Abstract
Objective
We conducted a randomized, double-blind, placebo-controlled efficacy and tolerability trial of Matricaria recutita (chamomile) extract therapy in patients with mild to moderate Generalized Anxiety Disorder (GAD). We hypothesized that chamomile would be superior to placebo in reducing GAD symptoms with a comparable tolerability profile.
Materials & Methods
61 outpatients with mild to moderate GAD were enrolled and 57 were randomized to either double blind chamomile extract (n=28) or placebo (n=29) therapy for 8 weeks. The study was powered to detect a statistically significant and clinically meaningful group difference in change over time in total Hamilton Anxiety Rating (HAM-A) scores. Secondary outcomes included change in the Beck Anxiety Inventory score, Psychological Well Being score, Clinical Global Impression Severity score, and the proportion of patients with ≥50% reduction in baseline HAM-A score.
Results
We observed a significantly greater reduction in mean total HAM-A score during chamomile versus placebo therapy (p=0.047). Although the study was not powered to identify small to moderate differences in secondary outcomes, we observed a positive change in all secondary outcomes in the same direction as the primary outcome measure. One patient in each treatment group discontinued therapy for adverse events. The proportion of patients experiencing 0, 1, 2, or ≥3 adverse events was not significantly different between groups (p=0.417).
Conclusion
This is the first, controlled clinical trial of chamomile extract for GAD. The results suggest that chamomile may have modest anxiolytic activity in patients with mild to moderate GAD. Future studies are needed to replicate these observations.
INTRODUCTION
Anxiety disorders are among the most common psychiatric conditions. (1,2) In particular, generalized anxiety disorder (GAD) is characterized by a wide array of psychological and physical symptoms, and usually manifests as a chronic disorder with only 30% of patients experiencing spontaneous remission. (1) While benzodiazepine (BZ) anxiolytics have been the mainstay therapy of GAD, these agents are often associated with unwanted side effects and dependence. (3) More recently, selective serotonin reuptake inhibitor (and other) antidepressants have been used to treat GAD. (4) However, these agents can produce unwanted sexual and other side effects that can result in patient dissatisfaction and treatment discontinuation. (5,6)
Many individuals with mild to moderate symptoms of anxiety do not seek medical attention because they do not view their symptoms as the result of a medical condition. Rather, many individuals with self-diagnosed anxiety symptoms will seek non-conventional anti-anxiety treatment with a complementary and alternative medicine (CAM). (7) These remedies have now become widely available, and have increased in use in recent decades. (8) Common reasons for individuals choosing CAM therapy may include lack of health insurance, cultural proscriptions, concern over the stigma of having an anxiety disorder, or the association of mental illness with mental weakness. Moreover, individuals seeking CAM remedies often come from vulnerable populations such as the uninsured, or racial and ethnic minorities. Rigorous testing of candidate CAM therapies of mild to moderate anxiety is needed to expand available therapeutic options for this disorder (9,10,11).
Matricaria recutita or Chamomilla recutita (chamomile) has been used as a traditional herbal remedy for its relaxation and calming effect. It is well tolerated and demonstrates pharmacological activity in animal models of anxiety. (12,13–15) However, despite its widespread use as a calming agent, there have been no controlled clinical trials of chamomile’s anxiolytic efficacy or tolerability in individuals with GAD. We present preliminary results from the first 8-week, randomized, double-blind, placebo-controlled, parallel group trial of oral chamomile extract therapy of mild to moderate GAD. We hypothesized that chamomile would have a superior anxiolytic efficacy versus placebo, and that chamomile would have a comparable tolerability profile relative to placebo.
