell culture media.
2.4. Real-time PCR
For analysis of amelogenin and C/EBPα gene expression, primary EOE cells were treated with 1% bRGDS PA or 1% ScrRGDS PA for 4 hrs. Total RNA was extracted using the RNA-Bee reagent (TEL-TEST, Friendswood, TX). First strand cDNA was synthesized with 100 ng random oligodeoxynucleotide decamers (Ambion, Austin, TX). Real-time PCR was performed with an iCycler iQ multi-color real-time PCR detection system (Bio-Rad, Hercules, CA). Primer sequences for each target gene are as follows: for amelogenin, the forward primer was 5′-GGGGACCTGG ATTTTGTTTG-3′, and the reverse primer was 5′-AACCATAGGA AGGATACGGC TG-3′; for C/EBPα, the forward primer was 5′-CGCCTTCAAC GACGAGTTCC-3′, and the reverse primer was 5′-TAGTCAAAGT CACCGCCGCC AC-3′; for β-actin used as the internal control, the forward primer was 5′-GGGAAATCGT GCGTGACATC-3′ and the reverse primer was 5′-GCGGCAGTGG CCATCTC-3′. The iQ SYBR Green Supermix kit (Bio-Rad) was utilized in PCR assays according to the manufacturer’s protocol at a final concentration of 3 mM MgCl2 and 0.2 mM each primer for a 20 μl reaction. All real-time PCR was performed in triplicate and conducted with an initial denaturing interval (95°C, 15 min) followed by 40 cycles consisting of heating to 95°C (10 s) and cooling to 55°C (45 s). The iCycler iQ real-time PCR detection system software version 3.1 was used to analyze results, and the PCR baseline subtraction curve fit function was used to determine threshold cycle (CT) values [36]. Gene expression levels were quantified with the comparative CT method employing software provided by the manufacturer.
2.5. Western blotting
Whole cell lysates were prepared from primary EOE cells and treated as sham controls with cell culture medium, or were treated with either 1% ScrRGDS PA or 1% bRGDS PA for a selected period of time. Whole cell lysates were prepared from ameloblast-like LS8 cells transiently transfected using a gradient of concentrations for the expression plasmid c-Jun. Protein concentrations were determined by the Bradford protein assay (Bio-Rad) with extrapolation to a bovine serum albumin (BSA) standard curve. Equal amounts of protein (10–20 μg) were subject to 4%–12% SDS-polyacrylamide gel electrophoresis (Invitrogen, Carlsbad, CA). The resolved proteins on the gel were blotted onto an Immobilon-P membrane (Millipore, Billerica, MA). The membranes were incubated with a suitable dilution of primary antibody: polyclonal anti-FAK or phospho-FAK (Tyr397) (p-FAK Tyr397, Cell Signaling, Danvers, MA), phospho-SAPK/JNK (Thr183/Tyr185, Cell Signaling) (p-SAPK/JNK Thr183/Tyr185, Cell Signaling), monoclonal anti c-Jun (Cell Signaling), phospho-c-Jun (Ser63) (p-c-Jun Ser63, Cell Signaling), JNK rabbit antibody (Cell Signaling) or monoclonal anti-GAPDH mouse antibody (Millipore) overnight at 4°C. The membrane was washed several times with Tris-buffered saline with 0.1% Tween 20 (TBST) and incubated with the corresponding horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG or goat anti-mouse IgG (Invitrogen, Valencia, CA) for 1 hr at room temperature. A chemiluminescent substrate (Thermo Scientific, Logan, UT) for HRP was used to detect the bound antibodies.
2.6. Immunofluorescence microscopy
Primary EOE cells were cultured on chamber slides (Millipore, Billerica, MA), fixed in 4% formaldehyde in PBS for 30 min, permeabilized in 0.5% Triton X-100/PBS for 5 min and rinsed in PBS prior to staining. Immunostaining was performed as previously described [37]. Non-specific staining was blocked with 2% BSA/PBS and all subsequent antibody reactions were performed in 1% BSA/PBS. Slides were incubated with the indicated primary antibody overnight at 4°C, and then stained with FITC-conjugated goat anti-rabbit IgG (Invitrogen, Valencia, CA) for 1 hr at ambient temperature. P-FAK, or p-SAPK/JNK or p-c-Jun was detected with Alexa Fluor 488 anti-rabbit IgG (Invitrogen). F-actin was stained with Alexa Fluor 594 phalloidin (Invitrogen), and nuclei were stained with DAPI (Invitrogen). Imaging was performed using a Nikon TE300 Quantum upright confocal microscope.
2.7. Cell transfection and luciferase assay
The wild type luciferase reporter construct for the amelogenin promoter was used as previously described [19]. The promoter region was subcloned into pGL3-Basic to drive the firefly luciferase (luc) reporter gene. The expression plasmid for c-Jun, a kind gift from Dr. Alan Friedman containing the EcoRI cDNA fragment of c-Jun (1.8kb, verified by DNA sequencing) was cloned into the PvuII site of the poly-linker. Various reporter constructs were transiently transfected into an ameloblast-like cell line LS8, with c-Jun expression plasmid or empty vector pcDNA3 alone (as control). In all cases, the pGL4.75 [hRluc/CMV] vector (50 ng/well) encoding the luciferase reporter gene hRluc (Renilla reniformis) (Promega, Madison, WI) was co-transfected as an internal control.
Transient transfection of 2 × 105 cells/well were performed in 12-well plates using a calcium phosphate-mediated transfection procedure (Promega, Madison, WI). Forty-eight hours after transfection, ameloblast-like LS8 cells were subjected to a dual-luciferase reporter assay (Promega, Madison, WI) according to the manufacturer’s recommendation. Briefly, cells were lysed with passive lysis buffer (PLB) at room temperature for 15 min. Subsequently cell lysates were cleared of residual cell debris by centrifugation and mixed with luciferase assay reagent II (LAR II) to obtain firefly luminescence quantitation using a luminometer (Turner Biosystem, Promega, Madison, WI), followed by adding a mixture of Stop & Glo Reagent to obtain Renilla luminescence quantitation. The ratio of firefly luminescence to Renilla luminescence was calculated and normalized to the ratio of control wells that were treated identically.
2.8. Site mutagenesis
Nucleotides within selected c-Jun transcription factor binding sites for the amelogenin promoter were altered using the Quickchange XL site-directed mutagenesis procedure (Agilent Tech, Santa Clara, CA) according to the manufacturer’s instructions. The reporter construct with a single mutation (MUT (213)-luc or MUT (364)-luc) was generated at either position -213 in the wild-type reporter construct (WT (213)-luc), or at -364 in the wild-type reporter construct (WT (364)-luc). A reporter construct with double mutations (MUT (213)-MUT(364)-luc) was generated at both positions -213 and -364 in the wild-type reporter construct. The mutagenesis primers were designed to contain the desired mutation and to anneal to the nucleotide sequence on the balancing strand of the plasmid. Primers were purified by polyacrylamide gel electrophoresis. The mutagenesis reaction was set up using 18 cycles with heating to 95°C for 50 sec, holding at 60°C for 50 sec, and holding at 68°C for 1 min in a reaction system containing the reporter construct or pWhitescript 4.5-kb as control. After the mutagenesis reaction, DpnI restriction enzyme was added to each amplification reaction and incubated at 37°C for 1 hr to digest the parental supercoiled dsDNA. The DpnI-treated DNA was then transformed into XL10-Gold Ultracompetent cells to reduce background from wild-type plasmids. To confirm the mutation, the plasmids were analyzed by DNA sequencing. Primer sequences for site-directed mutagenesis are as follows: SN213: 5′-CAGCATATGC AGTCAACTAA TTTGCTATTT TGAAGACAGCT TCCCAAACCT ATTA-3′, SN213 antisense: 5′-TAATAGGTTT GGGAAGCTGTC TTCAAAATAG CAAATTAGTT GACTGCATA TGCTG-3′; SN364: 5′-TGATATGAA AAGTCTGGAG AACCTTAAG TATTTGTTT TGAGTACAC TGGAGAAACT-3′, SN364 antisense: 5′-AGTTTCTCCA GTGTACTCAA AACAAATACT TAAGGTTCTC CAGACTTTTC ATATCA-3′.
