Abstract
Bacterial pathogens secrete chemically diverse iron chelators called siderophores, which may exert additional distinctive functions in vivo. Among these, uropathogenic E.coli often co-express the virulence-associated siderophore yersiniabactin (Ybt) along with catecholate siderophores. Here we used a novel mass-spectrometric screening approach to reveal that yersiniabactin is also a physiologically favorable copper (II) ligand. Direct mass-spectrometric detection of the resulting Cu(II)-Ybt complex in mice and humans with E. coli urinary tract infections demonstrates copper binding to be a physiologically relevant in vivo interaction during infection. Yersiniabactin expression corresponded to higher copper resistance among human urinary tract isolates, suggesting a protective role for this interaction. Chemical and genetic characterization showed that yersiniabactin helps bacteria resist copper toxicity by sequestering host-derived copper (II) and preventing its catechol-mediated reduction to copper (I). Together, these studies reveal a new virulence-associated function for yersiniabactin that is distinct from iron binding.
Introduction
Siderophores are a chemically diverse group of secondary metabolites used extensively by microbes – and possibly higher vertebrates – to bind and acquire ferric iron (see review)1,2. While chemists recognize the ability of some siderophores to bind non-ferric metal ions3–8, investigations into pathophysiologic functions of siderophores solely address their favorable interaction with ferric iron9–12. The marked chemical diversity and differential iron-binding affinities among siderophores raises the possibility that some may have evolved to bind non-ferric metal ions to fulfill additional physiologic functions. Indeed, alterations in physiologic metal composition at sites of infection may drive bacterial pathogens to secrete siderophores with differential metal specificity to maintain fitness within the host (see review)13,14. Understanding the functional consequences arising from this chemical coevolution between host and pathogen may provide new insights into the selective pressures driving siderophore chemistry and bacterial pathogenesis.
Genetic and metabolomic studies associate siderophore production with virulence among multiple human pathogens, particularly among E. coli and related Gram-negatives15–21. Although bacterial expression of a single siderophore type is sufficient for iron acquisition in vitro, most uropathogenic E. coli (UPEC) express multiple siderophore types17,22, often including the virulence-associated phenolate/thiazolidine siderophore yersiniabactin. All yersiniabactin-expressing UPEC strains described to date co-express the chemically distinct, catecholate siderophore enterobactin and occasionally the enterobactin derivative salmochelin17. Epidemiologic studies suggest that E. coli strains that progress from bladder infection to kidney or bloodstream infection are more likely to carry the fyuA gene, a correlate of yersiniabactin-producing strains19,23. Exactly how yersiniabactin expression facilitates invasive infections has been unclear.
In this study we devised a mass spectrometry-based screen to determine whether non-ferric metals bind yersiniabactin in physiologically relevant fluids. This approach identified prominent copper (II) binding by yersiniabactin in human urine. Direct mass spectrometric analyses of urine from humans and mice confirmed the presence of copper(II)-yersiniabactin complexes during infection with yersiniabactin-expressing strains. Functional studies demonstrated that this binding interaction is competitive with iron (III) and protects uropathogens by binding copper and preventing its catecholate-mediated reduction. Together, these studies reveal a new activity for yersiniabactin as a pathogenic countermeasure to copper-based antibacterial functions in humans.
Results
Identification of a copper (II)-yersiniabactin complex
To screen for metals that bind yersiniabactin (Ybt) in a biologically relevant environment, we devised a liquid chromatography-constant neutral loss (LC-CNL) mass-spectrometric screen based on a common ion-fragmentation pathway identified for model metal-yersiniabactin complexes. This fragmentation pathway resulted in a 187 mass unit neutral loss, consistent with rearrangement of the C13-C14 bond to lose the carboxy-terminal thiazoline (C8H13NO2S, Fig. 1a and 1b). To identify biologically relevant metal-Ybt complexes, we added apo-Ybt to pooled urine samples from six healthy donors and analyzed the mixture using LC-CNL over a mass range encompassing the calculated range of naturally-occurring terrestrial metal complexes (6Li to 239Pu). The LC-CNL ion chromatogram revealed formation of a dominant, novel analyte with m/z 543 (peak 1, Fig. 1c) in addition to a peak corresponding to Fe(III)-Ybt and its deuterated internal standard (Supplementary Results, Supplementary Figure 1a and 1b). The new peak 1 was absent in urine alone treated with internal standard or when apo-Ybt was added to water (data not shown). Although urine is a highly complex mixture containing thousands of small biomolecules, the simplicity of the resulting chromatograms suggested that the 187 mass unit loss is highly specific to Ybt. Peak 1 matched no previously reported spectra, also exhibited a neutral loss of 187 mass units, and was formed by adding apo-Ybt to urine, suggesting formation of a new and biologically plausible yersiniabactin complex.
