Abstract
Background and Purpose
Therapeutic effects of early insulin glycemic control for post-stroke hyperglycemia in combination with tissue-type plasminogen activator (tPA) thrombolytic therapy has not yet been studied but is of great clinical interest. In this study we tested the effects of insulin plus tPA combination in a model of focal embolic stroke in type I diabetic rats.
Method
STZ was used to produce Type I diabetes in male Wistar rats for 6 weeks then embolic focal strokes were induced. All rats were treated with insulin or saline at 1 h followed by tPA or saline at 1.5 h after stroke. Mortality, infarction, hemispheric swelling, hemorrhagic transformation and perfusion defects were examined at 24 hours after stroke. Total plasma plasminogen activator inhibitor-1 (PAI-1) antigen and activity levels were measured at before stroke, 1.5, 3 and 6 hours after stroke by ELISA assays.
Results
Early insulin glycemic control alone or tPA thrombolysis alone had no significant effects on ischemic infarction. However, early insulin glycemic control combined with tPA significantly reduced brain infarction and swelling , ameliorated tPA-associated hemorrhagic transformation, and improved plasma perfusion at 24 hours after stroke. We also found the combination significantly decreased plasma PAI-1 antigen level at 6 hours, and PAI-1 activity at 1.5 hours and 6 hours after stroke.
Conclusions
Early insulin glycemic control may be beneficial in combination with tPA thrombolysis for ischemic stroke with diabetes mellitus or post-stroke hyperglycemia.
Keywords: hyperglycemia, type I diabetes, rats, focal embolic stroke, insulin glycemic control, tPA, thrombolysis
Introduction
Post-stroke hyperglycemia is presented in all preexisting diabetes (about 37% of stroke patients) and 50% of non-diabetic stroke patients1, 2, and the severity of the post-stroke hyperglycemia correlates with worse neurological outcomes2,3. Furthermore, experimental and clinical investigations suggest that intravenous tPA in acute ischemic stroke patients with hyperglycemia may increase the risk of hemorrhagic transformation and worsen functional outcomes4, 5. Taken together, these findings provide a rationale for attempting glycemic control in hyperglycemic stroke patients in combination with tPA administration5, 6. However hyperglycemic correction with insulin in the acute phase of stroke did not show beneficial effects7, 8. Is it possible that the lack of efficacy in these past efforts is related to the delayed institution of insulin treatments, i.e. early correction of hyperglycemia is required for therapeutic benefit, in particular to tPA reperfusion therapy6? In this preclinical study, we tested the effects of early insulin glycemic control combined with tPA thrombolysis in STZ-induced type I diabetic rats subjected to focal embolic stroke.
Materials and Methods
Induction of Type I Diabetes in Rats
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Eight-week-old male Wistar rats (Charles River Laboratories, Wilmington, MA) with an initial body weight of 200 to 220 g were used for inducing type I diabetes by a standard intraperitoneal injection of streptozotocin (60 mg/kg; Sigma, St Louis, MO) as we previously described 9.
Focal Embolic Cerebral Ischemia and Treatment Groups
After 6 weeks of diabetes, all rats were subjected to a focal embolic stroke following our previously published methods9. This focal embolic stroke rat model was originally established by Dr. Chopp's group, its thrombolytic reperfusion time window and hemorrhagic transformation closely mimic clinical situation, which has been most commonly used for thrombolytic stroke studies 10. For rapid and sustained glycemic control, insulin (2 units/rat intravenous injection of Humulin® regular insulin, combined with 4 units/rat subcutaneous injection of Humulog® Mix75/25™ insulin, purchased from Lilly USA) was given at 1 hour after stroke onset. A standard rat dose of tPA (10 mg/kg Activase, Genentech) was intravenously injected at 1.5 hours after stroke. Blood glucose levels were measured before stroke, and 1.5, 2.5 and 24 hours after stroke. Forty-four rats were blindly and randomly assigned into 4 treatment groups (n=11 per group): (1) saline at 1.5 hours, (2) tPA at 1.5 hours, (3) insulin at 1 hour plus saline at 1.5 hours, (4) insulin at 1 hour plus tPA at 1.5 hours, after stroke. All drug treatments and outcome assessments were performed by investigators blinded to the surgical groups.
Analysis of acute brain tissue outcomes
Rats were euthanized at 24 hours after ischemia and brain slices were stained with 2,3,5-triphenyltetrazolium chloride (TTC). Ischemic infarction volumes and hemispheric swelling were assessed using computer-assisted image analysis. Thereafter, hemorrhage volume was quantified with a spectrophotometric hemoglobin assay 9.