3. Results
3.1. Effect of bioactive peptide amphiphiles on enamel regeneration
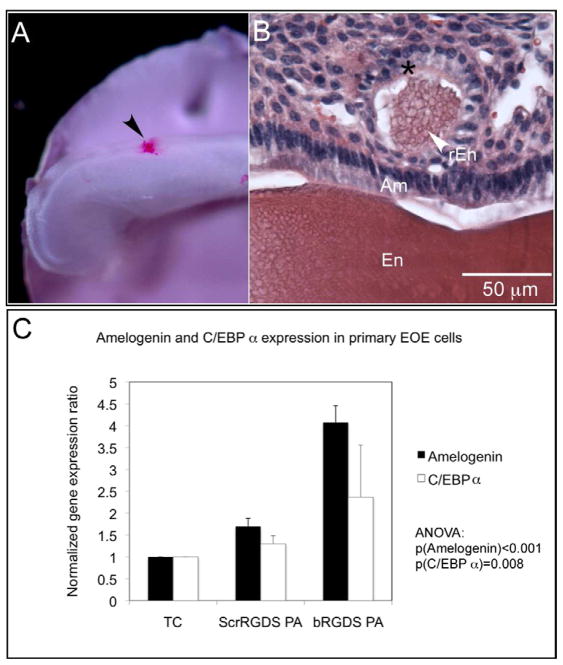
The regenerative capacity of the bioactive, branched RGDS-containing peptide amphiphile (bRGDS PA) was demonstrated by injecting the material into the developing mouse incisor. A control PA containing a scrambled RSDG sequence (ScrRGDS PA) that conserved the overall charge of the molecule was used as a control. To distinguish the site of injection, 1% (w/v) PA matrix (bRGDS PA or ScrRGDS PA) was mixed with a rhodamine containing PA (20:1) and injected adjacent to the ameloblasts along the rostral-caudal gradient of E18.5 mouse incisor development to provide a fluorescent label for ease of identification (Fig. 1A, arrowhead). Following an 8-week graft period under the kidney capsule of a murine host, histologic analysis of the injected organs showed no enamel regeneration at the ScrRGDS PA injection site, while a regenerated enamel nodule (Fig. 1B, arrowhead) formed at the bRGDS PA injection site, as previously described [32, 38]. Dental epithelial cells surrounding the regenerated enamel nodule revealed themselves as elongated and polarized, identical in their morphology to authentic ameloblasts (Fig. 1B, asterisk).
Figure 1. Bioactive peptide amphiphiles (PA) and enamel regeneration.
(A) Peptide amphiphiles were injected into the enamel organ epithelia of an E18.5 mouse incisor grown on a nitrocellulose disc in organ culture. bRGDS PA matrix was mixed with a rhodamine containing PA (20:1) to permit fluorescent detection of the injection site (arrowhead) along the rostral-caudal gradient of incisor development and one that corresponds to an early secretory stage of enamel formation;
(B) A representative cross-sectional view of a tissue section from a bRGDS PA injected incisor stained with hematoxylin and eosin. A regenerated enamel nodule (arrowhead) was formed at the PA injection site after transplantation under the kidney capsule for an 8-week period. The cross sectional view of the nodule reveals a rosette of polarized dental epithelial cells (*) that surround a nodule of regenerated enamel matrix. rEn: regenerated enamel; Am: ameloblast; En: Enamel;
(C) Detection of amelogenin mRNA and ameloblast-specific transcription factor, C/EBPα in primary enamel organ epithelial (EOE) cells treated with different PAs by real-time PCR. Amelogenin transcripts were approximately 4 times as abundant in primary EOE cells grown on bRGDS PA compared with identical EOE cells grown on plastic (tissue culture plates, TC) or scrambled RGDS PA (ScrRGDS PA) (p < 0.05, ANOVA). C/EBPα transcripts were twice as abundant in primary EOE cells grown on bRGDS PA compared with cells grown on plastic (TC) or ScrRGDS PA (p < 0.01, ANOVA).
To better characterize the immediate molecular response of the primary enamel organ epithelial (EOE) cells to the artificial matrix that led to enamel regeneration and biomineralization, gene expression analysis of EOE cells treated with either bRGDS PA or ScrRGDS PA was performed. These EOE cells correspond to the same developmental stage as the injected incisors and were chosen for this analysis because the number of cells surrounding an injected PA site was insufficient for analysis. For this study, as performed previously, the primary EOE cells from E18.5 mouse incisors were co-cultured with the selected PA by first coating the dish with the PA, growing the cells on the assembled PA surface for 24 hr, and covering the cells with the same PA to enmesh the cells in the assembled PA for 4 hr before harvesting [32, 38]. Amelogenin is the dominant extracellular matrix protein of developing enamel and amelogenin transcripts were approximately 4-times more abundant in primary EOE cells treated with bRGDS PA compared with either sham treated cells grown on tissue culture plastic (TC) or to cells treated with ScrRGDS PA (p < 0.001, ANOVA) (Fig. 1C). CCAAT-enhancer binding protein alpha (C/EBPα) is the principle known activator of amelogenin transcription and C/EBPα transcripts were twice as abundant in primary EOE cells treated with bRGDS PA compared with sham-treated cells grown on tissue culture plastic (TC) or with ScrRGDS PA (p = 0.008, ANOVA). As expected, enhanced C/EBPα levels correlate with increased amelogenin expression. These data prompted further experiments to elucidate the intracellular signaling responsible for mediating these differences in gene expression conditional for enamel regeneration.
3.2. Phosphorylation of focal adhesion kinase in primary enamel organ epithelial cells treated with peptide amphiphiles
The RGDS epitopes displayed at the surface of bRGDS PA assemblies were chosen due to their ability to bind integrins on the cell surface and mediate intrinsic signals for enamel differentiation. To evaluate if integrin binding occurs, we visualized and quantified the amount of phosphorylated focal adhesion kinase (FAK) in response to bRGDS PA or ScrRGDS PA. FAK is a protein that is constitutively associated with β-integrin subunits and undergoes phosphorylation when the ligand binds integrin receptors. Confocal immunofluorescent analysis of primary EOE cells treated with ScrRGDS PA for 2 min revealed marginal stimulation of phospho-FAK (p-FAK) in the cytoplasm (Fig. 2A and 2A1, arrowhead). In contrast, EOE cells treated with bRGDS PA revealed increased amounts of p-FAK, which was distributed across the cytoplasm (Fig. 2B and 2B1, arrowheads). Western blot analysis was used to confirm the response and to quantitate p-FAK in relation to total FAK in primary EOE cells treated with bRGDS PA, compared to sham-treated cells grown on tissue plastic (TC) and cells grown with ScrRGDS PA. Equal amounts (15 μg) of protein lysate were resolved to size using SDS-PAGE gel electrophoresis, transferred to a solid support and probed with specific antibodies that distinguished total FAK from p-FAK (Fig. 2C). We observed that the level of p-FAK to total FAK (Fig. 2E) was up-regulated approximately 2 times in primary EOE cells treated with bRGDS PA compared with sham control, and approximately 1.5 times compared with ScrRGDS treated group within 2 min (p<0.05, ANOVA). These levels returned to but a mild increase within 15 min (Fig. 2D, upper panel), with no significant difference in the expression of total FAK (Fig. 2D, lower panel).
Figure 2. Effect of bRGDS PA on focal adhesion kinase (FAK) in primary enamel organ epithelial cells.