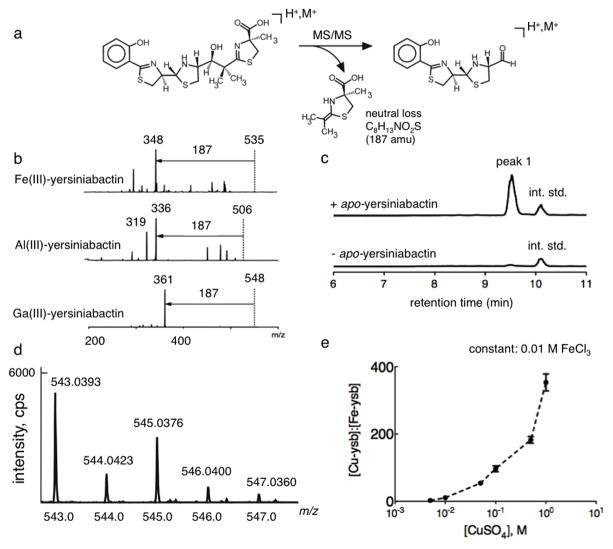
Figure 1. A yersiniabactin neutral loss screen reveals formation of a novel copper(II)-yersiniabactin complex in human urine.
(a) Yersiniabactin and its metal complexes exhibit a dominant 187 mass unit neutral loss upon CID fragmentation of the positive ESI-derived ion. This neutral loss is consistent with rearrangement to lose the terminal carboxylated thiazoline. (b) A 187 mass unit neutral loss is evident in MS/MS product ion spectra of ferric-yersiniabactin (Fe(III)-Ybt) at m/z 535, aluminum-yersiniabactin (Al(III)-Ybt) at m/z 506, and gallium-yersiniabactin (Ga(III)-Ybt) at m/z 548. A constant neutral loss (CNL) scan based on this conserved fragmentation pathway was used as a metallomic screen to identify physiologic yersiniabactin binding partners. (c) Representative constant neutral loss chromatograms of urine samples in the presence and absence of purified apo-yersiniabactin. The combination of apo-yersiniabactin and urine results in formation of a prominent new peak (peak 1). Peaks corresponding to the internal standard (int. std) are indicated. These results were confirmed in three independent experiments. (d) High resolution positive ion ESI mass spectrum is consistent with the empiric formula for a singly charged Cu(II)-Ybt ion and demonstrates the prominent natural abundance M+2 ion expected from 65Cu. (d) Competitive binding experiments were conducted by titrating cupric sulfate into solutions containing a fixed concentration of 0.01 M ferric chloride and 0.01 M apo-yersiniabactin. Data indicate competitive binding between cupric and ferric ion for the ligand. Cu(II)-Ybt/Fe(III)-Ybt ratios were determined by comparing selected ion chromatogram peak ratios to those from a standard curve.
Peak 1 was first subjected to additional mass analyses. The CNL mass spectrum of this complex exhibited a prominent M+2 peak at m/z 545, approximately one third the height of the base peak. MS/MS analysis of the M and M+2 peaks at m/z 543 and 545 exhibited product ion spectra with identical fragmentation patterns differing by 2 mass units (Supplementary Figure 2a). The apo-Ybt spectrum lacked this prominent M+2 peak, suggesting a prominent isotope-pattern contribution from the unknown Ybt binding partner (Supplementary Figure 2b). A Ybt-derived base peak ion 61 mass units higher than the Ybt [M+H]+ ion with a prominent M+2 isotope peak was consistent with a singly charged cupric complex of the form [Ybt+Cu(II)-H]+. The observed isotopic pattern was consistent with the natural 63Cu and 65Cu isotope abundances of 77% and 23%, respectively24. The m/z value of this complex was consistent with the presence of copper (II), rather than copper (I), which would require an additional proton to yield a singly charged ion at m/z 544, rather than the observed 543.