Measurements of cerebral perfusion
Regional cerebral perfusion of stroke rats was monitored by laser Doppler flowmetry for 1.5 hours after induction of ischemia and then continuously monitored for 1 hour after treatment (n=11 per group). At 24 hours after stroke, intravenous infusion of fluorescein isothiocyanate–dextran was used to examine the microvascular perfusion in stroke rats treated with tPA alone or insulin plus tPA as we previously described (n=5 per group) 9.
Measurements of total plasma PAI-1 antigen and activity levels
Since plasminogen activator inhibitor-1 (PAI-1) is the main and potent endogenous t-PA inhibitor, its plasma concentration and activity are increased in diabetes and after stroke. Both experimental and clinical investigations were indicative of a link between PAI-1 and tPA stroke therapy outcomes 11. It has been reported that insulin may lower circulating PAI-1 concentration and activity to diabetes 12, 13. Thus in this experiment, platelet free plasma samples were collected at before ischemia, 1.5 hours (30 min after insulin treatment, right before tPA treatment), 3 hours and 6 hours after ischemia. The total plasma PAI-1 antigen and activity levels were measured by ELISA kits PRAIKT-TOT and PRAIKT (Molecular Innovation, Novi, MI) according to the manufacturer's instructions, respectively. 50 μl of plasma sample was applied in each well. Data were expressed as relative fold changes of pre-stroke levels (n=7 per group).
Statistical Analysis
Data were expressed as mean±sd. The LDF perfusion levels, infarct volumes, hemispheric swelling. hemorrhage volumes, plasma PAI-1 antigen and activity levels were analyzed with variance followed by Tukey-Kramer tests. Plasma perfusion ratio was assessed with Independent-Samples T test. P<0.05 was considered statistically significant.
Results
Early insulin combined with tPA reduced acute brain tissue damages
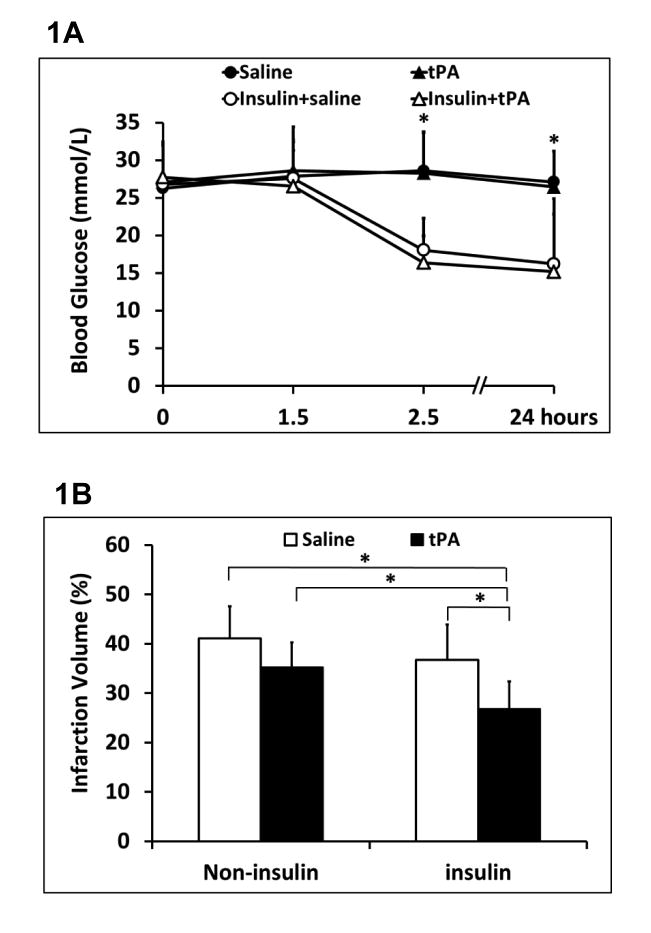
Insulin significantly lowered blood glucose levels up to 24 hours after stroke compared to non-insulin treated animals (Figure 1A). At 24 hours after stroke, early insulin glycemic control showed a significant reduction in ischemia-induced hemispheric swelling, but a slight decrease in brain infarction (10.7% reduction, P=0.48) (Figure 1B, 1C). tPA thrombolytics alone exhibited a trend for infarct reduction (14.2% reduction, P=0.16), but as expected, significantly elevated ICH (Figure 1B, 1D). However, insulin combined with tPA significantly reduced brain infarction and swelling, and ameliorated tPA-associated ICH transformation. There were also no differences in mortality; at 24 hrs mortality was 2/11 for saline alone, 2/11 for insulin plus saline, 1/11 for tPA alone, and 2/11 for insulin plus tPA.