(A) Confocal immunofluorescent analysis of primary enamel organ epithelial cells treated with ScrRGDS PA for 2 min using phospho-FAK Tyr397 (p-FAK) antibody (green), Alexa Fluor 594 phalloidin for F-actin (red) and DAPI for nuclei (blue). Slight stimulation of p-FAK was located mainly in the cytoplasm (arrowhead). Panel A1 is a higher magnification of the box outlined in panel A;
(B) Confocal immunofluorescent analysis of primary enamel organ epithelial cells, treated with bRGDS PA for 2 min using p-FAK antibody (green), Alexa Fluor 594 phalloidin for F-actin (red) and DAPI for nuclei (blue). Abundant stimulation of p-FAK, distributed in both the cytoplasm and cellular protrusions (arrowheads). Panel B1 is a higher magnification of the box outlined in panel B;
(C) Western blot analysis of protein from primary enamel organ epithelial cells to identify phospho-FAK Tyr397 (p-FAK) and total FAK. Cells were treated for 2- or 15-min with ScrRGDS PA or bRGDS PA or grown on tissue culture (TC) as a sham control. Equal amounts (15 μg) of lysate for each sample were used in each lane and probed with p-FAK or FAK antibodies. GAPDH is used as loading control;
(D) Densitometry tracing of phospho-FAK Tyr397 (p-FAK) (top panel) and total FAK (bottom panel) detected by Western blot analysis shown in panel D. The p-FAK and total FAK levels were normalized to GADPH values;
(E) The ratio of activated p-FAK to total FAK was significantly increased in enamel organ epithelial cells treated with bRGDS PA within 2 min (p<0.05, ANOVA) and returned to basal levels within 15 min.
3.3. Up-regulation of amelogenin gene expression and its transcriptional activator C/EBPα through FAK
To evaluate the specificity of FAK activation for up-regulation of C/EBPα and amelogenin gene expression in response to PA treatment, we inhibited the FAK pathway using a specific inhibitor, PF 573228, which has been found to efficiently inhibit FAK phosphorylation on tyrosine 397 without inhibiting cell growth or inducing apoptosis when used at a concentration of 1 μM [39]. To determine the minimal concentration of PF 573228 required for inhibition of PA-mediated FAK phosphorylation in the ameloblast-like cell line LS8, we treated LS8 cells with bRGDS PA for 2 min after incubation with the specific FAK inhibitor PF 573228 using a stepwise gradient of inhibitor concentrations: 0, 0.05, 0.1, 0.3, 0.5, 0.9, 1.0, 1.5, 2.0 μM for 1 hr. Subsequently, whole cell lysates were resolved to size by 4%–12% SDS-PAGE, transferred to a membrane and blotted with p-FAK (Tyr397) specific antibody. FAK phosphorylation levels dropped in a dose-dependent manner to low but detectable amounts in the 0.3–0.5 μM range (Fig. 3A). The 0.5 μM concentration was chosen for subsequent studies where FAK inhibition was sought.
Figure 3. FAK activation regulates amelogenin gene and ameloblast-specific transcription factor C/EBPα.
(A) Cellular FAK phosphorylation on tyrosine residue 397 was blocked by the FAK specific inhibitor PF 573228 in an ameloblast-like LS8 cell line. LS8 cells were treated with bRGDS PA after incubation with PF 573228 at a selected concentration ranging from 0, 0.05, 0.1, 0.3, 0.5, 0.9, 1.0, 1.5, 2.0 μM for 1 hr. Proteins from whole cell lysates were resolved and probed for phosphorylated FAK (p-FAK). FAK phosphorylation level revealed a sharp diminution at a FAK inhibitor concentration of approximately 300 nM;
(B) The effect of FAK inhibitor PF 573228 on the expression of mRNAs for amelogenin and C/EBPα in primary enamel organ epithelial (EOE) cells treated with different peptide amphiphile (PA) matrices using real-time PCR. Primary EOE cells were treated with either bRGDS PA or ScrRGDS PA for 4 hrs in the presence of 0.1% DMSO (vehicle control) or 500 nM FAK inhibitor PF 573228. The abundance of mRNAs for amelogenin and C/EBPα was markedly up regulated when cells were treated with bRGDS PA (bRGDS PA+DMSO), while the FAK inhibitor at 500 nM (bRGDS PA+FAK Inh) reduced the stimulating effect of bioactive PA on amelogenin (p=0.03, ANOVA), and C/EBPα expression (p=0.006, ANOVA), compared with mRNA abundance levels for the ScrRGDS PA treated group in either the presence or absence of PF 573228 (ScrRGDS PA+DMSO; ScrRGDS PA+FAK Inh).
Next, we studied the effect of the FAK inhibitor PF 573228 on the PA-mediated expression of amelogenin and C/EBPα in primary EOE cells using real-time PCR to measure gene expression profiles. Primary EOE cells were treated with bRGDS PA or ScrRGDS PA for 4 hrs in the presence of either 0.1% DMSO (vehicle control) or 0.5 μM FAK inhibitor. The expression of amelogenin and C/EBPα was markedly up-regulated when treated with bRGDS PA alone (bRGDS PA + DMSO) compared with the values obtained for the ScrRGDS PA treated group (ScrRGDS PA + DMSO), as expected. However, in the presence of the FAK inhibitor, the bRGDS PA induced enhanced gene expression for amelogenin was reversed (bRGDS PA + FAK Inh) (p=0.03, ANOVA), and the level of C/EBPα expression was similarly affected (p=0.006, ANOVA) (Fig. 3B). These results indicate that FAK is a central mediator for up-regulating gene expression associated with bRGDS PA mediated enamel regeneration.
3.4 Activation of Stress-activated protein kinase/c-Jun N-terminal kinase in primary enamel organ epithelial cells treated with peptide amphiphile matrices
Since FAK was demonstrated to play a crucial role for downstream signaling, we sought to further elucidate the signaling pathway by probing the downstream effector SAPK/JNK. Confocal immunofluorescent analysis showed that primary EOE cells, treated with bRGDS PA for 15 min expressed increased phosphorylated SAPK/JNK (p-SAPK/JNK), which accumulated in the nuclei (Fig. 4B, arrowhead) as compared with EOE cells treated with ScrRGDS PA matrix (Fig. 4A).
Figure 4. Activation of stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in primary enamel organ epithelial cells treated with signaling (bRGDS) peptide amphiphile matrix.
(A) Confocal immunofluorescent analysis of primary enamel organ epithelial (EOE) cells treated with ScrRGDS PA for 15 min, using a phosphorylated SAPK/JNK Thr183/Tyr185 (p-SAPK/JNK) antibody (green) and Alexa Fluor 594 phalloidin for F-actin (red);
(B), primary EOE cells treated with bRGDS PA for 15 min. Compared with primary EOE cells treated with control (scrambled) PA matrix, EOE cells treated with the integrin-binding bRGDS PA showed an increase in p-SAPK/JNK that accumulated in both the cytoplasm and nuclei (arrowhead);
(C), primary EOE cells were treated with bRGDS PA for 15 min in the presence of the JNK inhibitor SP 600125 at 0.9 μM, a concentration that demonstrates clear inhibition of the p-SAPK/JNK signal by Western blot analysis;
(D) Western blot analysis of p-SAPK/JNK in primary enamel organ epithelial (EOE) cells treated with control PA matrix (ScrRGDS PA) or with the bioactive bRGDS PA detected by antibody specific to p-SAPK/JNK Thr183/Tyr185. The intensity of the detected p-SAPK/JNK band was normalized to a constitutively expressed protein, GAPDH. The level of p-SAPK/JNK protein was approximately 3-times as abundant in primary EOE cells treated with bRGDS PA compared with ScrRGDS PA treated cells (*p<0.01, student t-test);
(E) The effect of JNK inhibitor SP 600125 on the protein levels for p-SAPK/JNK and phosphorylated c-Jun Ser63 (p-c-Jun) in primary enamel organ epithelial (EOE) cells treated with different PA matrices by Western blot analysis. Primary EOE cells were pre-incubated with 0.9 μM JNK inhibitor SP600125 for 1 hr, and then treated with bRGDS PA or ScrRGDS PA for 15 min. The expression of p-SAPK/JNK and p-c-Jun expression was markedly up regulated when cells were treated with bRGDS PA, while treatment with the JNK inhibitor SP600125 at 0.9 μM significantly reduced the activation of the bioactive bRGDS PA on p-SAPK/JNK and p-c-Jun.