Further confirmation was pursued by adding molar excess of copper (II) sulfate to UTI89 ΔentB (which produces Ybt as the only siderophore) culture supernatant, followed by preparative chromatography. Application of this copper(II)-treated supernatant to a preparative C18 column resulted in retention of a blue-colored fraction on the column that was eluted with 80% methanol. The LC-MS chromatogram of this blue fraction was dominated by peak 1. This fraction was subjected to accurate mass determination on a Bruker Q-TOF Maxis using positive ion electrospray mode, which again showed the prominent M+2 ion and supported the formula C21H25CuN3O4S3 for peak 1 ion at 543.0393 (Fig. 1d). The presence of 21 carbons was confirmed by detection of 13C-labeled peak 1 at m/z 564 in supernatants from bacteria grown in [13C3] glycerol (Supplementary Figure 3a). MS/MS of this peak 1 isotopologue revealed a new dominant MS/MS neutral loss of 195 mass units, supporting the proposed common C8H13NO2S neutral loss (Supplementary Figure 3b). Together, these results supported the identity of peak 1 as a stable copper (II) complex of Ybt that formed spontaneously when Ybt was exposed to physiologic concentrations of copper in human urine.
Cu(II)-yersiniabactin is stable in the presence of iron
To determine whether cupric and ferric ion species bind competitively to Ybt, we quantified relative copper(II)- and iron(III)-yersiniabactin (Cu(II)-Ybt and Fe(III)-Ybt, respectively) yields in competitive binding experiments. Increasing concentrations of cupric sulfate were added to PBS containing 0.01 M ferric chloride at pH 7.0 at 25 C for one hour (Fig. 1e). One micromolar apo-yersiniabactin was then added to these samples and the Cu(II)-Ybt/Fe(III)-Ybt ratio was determined by LC-MS following a two-hour incubation. The Cu(II)-Ybt/Fe(III)-Ybt ratio exhibited a positive correlation with copper (II) concentration, consistent with competitive binding between aqeuous cupric and ferric species.
To determine whether iron displaces copper from Cu(II)-Ybt complexes in our experimental conditions, we monitored an extended time course of Cu(II)-Ybt levels in the presence of ferric ions. Twenty five micromolar apo-Ybt was incubated with 25 μM cupric sulfate for one hour to form Cu(II)-Ybt. Next, 0.025 M competing ferric chloride was added and the resulting Cu(II)-Ybt level was followed over a 24 hour time course (Supplementary Figure 4). The Cu(II)-Ybt level was unchanged, consistent with a low rate of ferric ion displacement in our analytical and experimental conditions.
Cu(II)-yersiniabactin forms in vivo during infections
To determine whether Ybt is expressed and binds copper during infection, we used LC-MS/MS to analyze murine urine and bladder tissue following experimental infection with the model uropathogen UTI89. A strong signal with the expected Cu(II)-Ybt MS/MS transition and retention time was observed in all 30 bladder tissue and urine sample from infected, but not mock infected, mice 24 hours after pathogen inoculation (Supplementary Figures 5a and 5b). The median Cu(II)-Ybt/Fe(III)-Ybt molar ratio was 15.3 (range = 0.38–84.8) (Supplementary Figure 5c). 29 of 30 (97%) urine samples from infected mice exhibited a Cu(II)-Ybt/Fe(III)-Ybt molar ratio >1, consistent with favorable in vivo Cu(II)-Ybt formation. These findings demonstrate that a typical urinary pathogen expresses Ybt during murine infections, and that a greater abundance of copper (II) complexed siderophore is subsequently detected in biological samples derived from infected mice.
To determine whether Ybt is expressed and binds copper during human urinary tract infections, we analyzed 32 midstream urine samples from a cohort of women with acute cystitis for Cu(II)-Ybt. LC-MS/MS analysis of cultured urinary pathogen isolates from these patients confirmed infection by a Ybt-expressor in 15 out of 32 subjects. Scanning neutral loss analysis of urine from a patient infected with a Ybt-expressing strain revealed a strong Cu(II)-Ybt signal with the expected m/z 543 ion and its characteristic 65Cu isotopomer at m/z 545, confirming in vivo formation of Cu(II)-Ybt (Fig. 2a). Quantitative analysis using 13C-labeled internal standards demonstrated significantly (p<0.0001) higher urinary Cu(II)-Ybt levels in patients infected with Ybt expressors (13/15 positive) than in patients infected with non-expressors (0 of 17 positive) (Fig. 2b). Together, these data demonstrate that uropathogenic E.coli can express Ybt during human urinary tract infections and that this Ybt binds host-derived copper.
Figure 2. Cupric-yersiniabactin is produced in cystitis patients infected with yersiniabactin-producing strains.