MATERIALS & METHODS
Patient Selection
Patients were referred from the Department of Family Medicine & Community Health outpatient clinic. All were ≥ 18 years old and had a DSM IV Axis I diagnosis of GAD that was ascertained using the Structured Diagnostic Interview for DSM IV (SCID) (16) interview format. Patients had a minimum baseline total Hamilton Anxiety Rating (HAM-A) (17) score ≥ 9. Patients with other co-morbid DSM IV Axis I disorders (e.g., minor depression) were not excluded from the trial if the co-morbid condition did not constitute the primary disorder. Patients were excluded from the trial if they had a current diagnosis of major depressive disorder, bipolar disorder, panic disorder, phobic disorder, obsessive-compulsive disorder, post-traumatic stress disorder, acute stress disorder, substance-induced anxiety disorder, psychosis, dementia, or substance abuse or dependence within the preceding 3 months. Other exclusion criteria were an unstable medical condition, hepatic or renal insufficiency, malignancy, abnormal serum thyrotropin level≥ 5 μIU/ml, or known sensitivity to chamomile, plants of the asteraceae family, mugwort, or birch pollen. Concurrent use of anxiolytics, antidepressants, mood stabilizers, sedatives, or CAM remedies (e.g., St. John’s Wort), or other chamomile preparations were not permitted. Women of child-bearing potential employed a medically proven form of contraception and had a negative pregnancy test before starting therapy.
Evaluation Procedures
Patients provided informed consent in accordance with the ethical standards of the Institutional Review Board. The study was conducted using the Principles of Good Clinical Practice Guidelines, with oversight by the local Office of Human Research and by an independent Data & Safety Monitoring Board. A psychiatric history was obtained using the SCID interview format. (16) A medical history, physical examination, and laboratory evaluation was performed that included complete blood count, electrolytes, hepatic, renal and thyroid panel, pregnancy test (in women of child-bearing potential), urinalysis, and urine drug screen. Structured symptom ratings were obtained at each study visit using the HAM-A rating (17), Beck Anxiety Inventory (BAI) (18), the Psychological General Well Being (PGWB) index (19), Clinical Global Impressions Severity (CGI/S) rating (20), and a standardized treatment emergent side effects profile. (21) Sitting and standing blood pressure, pulse, and weight were obtained at each study visit. All evaluations took place at the Depression Research Unit at the University of Pennsylvania.
Materials
Identically appearing capsules containing either pharmaceutical grade German chamomile extract standardized to a content of 1.2% apigenin (Spectrum Pharmacy Products, New Brunswick, NJ) or placebo (i.e., lactose monohydrate NF) were prepared. Chamomile was prepared as 220 mg capsules. Blinding of the characteristic chamomile aroma was achieved by inserting a disk impregnated with 1 drop of chamomile oil (for placebo) or 1 drop of neutral oil (for chamomile) into the lid of each air-tight medication container.
Randomization & Blinding Procedures
Randomization was performed using blocked randomization with varying block sizes. First, we randomly selected a block size from among a small set of block sizes. Then we randomly permuted the group numbers within that block. We continued this procedure until all subjects were randomized into each of the treatment conditions. Random numbers were permuted within each block using the random number generator and user code in Stata software. All study results were analyzed under blinded conditions.
Treatment Procedures
Chamomile or placebo therapy was initiated at one capsule daily for the first week and increased to 2 capsules daily during the second week of therapy. Patients with a ≤ 50% reduction in total HAM-A score (versus baseline) were increased to 3 capsules daily during week 3, and then to 4 capsules daily during week 4 of therapy. Patients who continued to have a ≤ 50% reduction in baseline HAM-A score were increased to 5 capsules daily during study weeks 5 through 8 of therapy. Dose reductions could occur at any time based upon drug tolerability. Outcome measurements were obtained at baseline and after 2, 4, 6, and 8 weeks of treatment.
Sample Size Justification
The study was powered to detect relatively large statistically significant differences between treatment conditions in the primary outcome measure of change over time in total HAM-A score. Using an estimate of power based upon a longitudinal comparison model, the study had sufficient power to detect an effect size of 0.57 based on a sample size of 30 patients per group with 5 measurements per patient and an intra-subject correlation of 0.50. The detectable effect sizes with 80% (90% power) were 0.57 (0.68), 0.51 (0.60), 0.44 (0.52), 0.36, (0.43), and 0.26 (0.30), for correlations of 0.50, 0.60, 0.70, 0.80, 0.90, respectively.