As measured by Western blot analysis, the p-SAPK/JNK protein was approximately 3 times more abundant in primary EOE cells treated with bRGDS PA compared to cells treated with ScrRGDS PA (p<0.01, student t-test, Fig. 4D). Furthermore, the bRGDS PA induced activation of JNK was completely blocked by the JNK inhibitor SP 600125. The p-SAPK/JNK signal in primary EOE cell nuclei was significantly attenuated when cells were treated with bRGDS PA matrix in the presence of JNK inhibitor SP 600125 at 0.9 μM (Fig. 4C).
We also tested the effect of JNK inhibitor SP 600125 on p-SAPK/JNK and its downstream target phosphorylated c-Jun (p-c-Jun) and quantified the total amount of protein by Western blot analysis. Primary EOE cells were incubated with 0.9 μM JNK inhibitor for 1 hr prior to the addition of either bRGDS PA or ScrRGDS PA for 15 min (Fig. 4E). The level of p-SAPK/JNK and p-c-Jun was significantly up-regulated when cells were treated with bRGDS PA, while JNK inhibitor SP 600125 at 0.9 μM significantly reduced the activation of p-SAPK/JNK and p-c-Jun induced by the bRGDS PA matrix alone.
3.5. Phosphorylation of c-Jun in primary enamel organ epithelial cells treated with peptide amphiphile matrices
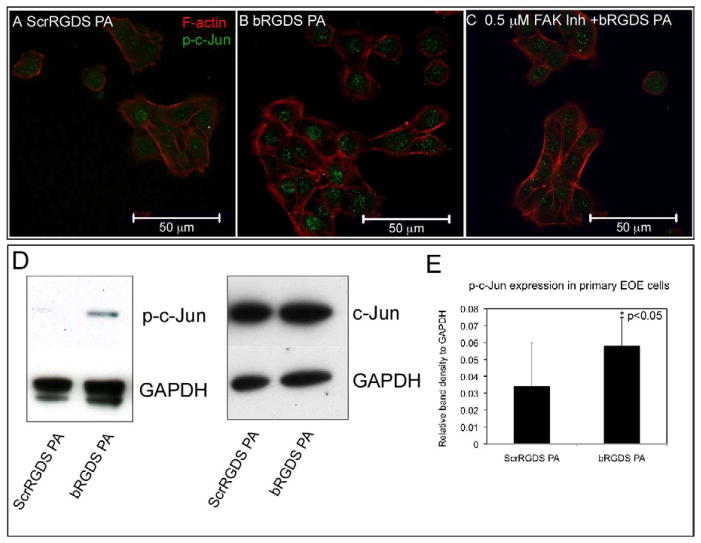
We next examined the phosphorylation of the transcription factor c-Jun by bRGDS PA matrix-induced activation of JNK. Confocal immunofluorescent analysis showed that compared with primary EOE cells treated with ScrRGDS PA matrix (Fig. 5A), primary EOE cells treated with bRGDS PA for 15 min expressed increased levels of phosphorylated c-Jun (p-c-Jun) which accumulated in the nuclei (Fig. 5B). Moreover, the enhanced nuclear localization of p-c-Jun was attenuated in primary EOE cells treated with bRGDS PA in the presence of 0.5 μM FAK inhibitor PF 573228 (Fig. 5C).
Figure 5. Phosphorylation of transcription factor c-Jun in primary enamel organ epithelial cells treated with signaling (bRGDS) peptide amphiphile matrix.
(A) Confocal immunofluorescent analysis of primary enamel organ epithelial (EOE) cells treated with ScrRGDS PA for 15 min and imaged with a specific antibody against phosphorylated c-Jun Ser63 (p-c-Jun) antibody (green) with Alexa Fluor 594 phalloidin staining for F-actin (red);
(B), primary EOE cells treated with bRGDS PA for 15 min. Compared to control PA matrix lacking signaling capacity, the primary EOE cells treated with bioactive bRGDS PA revealed increased p-c-Jun accumulation in the nuclei (arrowhead);
(C), primary EOE cells treated with bRGDS PA for 15 min in the presence of 500 nM FAK inhibitor PF 573228 revealed the reduction of nuclear localization signal for p-c-Jun;
(D) Primary enamel organ epithelial (EOE) cells were treated with either ScrRGDS PA or bioactive bRGDS PA and their lysates analyzed by Western blot analysis for expression of total c-Jun and activated phospho-c-Jun (p-c-Jun). The p-c-Jun expression was increased in primary EOE cells treated with bRGDS PA compared with ScrRGDS PA (*p<0.05, student t-test), while the levels of total c-Jun demonstrated little variation between treatment groups;
(E) Densitometry analysis of the intensity of the detected band normalized to GAPDH shown as a bar graph. The activated phospho-c-Jun protein level was approximately 1.5 times as abundant in primary EOE cells treated with bRGDS PA compared with scrambled PA control.
The p-c-Jun expression profile described above was confirmed and quantitated by Western blot analysis. Consistent with observations in the immunofluorescence experiments, c-Jun phosphorylation was low in primary EOE cells treated with ScrRGDS PA matrix. Robust phosphorylation of c-Jun was induced in response to bRGDS PA matrix, while the overall level of c-Jun was unaltered. The activated phosphorylated c-Jun protein was approximately 1.5 times more abundant in primary EOE cells treated with bRGDS PA compared to ScrRGDS PA treated cells (Fig. 5D and 5E).
3.6. Transcription regulation of the mouse amelogenin promoter by c-Jun
To directly probe amelogenin expression in response to c-Jun activation, putative cis-elements within the mouse amelogenin promoter that are potentially regulated by c-Jun [40] were identified in silco usingthe Biobase algorithm. This algorithm identifies complete binding sites, while excluding half-sites with no independent physiological context [41]. In the 5′-proximal region of the mouse amelogenin promoter, we identified two cis elements upstream of the transcription-start site (TSS; right handed arrow) at nucleotide position -213 (Fig. 6A, box, c-Jun1) and at nucleotide position -364 (Fig. 6A, box c-Jun2) based upon the conserved nucleotides core sequence of “TGACT”. For reference, the core binding sequence for the transcription factor activating amelogenin transcription, C/EBPα, was located at nucleotide position -70 (Fig. 6A, box, C/EBPα) as previously described by Zhou and colleagues [19, 20].
Figure 6. Transcriptional activation of the mouse amelogenin promoter by c-Jun.