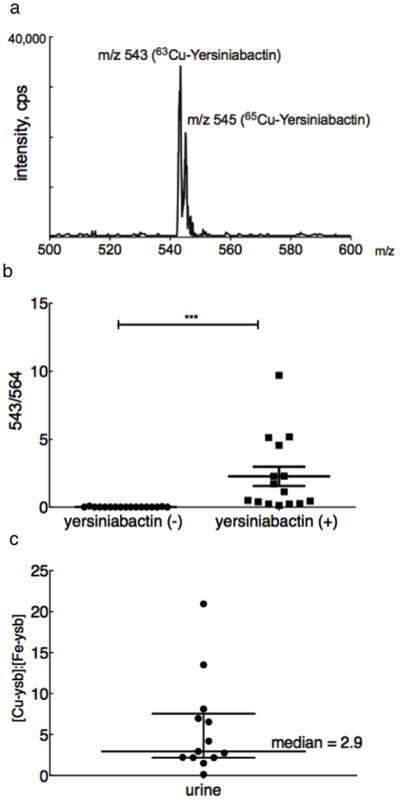
(a) A scanning constant neutral loss spectrum reveals the spectrum for Cu(II)-Ybt at its expected retention time. The expected copper isotope peaks at m/z 543 for 63Cu and m/z 545 for 65Cu peak are present at the expected ~2:1 ratio. (b) Urinary Cu(II)-Ybt was detected in 13 of 15 patients infected with a yersiniabactin-expressing pathogen and in none of the patients with yersiniabactin non-expressors. Cu(II)-Ybt levels are reported as a fraction of the corresponding 13C internal standard peak height. (c) In urine samples with detectable yersiniabactin complexes, the median Cu(II)-Ybt (m/z 543) to Fe(III)-Ybt (m/z 535) ratio is 2.941, indicating preferential in vivo copper (II) binding.
To determine whether the extent of in vivo copper and iron binding was similar, we measured the Cu(II)-Ybt/Fe(III)-Ybt molar ratio in urine (Supplementary Figure 6). In the 13 samples with detectable Ybt complexes, the median Cu(II)-Ybt/Fe(III)-Ybt ratio was 2.9 (range= 0.12–20.9) (Fig. 2c) with 92% (12/13) patients exhibiting a molar ratio >1. These data indicate that Ybt binds host-derived copper at least as extensively as ferric iron during human infections.
Yersiniabactin protects E.coli from copper toxicity
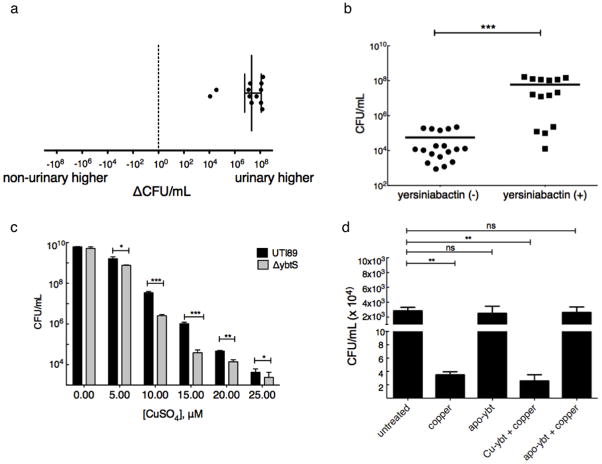
Copper ions are toxic to E. coli and other bacteria at low micromolar concentrations25,26. By binding copper ions, Ybt may act as a pathogenic countermeasure to copper toxicity. To determine whether copper resistance is a virulence correlate, we measured the effects of copper on bacterial growth in a previously described collection of co-existing urinary and non-urinary E. coli isolates from 13 UTI patients17. These experiments were conducted within the normal serum copper concentration range of 10 μM. This analysis revealed that the E.coli strains infecting the urinary tract were significantly (p<0.0005) more copper resistant than coexisting non-urinary strains from the same patients with 10 out of 13 urinary isolates exhibiting >107 higher CFU/mL (Fig. 3a).
Figure 3. Yersiniabactin promotes E. coli growth in copper-toxic conditions.
Urinary and non-urinary E. coli isolates from a UTI patient population were cultured in the presence of 10 μM copper (II) sulfate for 18 hours. Growth was determined and expressed as total CFU/mL. (a) Urinary strains demonstrate greater resistance to copper toxicity than coexisting rectal strains. For each patient, CFU/mL from the non-urinary strain was subtracted from CFU/mL from the coincident urinary strain to yield a difference. In the four patients from whom multiple coincident urinary and non-urinary strains were recovered, the mean difference in colony forming units is reported. The median value of these differences was 2.11 x 107 CFU/mL, with a range of −5.4 x 103 to 1.66 x 108. (b) Yersiniabactin-expressors were more resistant to copper toxicity than non-expressors (p<0.0013). These results were confirmed in three independent experiments. (c) Yersiniabactin-expressor (UTI89) and non-expressor (UTI89ΔybtS) cultures treated with 0–25 μM copper (II) sulfate revealed an average of ten-fold survival advantage for the yersiniabactin expressor (p-value = 0.012, 0.0004, 0.009, 0.002 and 0.023, respectively, t-test). (d) Purified apo-yersiniabactin or Cu(II)-Ybt was added in 1.5-fold molar excess over 10 μM copper (II) sulfate to yersiniabactin-deficient (UTI89ΔybtS) culture. Samples containing copper alone demonstrated a >3 log CFU/mL decrease in viability. Apo-yersiniabactin (apo-Ybt) addition restores growth to untreated wild type levels (p = ns). This cytoprotective effect is unique to apo-yersiniabactin, and is not observed upon addition of pre-formed Cu(II)-Ybt. These results were confirmed in three independent experiments.