Figure 1.
Acute brain tissue outcomes of insulin combined with tPA thrombolytics in diabetic stroke rats. A. Blood glucose levels were monitored over the times up to 24 hours after stroke. B. At 24 hours after stroke, ischemic infarct volumes were quantified on TTC-stained brain slices. C. Hemispheric swelling were quantified on the same TTC-stained brain slices. D. Intracerebral hemorrhage volumes were quantified with hemoglobin assay at 24 hours after stroke. Data were expressed as mean ± sd, * P<0.05, n=9 or 10 per group.
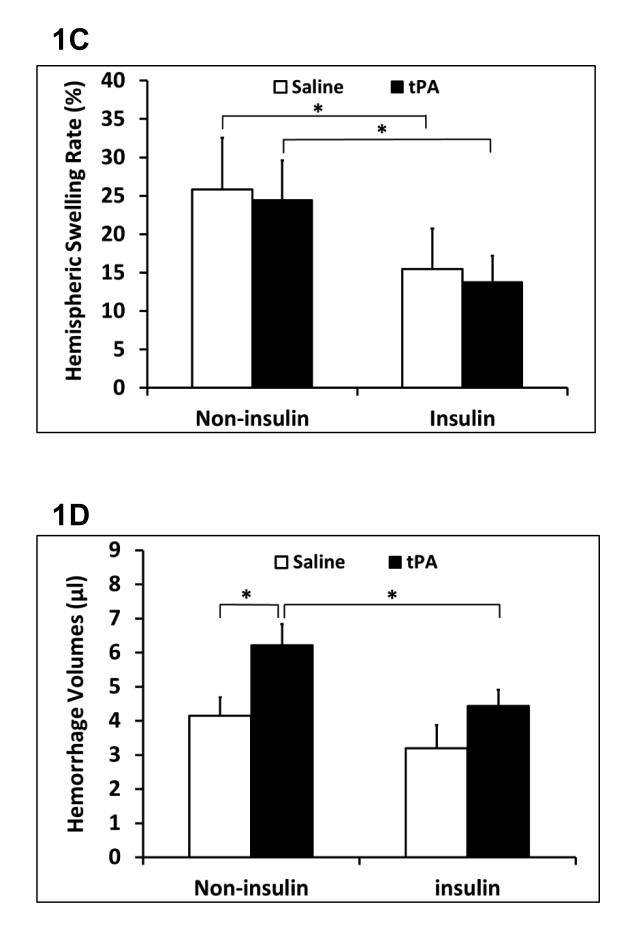
Early insulin combined with tPA improved cerebral blood perfusion
Focal embolic ischemia resulted in comparable reductions in cerebral perfusion in all rats. No change in perfusion was detected for up to 1 hr after thrombolysis in all four treatment groups (Figure 2A). At 24 hours after stroke, fluorescein isothiocyanate–dextran plasma perfusion imaging demonstrated persisting areas of perfusion defects in the expected middle cerebral artery territory (Figure 2B). However, the relative FITC–dextran plasma perfused area (ratio of ipsilateral/contralateral hemisphere) was significantly increased in the insulin plus tPA combination group compared to the tPA alone group (26% increase) (Figure 2C).
Figure 2.
Cerebral perfusion of tPA thrombolytics with/without early insulin glycemic control in diabetic stroke rats. A. Laser Doppler flowmetry monitored regional cerebral blood flow for up to 1 hour after treatments. B. Representative microvessels perfused with FITC–dextran of coronal rat brain sections treated with tPA or insulin plus tPA combination at 24 hours after stroke. C. Relative microvasculature area perfused with FITC-dextran. Data were expressed as mean ± sd. * P<0.05, n=5 per group.