Statistical Procedures
All primary and secondary outcome measures were analyzed under blinded conditions. Primary comparisons implemented quasi-least squares (QLS) with 2-sided tests of hypotheses and a p-value < 0.05 as the criterion for statistical significance using the xtqls procedure for Stata 10.0 (22). QLS was chosen to allow for use of the Markov correlation structure that models the correlation between repeated measures and is appropriate for unequal measurement times.
Regression models including intercept (β0), chamomile group indicator (β1), time (β2) and their interaction (β3) were used to test the primary hypothesis that change in HAM-A scores differed significantly between treatment conditions (H0:β3 = 0). Regression analysis was also used to examine change in the secondary BAI and PGWB measures. In order to assess for sensitivity of the results in relation to the choice of correlation structure, we also used the AR(1) correlation structure and both the model based and robust sandwich estimates of the covariance matrix were applied. The results did not differ between structures. Results presented here were based on application of the model based covariance matrix, because this approach yielded slightly larger p-values and was therefore viewed as more conservative for this analysis.
A last observation carried forward (LOCF) analysis was also conducted to examine change in total HAM-A scores between treatment conditions.
The Pearson chi-square test was used to compare the proportion of responders (with a ≥ 50% reduction in baseline HAM-A score) between treatment conditions. We used the intent-to-treat approach that assumed that patients who withdrew from treatment before the end of the trial were non-responders.
Wilcoxon Rank Sum tests were used to identify differences in continuous clinical and demographic values between treatment groups. Fisher’s exact test was used to compare frequencies of adverse events between treatment conditions. A t-test was used to examine whether the mean incidence rate of adverse events (i.e., the number of events per individual in days) was greater at higher chamomile doses (during weeks 5 to 8) versus lower chamomile doses (during weeks 1 to 4). We also compared the proportion of patients reporting 0, 1, 2, or ≥ 3 adverse events between treatment conditions using the χ2 test.
RESULTS
Enrollment
61 patients were enrolled in the trial and 57 patients had a baseline visit and at least one post-baseline measurement: chamomile (n=28) and placebo (n=29). Four patients (6.6%) were screen failures: 1 for noncompliance, and 3 for withdrawn consent. Eight patients (14.03%) discontinued treatment before completing the trial: 2 for adverse events, 3 for withdrawn consent, 2 lost to follow up, and 1 for noncompliance. [Note - One patient with a baseline measurement withdrew consent to participate in the trial after taking one dose of study drug. This patient was, nevertheless, retained in all efficacy and safety outcome analyses]. There were no significant group differences in any of the demographic or clinical variables (Table 2).
Primary Outcome
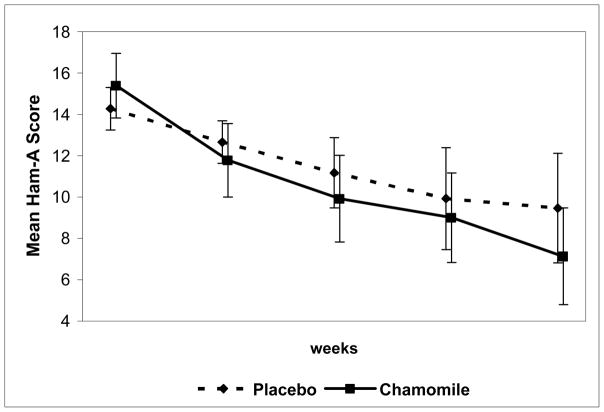
QLS analysis found a significantly greater reduction over time in the mean total HAM-A score for chamomile versus placebo (60β̂3 = −3.17, 95% CI, −6.29 to −0.45) (p=0.047). [Note - Because some of the week 8 HAM-A measurements occurred after day 56, we used day 60 in the estimated regression model to represent the mean day of the last outcome measurement].