It is known from previous work that 10 of 14 urinary isolates and 6 of 18 non-urinary isolates produce Ybt17. When growth in copper-supplemented media was grouped by Ybt expression, the Ybt-expressors exhibited significantly (p<0.0013) greater copper resistance (Fig. 3b). Together, these data show that urinary isolates exhibit greater copper resistance than non-urinary isolates in a UTI patient population and that enhanced copper resistance is strongly associated with Ybt production.
To determine whether Ybt production has a functional impact on copper resistance, we compared the Ybt-deficient mutant UTI89ΔybtS to the wild type uropathogen control UTI89. After an 18-hour incubation, copper (II) sulfate inhibited UTI89ΔybtS growth significantly more than that of wild type UTI89. Over a range of 5 to 25 μM copper (II) sulfate, wild type cultures yielded an average of 1.34 log more CFUs than UTI89ΔybtS cultures (p=0.0032, Fig. 3c). To confirm that Cu(II)-Ybt is expressed in this experimental system, we performed LC-MS analysis of culture supernatants treated with 25 μM copper (II) sulfate. Cu(II)-Ybt complexes were observed in wild type, but not UTI89ΔybtS, conditioned media (Supplementary Figure 7). Together, these findings show that intact Ybt gene plays a mechanistic role in copper resistance.
To determine whether Ybt directly protects bacteria from copper toxicity, we examined the effect of purified apo-Ybt on viability of UTI89ΔybtS (Ybt biosynthesis mutant) cultures exposed to 10μM copper sulfate (Fig. 3d). Copper addition alone resulted in a substantial >3 log CFU/mL decrease in bacterial viability. Apo-Ybt protected copper-treated bacteria almost completely from cytotoxicity, with only a 0.024 log decrease in viable cells compared to wild type (p = NS). Addition of pre-formed Cu(II)-Ybt complexes abolished the protective effect, suggesting that an unoccupied copper-binding site on apo-Ybt is required for cytoprotection. In the absence of copper, apo-Ybt alone had a negligible effect on bacterial viability. These results show that copper binding by exogenous Ybt directly protects bacteria from copper toxicity.
Yersiniabactin prevents Cu(II) reduction by catecholates
To determine whether other UPEC siderophores affect copper susceptibility, we evaluated copper toxicity in established UTI89 mutants with defined siderophore deficiencies17 (Fig. 4a). As noted above, UTI89ΔybtS was more susceptible to copper than the wild type strain (p = 0.0021). Conversely, additional deletion of the catecholate biosynthesis gene entB in this strain background (an ΔentBΔybtS double mutant), significantly (p = 0.0032) increased survival. The single ΔentB mutant, which expresses Ybt, exhibited a supraphysiological survival benefit in the presence of copper. These results are consistent with previous work describing copper (II) reduction by catechols to more toxic copper (I) ions27,28.
Figure 4. Catecholate siderophores and yersiniabactin exert opposing effects on copper cytotoxicity.