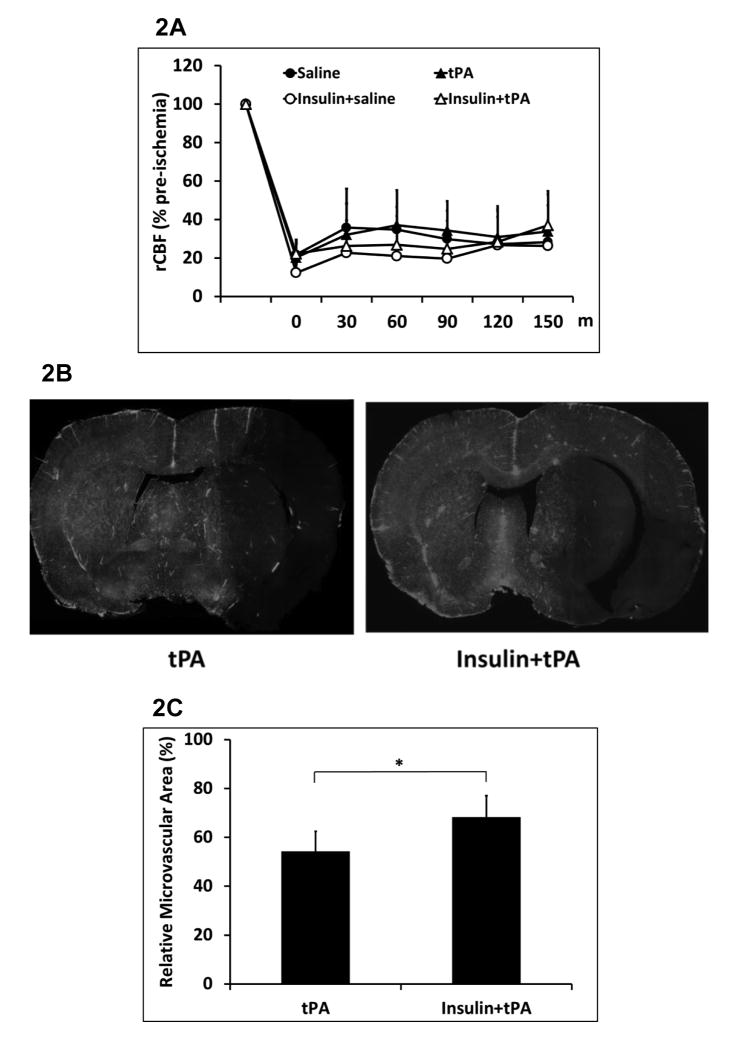
Early insulin infusion combined with tPA reduced total plasma PAI-1 antigen and activity levels
Animals were treated with tPA at 1.5 hours after stroke, or insulin at 1 hour combined with a followed tPA at 1.5 hours after stroke. Platelet free plasma samples were collected at 1.5 hours (right before tPA administration), 3 hours and 6 hours after stroke. The total plasma PAI-1 antigen levels were significantly elevated, and a significant reduction was detected at 6 hours after stroke in insulin plus tPA group compared to tPA alone group (Figure 3A). The total PAI-1 activity was almost neutralized by exogenous tPA at 3 hours after stroke, but it was significantly increased at 1.5 and 6 hours after ischemia in tPA alone group. However, insulin plus tPA group significantly decreased the elevated PAI-1 activity at 1.5 h and 6 h after ischemia compared to tPA alone treatment (Figure 3B).
Figure 3.
Effects of early insulin glycemic control combined with tPA in plasma PAI-1 antigen and activity levels. Platelet free plasma samples were collected at pre-stroke, 1.5, 3, and 6 hours after stroke. A. Changes of plasma PAI-1 antigen levels over the times up to 6 hours after stroke. B. Changes of plasma PAI-1 activity levels over the times up to 6 hours after stroke. Data were expressed as mean ± sd of relative fold changes of pre-stroke baseline. *P<0.05 vs pre-stroke baseline within the same treatment group; #<0.05 between groups at the same examining time point; n= 7 per group.
Discussion
In this study, only acute brain tissue outcomes of ischemic infarction, swelling, intracerebral hemorrhage were examined at 24 hours after focal embolic stroke in type 1 diabetic rats. One caveat is that we did not investigate long-term brain tissue and neurological functional outcomes. Since a clinical report showed that at 16 hours after stroke, only 11% (from 50% at early phase) of non-diabetic and 27% (from 100% at early phase) of diabetic patients remained hyperglycemic1. Additionally, post-hyperglycemia has been demonstrated in contribution of accelerating infarct formation and elevating tPA thrombolysis-associated acute ICH transformation6, 14. Thus in this proof-of-concept study, the improved acute 24 hours brain tissue outcomes by the insulin and tPA combination, especially in the context of tPA thrombolysis, that might ultimately translate into improved long-term neurological outcomes. Although the underlying mechanisms need to be further investigated, it may be assumed by the lowering blood glucose of insulin in concert with its direct and indirect anti-inflammatory, anti-oxidative stress, anti-hyperthrombotic and neuroprotective actions 13.