Secondary Outcomes
The study was not powered to detect small to moderate group differences in the secondary outcome measures, but rather to identify non-significant trends in these measures (if present). There were no statistically significant differences observed between treatment groups in any secondary outcome measure. However, clinically meaningful change in secondary outcome measures did occur in the same direction as the change seen in the primary outcome measure. We observed a somewhat greater reduction in mean total BAI score for chamomile versus placebo (60β̂3 = −2.09, 95% CI = −6.14 - 1.96) (p=0.411), a somewhat greater increase in mean PGWB score with chamomile versus placebo (60β̂3 = 6.33, 95% CI = −2.71 – 15.37) (p=0.170), and a somewhat greater reduction in the CGI/S score for chamomile versus placebo (60β̂3 = −0.43, 95% CI = −1.06 - 0.21 (p=0.187). There was a somewhat greater proportion HAM-A responders to chamomile (57.13%) versus placebo (37.93%) (p=0.146). Finally, the overall percentage change was numerically greater for chamomile (−52.97%) versus placebo (−34.92%) on the HAM-A (p=0.085), for chamomile (−42.48%) versus placebo (−20.79%) on the BAI (p=0.142), and for chamomile (27.58%) versus placebo (17.64%) on the PGWB (p=0.344). We again note that although the change over time for all secondary outcomes occurred in a direction favorable to chamomile, we also observed statistically significant results for the primary outcome measure for which the study was originally powered.
Safety
Two patients discontinued treatment for adverse events: 1 for allergic reaction (placebo) and 1 for abdominal discomfort (chamomile). There were a total of 33 adverse events rated as possible, probable, or definite: 11 on chamomile and 22 on placebo (p=ns). The proportion of patients with 0, 1, 2, or ≥ 3 adverse events was 35.7, 35.7, 10.7 and 17.9 for chamomile versus 24.1, 31.0, 27.6 and 17.3 for placebo, respectively (p=0.417).
We observed a somewhat greater reduction over time in resting pulse rate during chamomile versus placebo therapy (60β̂3 = −5.22, 95% CI = −13.64 - 3.20 (p=0.225), but no difference in resting systolic and diastolic blood pressure, or weight.
Dosage
There were no significant differences in mean daily capsule number taken between treatment groups during any treatment period. There was also no significant difference in the mean number of capsules taken at each treatment period for chamomile or placebo responders versus non-responders (with a ≥ 50% reduction in baseline total HAM-A score).
Finally, we examined whether the incidence rate of adverse events (i.e., the number of events per individual in days) was greater at higher chamomile doses (during weeks 5 to 8) versus lower chamomile doses (during weeks 1 to 4). This procedure adjusted for the influence of variable measurement times between treatment periods. We found a lower incidence rate of adverse events at higher chamomile doses (0.003; 2/717) versus lower chamomile doses (0.015; 11/734) (p=0.015), -suggesting that there is no increase in adverse events at higher chamomile doses.
DISCUSSION
This study represents the first randomized, double-blind, placebo-controlled, parallel group trial of oral chamomile extract therapy of mild to moderate GAD. We observed a statistically significant superiority of chamomile over placebo in reducing total HAM-A scores (p=0.047). While the study was not specifically powered to detect statistically significant differences between treatment groups for the secondary outcome measures, we nonetheless found clinically meaningful (albeit non-significant) changes over time in the secondary outcome measures that occurred in a direction favorable to chamomile. Chamomile also appeared it be exceedingly well tolerated relative to placebo.
Chamomile, in the form of teas and oils, is often used for its relaxation and calming effect. Its exact mode of action is unknown. However, several lines of evidence suggest that one or more of its flavonoid constituents may produce anxiolytic activity by affecting γ-amino butyric acid (GABA), noradrenalin (NA), dopamine (DA), and serotonin neurotransmission (23,24), or by modulating hypothalamic-pituitary-adrenocortical axis function (12,15). Apigenin, and other constituents of chamomile, have also been shown to bind to BZ receptors and reduce GABA-activated activity. (13,25,26,27).
Several caveats should be considered in the interpretation of the present findings. For example, the small sample size may have limited our ability to equally distribute covariates between treatment conditions during the randomization process. This factor could have contributed to the favorable outcome of chamomile in the present study. In addition, there was a slightly higher mean baseline HAM-A score for the chamomile group, which could suggest the possibility that chamomile’s superiority over placebo was the result of a regression toward the mean. Similarly, other unrecognized differences between treatment groups may have contributed to the present findings.