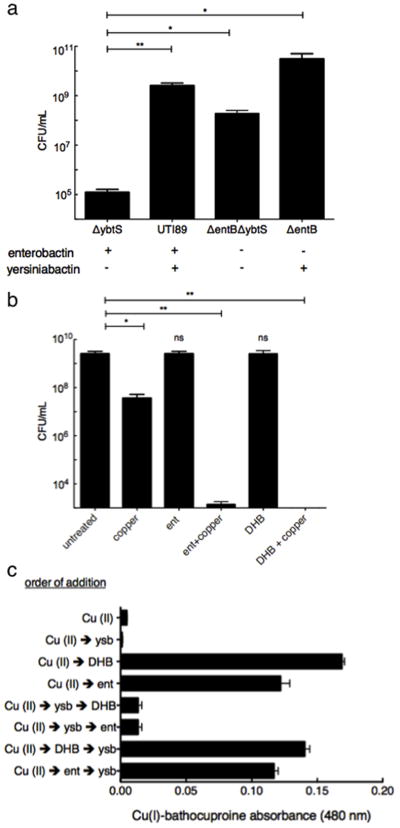
(a) Growth of wild type (UTI89), yersiniabactin (ΔybtS), catecholate siderophore (ΔentB), or total siderophore (ΔentBΔybtS) expression mutants in the presence of copper was determined. Results were consistent with copper-dependent cytoprotective effect for yersiniabactin and cytotoxic effect for catecholate siderophores. (b) Exogenous addition of 20 μM of the siderophore enterobactin, or its catecholate moiety 2,3-dihydroxybenzoate (DHB) enhances copper (II) sulfate toxicity in UTI89. (c) Apo-yersiniabactin prevents catechol-dependent reduction of copper (II) sulfate to copper (I) in an order-of-addition dependent manner. The complete reaction system consisted of 17.5 μM copper(II) sulfate, either 20 μM enterobactin (ent) or its catecholate moiety 2,3-dihydroxybenzoic acid (DHB), 25 μM apo-yersiniabactin (Ybt), and 25 μM of the copper(I) indicator bathocuproine sulfonate. Reagents were added in the order indicated and Cu(I)-bathocuproine absorbance was determined 30 min after addition of the last reagent. Results were confirmed in three independent experiments.
To test whether the absence of copper reduction by catecholate siderophores explains increased copper resistance in the the UTI89ΔentB mutant, we examined copper toxicity to UTI89 in the presence and absence of enterobactin or 2,3-dihydroxybenzoic acid (DHB), the catecholate moiety incorporated into enterobactin and salmochelin. Treatment with 10 μM copper (II) sulfate alone caused a 2.5 log reduction of viable colonies. While 20 μM enterobactin or DHB alone had a minimal effect, their addition to copper (II) sulfate significantly (p<0.0072 and p<0.0039, respectively) increased copper cytotoxicity, decreasing viable cells to below the level of detection (LOD = 20 CFU/mL) (Fig. 4b). These data show that antibacterial synergy between enterobactin and copper (II) is attributable to enterobactin’s catecholate groups.
To confirm that enterobactin reduces copper (II) and to determine whether Ybt affects this reaction, we monitored copper (I) formation by purified reagents using a bathocuproine-based spectrophotometric assay (Fig. 4c). Addition of either 20 μM enterobactin or DHB to samples containing 17.5 μM copper (II) sulfate resulted in a strong bathocuproine signal corresponding to 84.7% and 93.1% reduction of copper (II) to copper (I), respectively. This signal was almost completely blocked when apo-Ybt was added before enterobactin or DHB. In contrast, changing the order of addition such that enterobactin or DHB were added before apo-Ybt restored the copper (I) signal. Together, these results demonstrate that the catecholate groups of enterobactin and related siderophores reduce copper (II) to more cytotoxic copper (I) and that yersiniabactin protects bacteria not only by sequestering copper (II) but also by inhibiting its catecholate-mediated reduction.
Discussion
Although yersiniabactin and catecholate siderophores are regarded as redundant iron-acquisition molecules, this study shows that they assume disparate roles in modulating copper chemistry and toxicity at the host-pathogen interface. These results suggest that the chemical biology of pathogenic siderophores extends beyond issues of iron binding and acquisition. Precedent for microbial copper chelation is found among environmental bacteria29,30. While not a siderophore, methanobactin serves an analogous function by binding Cu(I) to satisfy a high nutritional copper requirement driven by synthesis of a particulate methane-monooxygenase, which accounts for up to 20% of methanotrophic bacterial proteins4,31,32. The algal hydroxamate siderophore schizokinen may weakly bind Cu(II), with differing effects on copper toxicity to environmental bacteria5. Although our data are consistent with protective copper sequestration as a yersiniabactin function, yersiniabactin could benefit bacteria by obtaining copper as a nutritional source under other circumstances. Yersiniabactin’s ability to bind copper during infection may thus derive from an ancestral function or reflect an example of convergent chemical evolution.
Although copper resistance proteins are described in a wide variety of bacteria33–36, it is unclear if copper resistance represents a virulence-associated adaptation. This question is readily addressed in E. coli, where disease-associated strains exhibit evidence of multiple virulence-associated adaptations. By demonstrating enhanced copper-resistance among disease-associated isolates, this study suggests an important role for copper ions as an antimicrobial defense in human UTI pathogenesis. A role for copper-based immune defenses is further supported by observations of infection-associated increases in copper14,37 and use of copper transporters by phagocytic cells to kill internalized bacteria38. It is possible that disease-associated isolates without high level copper resistance (in the present study, 4/14 isolates) are indicative of patients with subtle differences in pathophysiology or copper-based antimicrobial responses (Fig. 3b). Regardless, the sum of bacteriologic and bioanalytic findings suggests a role for host-derived copper as a virulence-associated selection factor among uropathogenic E. coli.