(A) Graphical representation of the cis-elements within 5′-proximal region of the mouse amelogenin promoter. The software program “Biobase” was used to identify the “TGACT”-cis element sequence for c-Jun which is shown outlined by boxes located at c-Jun1 (-213nt) and c-Jun2 (-364nt) and the C/EBPα binding “GAAA” element is outlined by box located at around -70nt upstream of the transcription initiation site (TSS, right handed arrow) within the 5′-proximal region of the mouse amelogenin promoter;
(B) Ameloblast-like LS8 cells were transiently transfected with 250 ng of the full-length amelogenin promoter reporter construct, p2207-luc and with variable mass of the expression plasmid encoding c-Jun starting at 200 ng (lane 2), 500 ng (lane3), 1000 ng (lane4) or with 750 ng empty vector (pcDNA3, lane1) as the control. The transcriptional activity of the amelogenin promoter reporter construct p2207-luc activation was measured using a dual reported assay for Renilla luciferase (transfection control) or firefly luciferase from the amelogenin promoter and normalized to the Renilla value. The exogenous c-Jun activated the transcription activity of amelogenin promoter reporter in a dose dependent manner;
(C) Whole cell lysates from a concentration gradient for c-Jun construct transfected LS8 cells, resolved in size and detected with a specific c-Jun antibody. Increasing mass of the transfected c-Jun construct resulted in increased abundance of c-Jun protein in the LS8 cells;
(D) Schematic representation of site-directed mutagenesis generated in the 5′-proximal region of the mouse amelogenin promoter in the reporter construct. Nucleotide position -213 and -364 represent the c-Jun binding sites upstream of the transcription initiation site (TSS, right handed arrow) in the 5′-proximal region of the mouse amelogenin promoter, where the c-Jun binding sites were mutated as shown in bold font;
(E) Site-directed mutagenesis of c-Jun binding sites on the regulation of amelogenin transcription activity. Ameloblast-like LS8 cells were transiently transfected with 200 ng of Wild type (WT) or mutated (MUT) amelogenin promoter-luciferase reporter constructs in the presence of 500 ng of the expression plasmid encoding c-Jun. Reporter constructs with either the single mutation site at residue 213 (MUT (213)-luc) or at the residue 364(MUT (364)-luc) were generated. A reporter construct with double mutations MUT (213)-MUT (364)-luc was generated at the -213nt and -364nt c-Jun binding sites. Mutation at -213nt and/or -364nt upstream of the transcription initiation site of the mouse amelogenin promoter resulted in significant decrease in amelogenin transcription activity (*p<0.01; ** p<0.01; *** p<0.01, student t-test), indicating amelogenin mRNA expression is regulated by c-Jun;
(F) The endogenous amelogenin gene is transcriptionally activated by c-Jun. Amelogenin gene expression from the genomic locus was increased about 1.5 fold.
We first tested the ability of the putative c-Jun sites to participate in regulating transcription activity of the amelogenin promoter. To do this, we used the reporter construct named p2207 containing 2263-bp from the mouse amelogenin promoter that was previously shown to be sufficient to mimic the temporal and spatial expression pattern for the mouse amelogenin gene [18, 19, 42]. Ameloblast-like LS8 cells were transiently transfected with 250 ng of full-length amelogenin promoter p2207-luc reporter construct in the presence of 750 ng empty vector pcDNA3 alone, or 200 ng, 500 ng, 1000 ng of an expression construct encoding c-Jun. The pGL4.75 plasmid (50 ng) was included in all experiment groups as an internal control to normalize for transfection efficiency. Transcription activity from the amelogenin promoter gradually increased compared with the control (empty vector) in response to the increasing amounts of c-Jun (Fig. 6B). To identify and quantitate the amount of c-Jun protein, whole cell lysates from c-Jun transfected LS8 cells were resolved to size by 4%–12% SDS-PAGE, transferred to a solid support and blotted with c-Jun antibody (Fig. 6C). The expression of c-Jun protein increased proportionally with increasing amounts of the c-Jun expression construct indicating that c-Jun protein activates the mouse amelogenin promoter in a dose-dependent manner in LS8 cells.
We next performed site-directed mutagenesis of the putative c-Jun binding sites in the mouse amelogenin promoter constructs. The wild type core c-Jun sequence “TGACT” at residue -213 (WT 213) was mutated to “TattT” (MUT 213), while the wild type core sequence at residue -364 (WT364) was mutated to “TattT” (MUT364) (Fig. 6D). Ameloblast-like LS8 cells were transiently transfected with 200 ng of the wild type (WT) or each of the mutated (MUT) amelogenin promoter-luciferase reporter constructs in the presence of 500 ng of expression plasmid for c-Jun or empty vector pcDNA3. Again, the pGL4.75 plasmid (50 ng) was included in all experiment groups as an internal control. Mutation at c-Jun binding sites -213 and/or -364 of the mouse amelogenin promoter resulted in a significant decrease in amelogenin promoter activity regulated by c-Jun (Fig. 6E). These findings support the predicted sites at -213 and -364 as authentic c-Jun responsive elements serving to regulate amelogenin gene expression in ameloblast-like LS8 cells.
To corroborate the c-Jun binding sites as regulators for amelogenin expression, quantitative real-time PCR was performed in LS8 cells transfected with 500 ng c-Jun, we observed greater than 1.5 fold increase in amelogenin mRNA expression from the endogenous amelogenin gene locus (Fig. 6F) when challenged with c-Jun. An increase in the abundance of C/EBPα is induced in EOE cells exposed to the PA matrix (Fig. 1C). Taken together, c-Jun can be considered a co-activator of amelogenin gene transcription that works in concert with increasing levels of the amelogenin transactivator C/EBPα.
4. Discussion
Spatiotemporal regulation of signaling events between ectoderm-derived oral epithelium with the underlying neural crest-derived ectomesenchyme dictate enamel formation during embryonic development. We have employed a synthetic biomaterial to mimic these biological signals at a time and place of our choosing in order to devise strategies for enamel regeneration. We have previously shown that the bRGDS PA artificial matrix serves as a cell-free equivalent of the dental ectomesenchyme, mimicking the signals needed to induce naïve enamel organ epithelial cells to enter the ameloblast differentiation pathway and synthesize a mature enamel matrix [32]. In this study, we confirm our prior observation that regenerated enamel is formed adjacent to the site of bRGDS PA injected among the enamel organ epithelia of developing mouse incisors (Fig. 1A, 1B). We observe that naïve dental epithelia cells proliferate, differentiate and establish cell polarity upon contact with the artificial bRGDS PA matrix, yielding functional ameloblasts with increased production of an enamel matrix (Fig. 1B) [32]. Ameloblast function on the level of gene transcription can be validated by amelogenin gene expression, the dominant matrix protein of mammalian enamel, which is orchestrated by stage-specific growth factors, cytokines and transcription factors triggered by reciprocal interactions between the epithelium and mesenchyme [6, 11, 43]. Transcriptional activation of amelogenin, ameloblastin and other members of the gene repertoire required for enamel formation are significantly up-regulated by the leucine-zipper transcription factor C/EBPα [19–21, 36]. The differentiation response induced by the biomaterial is further evidenced here by increased abundance of both amelogenin mRNA and C/EBPα mRNA (Fig. 1C) compared to controls where cells were exposed to a sham or control ScrRGDS PA matrix. The strong correlation of bRGDS PA to promote enamel regeneration prompted our investigation into the molecular pathway responsible for this biomaterial-induced regeneration. Elucidating this mechanism provides both design guidelines for future synthetic, cell-based strategies and opportunities to manipulate pathways for regulating enamel regeneration.
During the early stages of enamel formation, the extracellular matrix (ECM) coordinates signals that guide cellular proliferation and differentiation, mediated in part through integrin receptors located on odontogenic epithelial cells [44–46]. One of the principle amino acid sequence motifs for integrin binding is RGDS, which is found in fibronectin, a major component of the ECM. An equivalent domain was identified in ameloblastin (Ambn) [47], the second most abundant enamel matrix protein and shown to be involved with ameloblast cell adhesion to the enamel ECM during tooth development. Truncation of the Ambn protein disrupts ameloblast adhesion to the forming ECM and offers further evidence for the essential role of cell-to-matrix interactions during enamel tissue development [25]. In addition, pre-secretory ameloblasts transiently express the ECM molecule dentin sialophosphoprotein (DSPP), which is cleaved to release two mature dentin matrix proteins thought to contribute to enamel formation and to the dentino-enamel junction with its unique mechanical properties linking enamel to the underlying ectomesenchyme derived dentin [48–50]. DSPP is a member of the small integrin-binding ligand, N-linked glycoproteins (SIBLING) family [51] which shares an RGD tripeptide sequence that binds integrin receptors. Other important integrin interactions with the ECM during enamel formation involve laminin molecules and their respective integrin receptors [52–54].