It is possible that chamomile could have resulted in a more robust anxiolytic activity if we had employed a larger dose of the extract. The selection of the chamomile dose was based upon authoritative reviews (28) with no published dose range or tolerability studies to guide our selection of a optimal chamomile dose. Thus, the maximum chamomile dose of 1,100 mg daily may not have been the optimal anxiolytic dose of oral this chamomile extract. Moreover, the time of chamomile dosing was not standardized. Patients were instructed to take their study medication once daily or in divided doses, depending upon the time of their greatest anxiety symptoms. For example, patients may have taken their study medication in divided doses throughout the day to reduce anxiety symptoms, or may have taken the majority of their daily dosage at bedtime to reduce insomnia. This non-standardized dosing regimen may have introduced response variability and may have altered the true efficacy of the chamomile therapy. However, differences in pharmacokinetic and pharmacodynamic profiles of the ‘active’ constituents of chamomile extract, together with a paucity of controlled data on these constituents in humans, made it difficult to determine the optimal dosing strategy. Furthermore, because we used a dose escalation design, we were unable to determine whether or not there might be a dose-response relationship for chamomile extract in reducing anxiety.
It is possible that another chamomile preparation standardized to constituents other than apigenin may have produced different results. In addition, the use of non-pharmaceutical grade chamomile or chamomile from a species other than Chamomilla recutita may have produced different results. It is also possible that other chamomile formulations (e.g., oil, vapor, tea) may have produced different results with a different tolerability profile.
We did not employ a specific patient or clinician-rated measure to verify the adequacy of the blinded conditions. Thus, it is possible that unrecognized rater or patient or clinician bias may have contributed to the superiority of chamomile over placebo.
It is possible that the modest benefit of chamomile was an artifact of limited sample size. Nevertheless, despite this limitation, we observed a statistically significant, and clinically meaningful, group difference in the primary outcome measure, and fairly large effect sizes for change in the secondary outcome measures that occurred in the same direction as that of the primary measure.
We note that the patients enrolled in the study had more mild GAD symptoms and may have qualitatively differed from populations of more severely ill GAD patients included in GAD trials of conventional anxiolytic agents. It is possible that the beneficial effect of chamomile seen in this study is limited to individuals with mild to moderate GAD, and patients with more severe GAD would not benefit from chamomile therapy. We would note, however, that the change in GAD symptoms in the present study was not limited to patients with milder GAD symptoms.
Finally, the treatment duration of the current study was limited to 8 weeks, and future studies of longer duration will be needed to more fully assess the durability of chamomile’s anxiolytic effect.
CONCLUSION
The identification of a safe and effective alternative therapy for GAD would be of relevance for many individuals unable, or unwilling, to use conventional anxiolytic therapy. The current double-blind, placebo-controlled trial represents the first controlled clinical trial of the efficacy and tolerability of oral chamomile extract for mild to moderate GAD. The demonstration of chamomile’s efficacy and tolerability in patients with milder GAD may provide a wider acceptability of anxiolytic treatment in the general medical community. Despite the limitations of this initial study, the present findings suggest that chamomile may possess modest anxiolytic effect in some patients with mild GAD.
Figure 1.
Mean total HAM-A scores at each study visit for chamomile and placebo conditions.
Table 1.
Descriptive characteristics of patients.