While catechols are excellent iron-binding functional groups, their ability to directly reduce cupric ions provides a chemical rationale for acquisition of non-catecholate siderophores by bacterial pathogens27,39–41. Copper (II) is significantly more bactericidal when reduced to its copper (I) valence, which has been attributed to an improved ability of copper (I) to generate reactive oxygen species or to freely penetrate bacterial membranes and inactivate intracellular iron-sulfur clusters25. Thus, yersiniabactin’s ability to prevent copper (II) reduction and intracellular penetration may additionally protect pathogenic bacteria during infection. Targeting yersiniabactin biosynthesis or the strains that perform this may thus be a useful therapeutic approach. Although it is unclear which yersiniabactin coordination sites facilitate copper binding, it is notable that this molecule contains three nitrogenous heterocycles (thiazolines/thiazolidine), reminiscent of imidazole-rich copper coordination sites in ceruloplasmin42 and the nitrogen heterocycles in methanobactin6,32,43. Similar structural features in other siderophores and microbial products may facilitate similar copper-binding functions 44.
Siderophore profiling by mass spectrometric methods has yielded important insights into pathogenicity of E. coli and other important pathogens17,21,39–41. To date, neither pathogenic siderophore secretion nor in vivo metal ion selectivity of these siderophores have been directly observed and quantified. This work shows how tandem mass spectrometry, together with an understanding of gas phase ion chemistry, can also identify metal-binding interactions of these diverse molecules. Its notable sensitivity also permits use of LC-MS/MS to directly interrogate in vivo metal binding rather than relying solely upon attempts to simulate in vitro the complex metal availabilities at sites of infection.
Bacterial pathogens have adapted to a shifting array of evolutionary challenges by secreting a chemically diverse range of secondary compounds and proteins. Yersiniabactin may be one of many multifunctional, virulence-associated metal binders secreted by pathogenic bacteria. In future studies, similar binding interactions could be discovered and validated for other important biomolecules or other secondary compounds using an analogous chemical biology approach.
Methods
Bacterial strains and cultivation
The uropathogenic E.coli isolate UTI89 was used as the model pathogen in this study16,45. Urinary and rectal isolates are from a previously described collection17. Briefly, distinct, coexisting strains were identified by pulsed-field gel electrophoresis (PFGE) from a longitudinal patient study. Strains were not considered to be “rectal” if they were isolated from a urinary source at any time during the study.
Bacteria were cultured in for siderophore product analysis in M63 medium supplemented with 0.2% glycerol and 10 mg/mL niacin (Sigma) as previously described17. Bacterial production of yersiniabactin and its 13C and deuterium-labeled internal standards is described in Supplementary Methods. To assess copper sensitivity, overnight cultures in 50 mL M63 minimal media were washed twice in phosphate buffered saline (PBS, Sigma), centrifuged at 6,500 rpm, and resuspended in fresh M63 minimal media at a density of 108 colony forming units per milliliter (CFU/mL). Bacterial viability was determined as CFU/mL of the culture media 20 hours after addition of defined copper (II) sulfate (Sigma, ~99% pure) concentrations with test agents in 2 mL reaction volumes in 6-well tissue culture plates.
Liquid chromatography-mass spectrometry
LC-MS analyses were conducted using a Shimadzu UFLC-equipped AB-Sciex 4000 QTrap operated in positive ion mode using the Turbo V ESI ion source and a Thermo LCQ Deca as previously described17. The QTrap samples were injected onto a Fused-core™phenylhexyl column (100 × 2mm, 2.7 μm particle, Ascentis Express, Supelco) with a flow rate of 0.4 mL/min. The gradient used was as follows: Solvent A (0.1% formic acid) was held constant at 98% and solvent B (100% acetonitrile in 0.1% formic acid) was held constant at 2% for 2 minutes, solvent B was increased to 65% by 10 minutes and then to 98% by 12 minutes. The ion spray voltage was set to 5 kV. The heater temperature was 500°C. The declustering potential, nebulizer gas (G1), auxiliary gas (G2) and collision energy were set at 110, 40, 35 and 35V, respectively. In vivo Cu(II)-Ybt quantification was carried out in the MRM mode using 13C-labeled siderophore standards with previously identified CID fragmentations17. Cu(II)-Ybt/Fe(III)-Ybt ratio determinations were made by calibrating with standard curves conducted in 1x PBS buffer for in vitro or human urine for in vivo determinations (Supplementary Figure. 6).