In our approach, the synthetic PA matrix mimics the ECM and exploits the use of RGDS to activate cell surface receptors such as integrins for initiating cytosolic and nuclear events that occur in enamel regeneration. During enamel development, integrins are expressed by pre-secretory ameloblasts and dental epithelial stem cells, the precursor cell population for differentiated ameloblasts [24, 55]. The integrin subunit β1 is known to specifically bind RGDS [56] and is also critical for tooth bud morphogenesis where it plays critical role(s) in events such as ameloblast polarization, cusp formation and cervical loop extension [46]. Integrin binding can serve to trigger FAK (focal adhesion kinase), a kinase that is associated with β integrin subunits and phosphorylates upon activation to regulate multiple cell signaling pathways responsible for cell growth, survival, and morphogenesis [57].
We evaluated the specificity of the bRGDS PA to facilitate integrin engagement by investigating its ability to trigger FAK phosphorylation in primary EOE cells. A rapid and striking stimulation of p-FAK was observed when cells were treated for 2 minutes with bRGDS PA compared with cells exposed to controls of either sham or ScrRGDS PA treatment (Fig. 2A–2D). Rapid and efficient focal adhesion turnover takes place on the order of minutes, particularly when FAK and other intracellular proteins are recruited to form the new focal adhesions [58]. Consistent with this timeframe for focal adhesion turnover, we observe that the stimulation of p-FAK induced by bRGDS PA in primary EOE cells is attenuated after 5 min (data not shown) and returns to a only a slight increase within 15 min. Compared with cells treated with control ScrRGDS PA, EOE cells treated with bRGDS PA take on a polarized migratory shape with well-organized actin stress fibers and p-FAK distributed throughout the cytoplasm and cell filopodia, (Fig. 2). Furthermore, we show that the FAK specific inhibitor, PF 573228, at 0.5 μM attenuates the stimulatory effect of bRGDS PA, nullifying the enhanced downstream expression of amelogenin and C/EBPα that is observed without inhibitor (Fig. 3B). These results suggest that the bRGDS PA matrix is activating integrin-mediated signaling and cytoskeleton organization required for the activation and recruitment of FAK in primary dental epithelial cells. Furthermore, it is interesting to note that when adhesive cell types such as fibroblasts are treated with synthetic RGDS peptides alone, FAK inhibition is observed, as the free peptide competes with native ECM proteins for integrin binding [59]. In the case of bRGDS PA, FAK is clearly activated, perhaps due to the support from the self-assembled nanofibers, enabling conformational change of the integrin cytoplasmic domain and activation of proteins participating in focal adhesion formation.
Since the bRGDS PA matrix was found to activate FAK, we further explored the downstream effectors of the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) signaling pathway. The c-Jun N-terminal kinase (JNK) can be phosphorylated by FAK [60], and conversely, JNK signaling may coordinate integrin and actin functions during epithelial development [61]. It has been found that activation of the SAPK/JNK pathway, rather than the extracellular-signal regulated kinase (ERK) or p38 mitogen-activated protein kinase (MAPK) pathways, plays a major role in signaling between fibronectin and FAK in fibroblasts for cell survival in the absence of serum [16, 62, 63]. Phosphorylated forms of JNK, p38, and ERK are localized in ameloblasts at different developmental stages, and selectively activating JNK and p38 kinase promotes the expression of amelogenin, follistatin and BMP4, factors all active during tooth development [64, 65]. Activated SAPK/JNK can lead to c-Jun transcriptional activity, which is mediated by phosphorylation of Ser63 and Ser73 residues. Activated c-Jun has been found to be localized mostly with the nuclei of enamel-secreting ameloblasts, while remaining uniformly distributed among other enamel organ-derived cells during tooth development [64, 66], suggesting that c-Jun is involved in cell growth and differentiation during amelogenesis. Furthermore c-Jun acts to regulate epithelial cell proliferation, organization and migration as part of the activator protein 1, early response transcription factor family [67]. Thus, the SAPK/JNK pathway and c-Jun have implications for driving amelogenesis and possibly bRGDS PA-mediated enamel regeneration.
To this end, we discovered that the bRGDS PA matrix was capable of inducing the expression of p-SAPK/JNK and its downstream target, p-c-Jun in primary EOE cells, while the JNK inhibitor SP 600125 at 0.9 μM nearly abolished the effect of bRGDS PA on activating p-SAPK/JNK or p-c-Jun (Fig. 4A–4E). These findings suggest that activated JNK induced by the interactions between cells and bioactive bRGDS PA matrix is able to localize efficiently to the nucleus and phosphorylate a nuclear target. Furthermore, we demonstrated that the bRGDS PA-induced activation of c-Jun could be attenuated by the FAK specific inhibitor PF 573228 (Fig. 5A–5E). Therefore, JNK-c-Jun activation is implicated to regulate amelogenin expression during enamel regeneration. It has also been found that c-Jun regulates matrix metalloproteinase-20, a protease that cleaves amelogenin and promotes matrix removal during the final stages of mineral maturation [68]. We suggest that orchestrated expression of a family of enamel matrix genes and proteases is regulated by C/EBPα and c-Jun and that the extracellular matrix serves to integrate and coordinate these events at the time of enamel matrix formation.
To date, c-Jun has not been recognized to participate in regulating amelogenin gene transcription. However, the bone fide activator of amelogenin transcription is C/EBPα, a protein capable of forming a leucine zipper with c-Jun and forming a heterodimeric complex for DNA binding [69]. Since p-c-Jun activity was increased in bRGDS PA matrix treated EOE cells, we attempted to identify c-Jun binding motifs within the promoter region of the mouse amelogenin gene by computational analysis. In the 5′-proximal region of the mouse amelogenin promoter we identified two cis-elements upstream of the amelogenin transcription initiation site (TSS) at residues -213nt and -364nt (Fig. 6A) using the c-Jun core binding sequence “TGACT”. We then tested the ability of c-Jun to regulate transcription activity of the amelogenin promoter through these two sites. Ameloblast-like LS8 were transiently transfected with full-length amelogenin promoter p2207-luc reporter construct in the presence of a gradient of c-Jun expressed in these cells. We found increased transcription activity from the amelogenin promoter when challenged by c-Jun in a dose-dependent manner (Fig. 6B), indicating that ectopic c-Jun activated the amelogenin promoter. Site-directed mutagenesis of the c-Jun binding site in the mouse amelogenin promoter at -213nt and/or -364nt resulted in significantly decreased amelogenin promoter activity by optimal doses of c-Jun (Fig. 6E). This finding suggests that the c-Jun binding sites at -213nt and -364nt participate in regulating amelogenin transcription responsiveness to p-c-Jun. Furthermore, ameloblast-like LS8 cells transfected with c-Jun expressed increased transcripts from the endogenous amelogenin gene (Fig. 6F), providing further evidence that c-Jun is involved in regulating the transcription activity for the amelogenin gene in ameloblasts.
We have elucidated here a signaling mechanism by which an artificial, self-assembled matrix can promote enamel regeneration. From integrin engagement at the cell-biomaterial interface to the intracellular signaling pathway that includes activation of FAK and JNK/SAPK in the cytosol, and finally gene transcription in the nucleus by c-Jun, we have identified biomaterial-induced pathway for enamel regeneration. Furthermore, we have determined two c-Jun binding sites that may participate in amelogenin gene transcription. These signaling events may also have a broader context in understanding how to implement cell-based strategies for regeneration of enamel.
5. Conclusions
Using a self-assembling matrix as a biomaterial tool to manipulate the cell microenvironment, our study demonstrates that two transcriptional activating factors, C/EBPα and c-Jun, are involved in the regulation of ameloblast differentiation through the FAK signaling pathway. The study of these specific signaling molecules provides insight into the complex intrinsic regulation that is responsible for this cellular response. Activation of integrin-mediated signals by a synthetic biomaterial can determine the gene expression profiles necessary to achieve a cellular pathway for enamel regeneration.