| Placebo (n=29) | Chamomile (n=28) | P | |
|---|---|---|---|
| Gender (F/M) | 15:14 | 19:09 | 0.21 |
| Caucasian (%) | 68.97 | 78.57 | 0.41 |
| Age (mean ± SD) | 45.9 (10.88)/33–69 | 45.5 (14.53)/25–67 | 0.98 |
| Age at onset GAD (mean ± SD)/range | 25.3 (13.5)/7–75 | 27.9 (11.3)/14–58 | 0.25 |
| Illness duration (mean ± SD)/range (yrs) | 20.4(13.4)/0.3–54 | 15.0 (14.4)/1–51 | 0.11 |
| Episode duration (mean ± SD)/range (mos) | 42.9 (58.6)/2–240 | 54.3 (64.0)/6–256 | 0.22 |
| # prior episodes (mean ± SD)/range | 3.6 (5.7)/0–30 | 4.7 (9.1)/0–43 | 0.74 |
| Baseline HAM-A (mean ± SD)/range | 14.3 (2.8)/10–20 | 15.4 (4.2)/9–26 | 0.39 |
| Baseline BAI (mean ± SD)/range | 12.0 (6.2)/3–23 | 9.5 (5.6)/2–21 | 0.13 |
| Baseline PGWB (mean ± SD)/range | 58.9 (14.1)/31–91 | 62.0 (14.7)/23–86 | 0.31 |
Acknowledgments
This research was funded by the National Institute of Health/National Center for Complementary and Alternative Medicine grant AT001916. The authors’ work was independent of the NIH/NCCAM, and the NIH/NCCAM had no involvement in the study design of this trial.
Footnotes
Trial Registration – Chamomile Therapy for Generalized Anxiety, NCT00645983, http://www.clinicaltrials.gov/ct2/show/NCT00645983?term=Chamomile+Therapy+for+Generalized+Anxiety&rank=1
FINANCIAL DISCLOSURES
At the time this study was conducted, Dr. Amsterdam received grant support from: NIH grants AT002289 and AT001916; NIMH grants MH060353, MH070753, MH060998, MH060713, MH063818; Stanley Medical Research Institute; Lilly Research Laboratories; Sanofi Aventis, Inc; and Novartis, Inc. He is not a member of any industry-sponsored advisory board or speaker’s bureau, and has no significant financial interest in any pharmaceutical company.
At the time this research work was conducted, Ms. Li had no potential conflict of interest.
At the time this research work was conducted, Ms. Soeller received research support from NIMH grant MH060998. She is not a member of any industry-sponsored advisory board or speaker’s bureau, and has no significant financial interest in any pharmaceutical company.
At the time this research work was conducted, Dr. Mao received research support from HRSA D55-HP-05164. He is not a member of any industry-sponsored advisory board or speaker’s bureau, and has no significant financial interest in any pharmaceutical company.
At the time this research work was conducted, Dr. Rockwell was a consultant to Elan Pharmaceuticals, Inc.
At the time this research work was conducted, Dr. Shults received research support from NIH grants CA096885, AT002289, AT001916, MH060353, MH070753, MH060998, and MH63818. She is not a member of any industry-sponsored advisory board or speaker’s bureau, and has no significant financial interest in any pharmaceutical company.
References
- 1.Pollack MH. New advances in the management of anxiety disorders. Psychopharm Bull. 2002;36:79–94. [PubMed] [Google Scholar]
- 2.Wittchen H-U. Generalized anxiety disorder: prevalence, burden, and cost to society. Depression & Anxiety. 2002;16:162–171. doi: 10.1002/da.10065. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor K, Belanger L, Marchand A, et al. Psychological distress and adaptational problems associated with discontinuation of benzodiazepines. Addict Behav. 1999;24:537–541. doi: 10.1016/s0306-4603(98)00107-5. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin DS, Polkinghorn C. Evidence-based pharmacotherapy of generalized anxiety disorder. Int J Neuropsychopharmacol. 2005;8:293–302. doi: 10.1017/S1461145704004870. [DOI] [PubMed] [Google Scholar]
- 5.Amsterdam JD, Garcia-Espana F, Goodman D, et al. Breast enlargement during chronic SSRI therapy. J Affective Disord. 1997;46:151–156. doi: 10.1016/s0165-0327(97)00086-4. [DOI] [PubMed] [Google Scholar]
- 6.Sussman N, Ginsberg D. Rethinking side effects of the selective serotonin reuptake inhibitors: sexual dysfunction and weight gain. Psychiatry Annals. 1998;28:89–97. [Google Scholar]