In the context of reperfusion therapy, we were unable to detect early reperfusion after tPA, even though 24 hr perfusion images showed improvements with insulin plus tPA. In part, this may be related to our relatively brief period of LDF measurements and its restricted small cortex region15, but the molecular mechanism might be at least partially explained by the significantly reduced PAI-1 activity treated with early insulin infusion combined with tPA, which indicated more free form of tPA in circulation that might result in a more potent thrombolytic vascular reperfusion 11. In this study, we only tested relatively severer hyperglycemia of type I diabetic rats. We are aware that the prolonged perfusion profile, long term-neurological outcomes, and the underlying mechanisms, as well all safety aspects of the early insulin plus tPA combination need to be further investigated in stroke animals with different severities of hyperglycemia and different types of diabetes8.
In conclusion, this study suggests that early insulin glycemic control may be beneficial in combination with tPA thrombolytic therapy to ischemic stroke with diabetes mellitus or post-stroke hyperglycemia.
Acknowledgments
Sources of Funding: This work was supported in part by National Institute of Health grants R01-NS065998 and UO1-NS072324 (to X.W.).
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allport L, Baird T, Butcher K, Macgregor L, Prosser J, Colman P, et al. Frequency and temporal profile of poststroke hyperglycemia using continuous glucose monitoring. Diabetes Care. 2006;29:1839–1844. doi: 10.2337/dc06-0204. [DOI] [PubMed] [Google Scholar]
- 2.Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: Pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145–155. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- 3.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, et al. Treatment of hyperglycemia in ischemic stroke (this): A randomized pilot trial. Stroke. 2008;39:384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed N, Davalos A, Eriksson N, Ford GA, Glahn J, Hennerici M, et al. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: Results from the safe implementation of treatments in stroke international stroke thrombolysis register (sits-istr) Archives of neurology. 2010;67:1123–1130. doi: 10.1001/archneurol.2010.210. [DOI] [PubMed] [Google Scholar]
- 5.Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32:617–622. doi: 10.2337/dc08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by increasing superoxide production. Annals of neurology. 2011;70:583–590. doi: 10.1002/ana.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellolio MF, Gilmore RM, Stead LG. Insulin for glycaemic control in acute ischaemic stroke. Cochrane Database Syst Rev. 2011:CD005346. doi: 10.1002/14651858.CD005346.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Rosso C, Corvol JC, Pires C, Crozier S, Attal Y, Jacqueminet S, et al. Intensive versus subcutaneous insulin in patients with hyperacute stroke: Results from the randomized insulinfarct trial. Stroke. 2012;43:2343–2349. doi: 10.1161/STROKEAHA.112.657122. [DOI] [PubMed] [Google Scholar]
- 9.Fan X, Qiu J, Yu Z, Dai H, Singhal AB, Lo EH, et al. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2012;43:576–570. doi: 10.1161/STROKEAHA.111.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain research. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 11.Tjarnlund-Wolf A, Brogren H, Lo EH, Wang X. Plasminogen activator inhibitor-1 and thrombotic cerebrovascular diseases. Stroke. 2012;43:00–00. doi: 10.1161/STROKEAHA.111.622217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aljada A, Ghanim H, Mohanty P, Kapur N, Dandona P. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (egr)-1 expression in mononuclear cells (mnc) and reduces plasma tissue factor (tf) and plasminogen activator inhibitor-1 (pai-1) concentrations. The Journal of clinical endocrinology and metabolism. 2002;87:1419–1422. doi: 10.1210/jcem.87.3.8462. [DOI] [PubMed] [Google Scholar]
- 13.Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: A mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37:267–273. doi: 10.1161/01.STR.0000195175.29487.30. [DOI] [PubMed] [Google Scholar]
- 14.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 15.Henninger N, Bouley J, Bratane BT, Bastan B, Shea M, Fisher M. Laser doppler flowmetry predicts occlusion but not tpa-mediated reperfusion success after rat embolic stroke. Experimental neurology. 2009;215:290–297. doi: 10.1016/j.expneurol.2008.10.013. [DOI] [PubMed] [Google Scholar]