Acknowledgments
This work was supported by the National Institute for Dental and Craniofacial Research (NIDCR) of the National Institutes of Health, USPHS, 5R01 DE015920 (SIS, MLS)
Confocal fluorescent images were acquired at the USC Keck Center for Liver Diseases Confocal Microscopy Core, supported by NIH, USPHS grant P50 AA11999.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thesleff I, Tummers M StemBook, editor. Tooth organogenesis and regeneration. The Stem Cell Research Community, StemBook. 2009 doi: 10.3824/stembook.1.37.1. http://www.stembook.org. Available from URL: http://www.ncbi.nlm.nih.gov/books/NBK27071/ [DOI] [PubMed]
- 2.Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–61. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 3.Snead ML, Zhu DH, Lei Y, Luo W, Bringas PO, Jr, Sucov HM, et al. A simplified genetic design for mammalian enamel. Biomaterials. 2011;32:3151–7. doi: 10.1016/j.biomaterials.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paine ML, Snead ML. Tooth developmental biology: disruptions to enamel-matrix assembly and its impact on biomineralization. Orthod Craniofac Res. 2005;8:239–51. doi: 10.1111/j.1601-6343.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 5.Slavkin HC, Bessem C, Bringas P, Jr, Zeichner-David M, Nanci A, Snead ML. Sequential expression and differential function of multiple enamel proteins during fetal, neonatal, and early postnatal stages of mouse molar organogenesis. Differentiation. 1988;37:26–39. doi: 10.1111/j.1432-0436.1988.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 6.Fincham AG, Simmer JP. Amelogenin proteins of developing dental enamel. Ciba Found Symp. 1997;205:118–30. doi: 10.1002/9780470515303.ch9. discussion 130–4. [DOI] [PubMed] [Google Scholar]
- 7.Moradian-Oldak J. Amelogenins: assembly, processing and control of crystal morphology. Matrix Biol. 2001;20:293–305. doi: 10.1016/s0945-053x(01)00154-8. [DOI] [PubMed] [Google Scholar]
- 8.Fang PA, Conway JF, Margolis HC, Simmer JP, Beniash E. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc Natl Acad Sci USA. 2011;108:14097–102. doi: 10.1073/pnas.1106228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delak K, Harcup C, Lakshminarayanan R, Sun Z, Fan Y, Moradian-Oldak J, et al. The tooth enamel protein, porcine amelogenin, is an intrinsically disordered protein with an extended molecular configuration in the monomeric form. Biochemistry. 2009;48:2272–81. doi: 10.1021/bi802175a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gungormus M, Oren EE, Horst JA, Fong H, Hnilova M, Somerman MJ, et al. Cementomimetics-constructing a cementum-like biomineralized microlayer via amelogenin-derived peptides. Int J Oral Sci. 2012;4:69–77. doi: 10.1038/ijos.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong H, White SN, Paine ML, Luo W, Snead ML, Sarikaya M. Enamel structure properties controlled by engineered proteins in transgenic mice. J Bone Miner Res. 2003;18:2052–9. doi: 10.1359/jbmr.2003.18.11.2052. [DOI] [PubMed] [Google Scholar]
- 12.Paine ML, Zhu DH, Luo W, Bringas P, Jr, Goldberg M, White SN, et al. Enamel biomineralization defects result from alterations to amelogenin self-assembly. J Struct Biol. 2000;132:191–200. doi: 10.1006/jsbi.2000.4324. [DOI] [PubMed] [Google Scholar]
- 13.Thesleff I, Nieminen P. Tooth morphogenesis and cell differentiation. Curr Opin Cell Biol. 1996;8:844–50. doi: 10.1016/s0955-0674(96)80086-x. [DOI] [PubMed] [Google Scholar]
- 14.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–8. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 15.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–63. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takino T, Nakada M, Miyamori H, Watanabe Y, Sato T, Gantulga D, et al. JSAP1/JIP3 cooperates with focal adhesion kinase to regulate c-Jun N-terminal kinase and cell migration. J Biol Chem. 2005;280:37772–81. doi: 10.1074/jbc.M505241200. [DOI] [PubMed] [Google Scholar]
- 17.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–23. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 18.Snead ML, Paine ML, Chen LS, Luo BY, Zhou DH, Lei YP, et al. The murine amelogenin promoter: developmentally regulated expression in transgenic animals. Connect Tissue Res. 1996;35:41–7. doi: 10.3109/03008209609029173. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YL, Snead ML. Identification of CCAAT/enhancer-binding protein alpha as a transactivator of the mouse amelogenin gene. J Biol Chem. 2000;275:12273–80. doi: 10.1074/jbc.275.16.12273. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YL, Lei Y, Snead ML. Functional antagonism between Msx2 and CCAAT/enhancer-binding protein alpha in regulating the mouse amelogenin gene expression is mediated by protein-protein interaction. J Biol Chem. 2000;275:29066–75. doi: 10.1074/jbc.M002031200. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Zhou YL, Gonzalez FJ, Snead ML. CCAAT/enhancer-binding protein delta (C/EBPdelta) maintains amelogenin expression in the absence of C/EBPalpha in vivo. J Biol Chem. 2007;282:29882–9. doi: 10.1074/jbc.M702097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Shukla R, Casagrande L, Sedgley C, Nor JE, Baker JR, Jr, et al. Differentiating dental pulp cells via RGD-dendrimer conjugates. J Dent Res. 2010;89:1433–8. doi: 10.1177/0022034510384870. [DOI] [PubMed] [Google Scholar]
- 24.Fukumoto S, Yamada Y. Review: extracellular matrix regulates tooth morphogenesis. Connect Tissue Res. 2005;46:220–6. doi: 10.1080/03008200500344017. [DOI] [PubMed] [Google Scholar]
- 25.Fukumoto S, Yamada A, Nonaka K, Yamada Y. Essential roles of ameloblastin in maintaining ameloblast differentiation and enamel formation. Cells Tissues Organs. 2005;181:189–95. doi: 10.1159/000091380. [DOI] [PubMed] [Google Scholar]
- 26.Sur S, Matson JB, Webber MJ, Newcomb CJ, Stupp SI. Photodynamic control of bioactivity in a nanofiber matrix. ACS Nano. 2012;6:10776–85. doi: 10.1021/nn304101x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webber MJ, Tongers J, Renault MA, Roncalli JG, Losordo DW, Stupp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 2010;6:3–11. doi: 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storrie H, Guler MO, Abu-Amara SN, Volberg T, Rao M, Geiger B, et al. Supramolecular crafting of cell adhesion. Biomaterials. 2007;28:4608–18. doi: 10.1016/j.biomaterials.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 2008;108:4754–83. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–8. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 31.Newcomb CJ, Bitton R, Velichko YS, Snead ML, Stupp SI. The role of nanoscale architecture in supramolecular templating of biomimetic hydroxyapatite mineralization. Small. 2012;8:2195–202. doi: 10.1002/smll.201102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Z, Sargeant T, Hulvat J, Mata A, Bringas P, Koh C, et al. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J Bone Miner Re. 2008;23:1995–2006. doi: 10.1359/JBMR.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guler MO, Hsu L, Soukasene S, Harrington DA, Hulvat JF, Stupp SI. Presentation of RGDS epitopes on self-assembled nanofibers of branched peptide amphiphiles. Biomacromolecules. 2006;7:1855–63. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen LS, Couwenhoven RI, Hsu D, Luo W, Snead ML. Maintenance of amelogenin gene expression by transformed epithelial cells of mouse enamel organ. Arch Oral Biol. 1992;37:771–8. doi: 10.1016/0003-9969(92)90110-t. [DOI] [PubMed] [Google Scholar]