- 7.Mao JJ, Farrar JT, Xie SX, et al. Use of complementary and alternative medicine and prayer among a national sample of cancer survivors compared to other populations without cancer. Complement Ther Med. 2007;15:21–9. doi: 10.1016/j.ctim.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998;280:1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 9.Pagan JA, Pauly MV. Access to conventional medical care and the use of complementary and alternative medicine. Health Affairs. 2005;24:255–62. doi: 10.1377/hlthaff.24.1.255. [DOI] [PubMed] [Google Scholar]
- 10.Givens JL, Houston TK, Van Voorhees BW, et al. Ethnicity and preferences for depression treatment. Gen Hosp Psychiatry. 2007;29:182–91. doi: 10.1016/j.genhosppsych.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Givens JL, Katz IR, Bellamy S, et al. Stigma and the acceptability of depression treatments among African Americans and whites. J Gen Intern Med. 2007;22:1292–7. doi: 10.1007/s11606-007-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada K, Miura T, Mimaki Y, et al. Effect of inhalation of Chamomile oil vapour on plasma ACTH level in ovariectomized rat under restriction stress. Biol & Pharmacol Bull. 1996;19:1244–1246. doi: 10.1248/bpb.19.1244. [DOI] [PubMed] [Google Scholar]
- 13.Zanoli P, Avallone R, Baraldi M. Behavioral characterization of the flavonoids apigenin and chrysin. Fitoterapia. 2000;71:S117–123. doi: 10.1016/s0367-326x(00)00186-6. [DOI] [PubMed] [Google Scholar]
- 14.Nakazawa T, Yasuda T, Ueda J, et al. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol Pharm Bull. 2003;26:4. doi: 10.1248/bpb.26.474. [DOI] [PubMed] [Google Scholar]
- 15.Reis LS, Pardo PE, Oba E, et al. Matricaria chamomilla CH12 decreases handling stress in Nelore calves. J Vet Sci. 2006;7:189–192. doi: 10.4142/jvs.2006.7.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/PSY SCREEN) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 17.Hamilton M. The assessment of anxiety status by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult & Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 19.Grossi E, Groth N, Mosconi P, et al. Development and validation of the short version of the Psychological General Well-Being Index (PGWB-S) Health and Quality of Life Outcomes. 2006;4:88. doi: 10.1186/1477-7525-4-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC: US Department of Health, Education, and Welfare; 1976. pp. 218–222. [Google Scholar]
- 21.National Institute of Mental Health. Treatment Emergent Symptoms Scale (TESS) Psychopharmacol Bull. 1985;21:1069–1073. [Google Scholar]
- 22.Shults J, Ratcliffe SJ, Leonard M. The Stata Journal. Vol. 7. College Station: 2007. Improved generalized estimating equation analysis via xtqls for quasi-least squares in Stata; pp. 147–166. [Google Scholar]
- 23.Lorenzo PS, Rubio MC, Medina JH, et al. Involvement of monoamine oxidase and noradrenaline uptake in the positive chronotropic effects of apigenin in rat atria. Eur J Pharmacol. 1996;26:312:203–207. doi: 10.1016/0014-2999(96)00486-4. [DOI] [PubMed] [Google Scholar]
- 24.Marder M, Paladini AC. GABA(A)-receptor ligands of flavonoid structure. Curr Top Med Chem. 2002;2:853–867. doi: 10.2174/1568026023393462. [DOI] [PubMed] [Google Scholar]
- 25.Avallone R, Zanoli P, Puia G, et al. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol. 2000;59:1387–1394. doi: 10.1016/s0006-2952(00)00264-1. [DOI] [PubMed] [Google Scholar]
- 26.Campbell EL, Chebib M, Johnston GA. The dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABA(A) receptors. Biochem Pharmacol. 2004;15;68:1631–168. doi: 10.1016/j.bcp.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Losi G, Puia G, Garzon G, et al. Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur J Pharmacol. 2004;11;502:41–46. doi: 10.1016/j.ejphar.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Blumenthal M. The complete German Commission E monographs: therapeutic guide to herbal medicines. American Botanical Council; Austin: 1998. [Google Scholar]