- 35.DenBesten PK, Machule D, Zhang Y, Yan Q, Li W. Characterization of human primary enamel organ epithelial cells in vitro. Arch Oral Biol. 2005;50:689–94. doi: 10.1016/j.archoralbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Zhou YL, Erickson RL, Macdougald OA, Snead ML. Physical dissection of the CCAAT/enhancer-binding protein alpha in regulating the mouse amelogenin gene. Biochem Biophys Res Commun. 2007;354:56–61. doi: 10.1016/j.bbrc.2006.12.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couwenhoven RI, Luo W, Snead ML. Co-localization of EGF transcripts and peptides by combined immunohistochemistry and in situ hybridization. J Histochem Cytochem. 1990;38:1853–7. doi: 10.1177/38.12.2254649. [DOI] [PubMed] [Google Scholar]
- 38.Huang Z, Newcomb CJ, Bringas P, Jr, Stupp SI, Snead ML. Biological synthesis of tooth enamel instructed by an artificial matrix. Biomaterials. 2010;31:9202–11. doi: 10.1016/j.biomaterials.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–52. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 40.Kel AE, Gossling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, Wingender E. MATCH: A tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res. 2003;31:3576–9. doi: 10.1093/nar/gkg585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matys V, Fricke E, Geffers R, Gossling E, Haubrock M, Hehl R, et al. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003;31:374–8. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snead ML, Lau EC, Fincham AG, Zeichner-David M, Davis C, Slavkin HC. Of mice and men: anatomy of the amelogenin gene. Connect Tissue Res. 1989;22:101–9. [PubMed] [Google Scholar]
- 43.Robinson C, Brookes SJ, Shore RC, Kirkham J. The developing enamel matrix: nature and function. Eur J Oral Sci. 1998;106 (Suppl 1):282–91. doi: 10.1111/j.1600-0722.1998.tb02188.x. [DOI] [PubMed] [Google Scholar]
- 44.Yamada S, Yamada KM, Brown KE. Integrin regulatory switching in development: oscillation of beta 5 integrin mRNA expression during epithelial-mesenchymal interactions in tooth development. Int J Dev Biol. 1994;38:553–6. [PubMed] [Google Scholar]
- 45.Salmivirta K, Gullberg D, Hirsch E, Altruda F, Ekblom P. Integrin subunit expression associated with epithelial-mesenchymal interactions during murine tooth development. Dev Dyn. 1996;205:104–13. doi: 10.1002/(SICI)1097-0177(199602)205:2<104::AID-AJA2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 46.Chen B, Goodman E, Lu Z, Bandyopadhyay A, Magraw C, He T, et al. Function of beta1 integrin in oral epithelia and tooth bud morphogenesis. J Dent Res. 2009;88:539–44. doi: 10.1177/0022034509338008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beyeler M, Schild C, Lutz R, Chiquet M, Trueb B. Identification of a fibronectin interaction site in the extracellular matrix protein ameloblastin. Exp Cell Res. 2010;316:1202–12. doi: 10.1016/j.yexcr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 48.MacDougall M, Nydegger J, Gu TT, Simmons D, Luan X, Cavender A, et al. Developmental regulation of dentin sialophosphoprotein during ameloblast differentiation: a potential enamel matrix nucleator. Connect Tissue Res. 1998;39:25–37. doi: 10.3109/03008209809023909. discussion 63–7. [DOI] [PubMed] [Google Scholar]
- 49.Bleicher F, Couble ML, Farges JC, Couble P, Magloire H. Sequential expression of matrix protein genes in developing rat teeth. Matrix Biol. 1999;18:133–43. doi: 10.1016/s0945-053x(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 50.Paine ML, Luo W, Wang HJ, Bringas P, Jr, Ngan AY, Miklus VG, et al. Dentin sialoprotein and dentin phosphoprotein overexpression during amelogenesis. J Biol Chem. 2005;280:31991–8. doi: 10.1074/jbc.M502991200. [DOI] [PubMed] [Google Scholar]
- 51.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44 (Suppl 1):33–40. [PubMed] [Google Scholar]
- 52.Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145:1309–23. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bei M, Stowell S, Maas R. Msx2 controls ameloblast terminal differentiation. Dev Dyn. 2004;231:758–65. doi: 10.1002/dvdy.20182. [DOI] [PubMed] [Google Scholar]
- 54.Fukumoto S, Miner JH, Ida H, Fukumoto E, Yuasa K, Miyazaki H, et al. Laminin alpha5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J Biol Chem. 2006;281:5008–16. doi: 10.1074/jbc.M509295200. [DOI] [PubMed] [Google Scholar]
- 55.Chavez MG, Yu W, Biehs B, Harada H, Snead ML, Lee JS, et al. Characterization of dental epithelial stem cells from the mouse incisor with two-dimensional and three-dimensional platforms. Tissue Eng Part C Methods. 2013;19:15–24. doi: 10.1089/ten.tec.2012.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogel BE, Tarone G, Giancotti FG, Gailit J, Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1) J Biol Chem. 1990;265:5934–7. [PubMed] [Google Scholar]
- 57.Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell-fibronectin matrix interactions during tissue repair. J Investig Dermatol Symp Proc. 2006;11:73–8. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- 58.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol. 2009;21:676–83. doi: 10.1016/j.ceb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalla Costa AP, Clemente CF, Carvalho HF, Carvalheira JB, Nadruz W, Jr, Franchini KG. FAK mediates the activation of cardiac fibroblasts induced by mechanical stress through regulation of the mTOR complex. Cardiovasc Res. 2010;86:421–31. doi: 10.1093/cvr/cvp416. [DOI] [PubMed] [Google Scholar]
- 60.Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145:1461–9. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Homsy JG, Jasper H, Peralta XG, Wu H, Kiehart DP, Bohmann D. JNK signaling coordinates integrin and actin functions during Drosophila embryogenesis. Dev Dyn. 2006;235:427–34. doi: 10.1002/dvdy.20649. [DOI] [PubMed] [Google Scholar]
- 62.Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, et al. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149:741–54. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin TH, Aplin AE, Shen Y, Chen Q, Schaller M, Romer L, et al. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385–95. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishikawa S. Transient increase in anti-p-ATF2 immunoreactivity in the late secretion ameloblasts apical to the transition zone of rat incisors. Anat Sci Int. 2004;79:87–94. doi: 10.1111/j.1447-073x.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- 65.Abe K, Miyoshi K, Muto T, Ruspita I, Horiguchi T, Nagata T, et al. Establishment and characterization of rat dental epithelial derived ameloblast-lineage clones. J Biosci Bioeng. 2007;103:479–85. doi: 10.1263/jbb.103.479. [DOI] [PubMed] [Google Scholar]
- 66.Nishikawa S. Localization of transcription factor AP-1 family proteins in ameloblast nuclei of the rat incisor. J Histochem Cytochem. 2000;48:1511–20. doi: 10.1177/002215540004801108. [DOI] [PubMed] [Google Scholar]
- 67.Li G, Gustafson-Brown C, Hanks SK, Nason K, Arbeit JM, Pogliano K, et al. c-Jun is essential for organization of the epidermal leading edge. Dev Cell. 2003;4:865–77. doi: 10.1016/s1534-5807(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Li W, Chi HS, Chen J, Denbesten PK. JNK/c-Jun signaling pathway mediates the fluoride-induced down-regulation of MMP-20 in vitro. Matrix Biol. 2007;26:633–41. doi: 10.1016/j.matbio.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai DH, Wang D, Keefer J, Yeamans C, Hensley K, Friedman AD. C/EBP alpha:AP-1 leucine zipper heterodimers bind novel DNA elements, activate the PU. 1 promoter and direct monocyte lineage commitment more potently than C/EBP alpha homodimers or AP-1. Oncogene. 2008;27:2772–9. doi: 10.1038/sj.onc.1210940. [DOI] [PMC free article] [PubMed] [Google Scholar]