Liquid chromatography-constant neutral loss (LC-CNL) analysis
The UFLC-4000 QTrap was used with the chromatography and ion source settings described above to identify compounds with a common neutral fragment loss of 187 m/z units. The collision energy was set to 35 V and the first mass analyzer (Q1) was set to scan from m/z 200 to 700 amu while the second mass analyzer (Q3) simultaneously scanned at 187 m/z units less than Q1. Sensitivity was maximized by selecting the low-resolution (2 mass unit window, 0.7 FWHH) setting. In this manner, only ions exhibiting a neutral loss of 187 mass units were detected. As d4-ferric-yersiniabactin retained the 187 amu neutral loss, it was used as an internal standard in this analysis.
Human specimen collection
Study protocols were approved by the Institutional Review Board of the University of Washington. All patients provided written informed consent for the collection of samples and subsequent analysis. Clean-catch midstream urine specimens were obtained from female patients at the University of Washington, Seattle, WA with acute uncomplicated cystitis using previously described symptoms of dysuria, urinary frequency, or urinary urgency with a concentration of uropathogens in the urine of ≥1×102 colony-forming units (CFU/mL). One-tenth volume of Sigma FAST protease inhibitor solution (Sigma, St. Louis, MO) was added to freshly voided urines prior to clinical centrifugation to remove cellular material and the supernatant was frozen at −80°C. Uropathogens in midstream urine were identified using standard methods46. E. coli UTI urine specimens and pathogen isolates with urine white blood cell count ≥ 350 collected between 01/06/2010 and 11/15/2010 were selected for analysis.
Mouse infections
All animal studies using mice were approved by the Animal Studies Committee of Washington University. Six- to seven-week old female C3H/HeN mice obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN) were infected with 107 CFU/mL UTI89 or PBS control as described47. Murine urine samples were collected on the day of tissue harvest. Bladders were aseptically harvested at the indicated time point and homogenized in 1 mL PBS. CFU determination of viable bacteria in homogenates was conducted as described.
Biological specimen preparation
For the purposes of LC-CNL scans, urine samples from asymptomatic individuals were collected and pooled in metal-free Nalgene beakers and centrifuged in 50 mL Falcon flasks at 7,000 rpm for 15 minutes at 4°C. Apo-yersiniabactin and deuterated ferric-yersiniabactin internal standard were added to a final concentration of 20 μM to human urine supernatants to identify metal ion binding partners. To measure Cu(II)-Ybt in human urine, as well as murine urine and bladder homogenates, 2.5 μL each of 13C cupric- and ferric- internal standard was added to 850 μL of bladder homogenate or 500 μL urine, respectively. Samples were centrifuged at 14,000 rpm for 2 minutes. Yersiniabactin in the supernatant was extracted using preparative C18 chromatography (UCT, Inc., Bristol, PA) and eluted in 500 μL 100% methanol. Five microliters of the eluate was analyzed by LC-MS/MS to determine Cu(II)-Ybt levels.
Free copper (I) determinations
Free copper (I) was determined spectrophotometrically using the copper (I) indicator bathocuproinedisulfonic acid (Sigma, 92% pure)48,49. Briefly, 25 μM bathocuproine, 25 μM apo-yersiniabactin, and 17.5 μM copper (II) sulfate, were used in combination in order-of-addition experiments, as well as alone as controls in PBS. Either 20 μM enterobactin (Sigma) or its catecholate moiety, 2,3-dihydroxybenzoic acid (DHB, Sigma) were added as bioreductants. Following a 30 minute room temperature incubation, 25 μM bathocuproine was added and copper (I) levels were determined by measuring the visible absorbance at 480 nm of the cuprous-bathocuproine complex. Final copper (I) concentrations were determined by comparison to a standard curve.
Additional methods
For descriptions of strain preparation and statistical analyses, see Supplementary Methods.
Supplementary Material
Acknowledgments
The authors would like to thank M. Gross, H. Rohrs and Y. Zhang for high resolution mass spectrometry analysis and J. Pinker for assistance with small molecule purification. The authors are grateful to S. Hultgren, G. Marshall, and B. Ford for helpful discussions. J.P.H. holds a Career Award for Medical Scientists from the Burroughs-Wellcome Fund. We additionally acknowledge NIH grants K12 HD001459-09, AI 07172-24, P30 HL101263-01, P50 DK64540, U01 DK082315, and UL1 RR024992. Mass spectrometry was supported by P41-RR00954, 8-P41 GM103422-35 (NCRR), P60-DK20579, and P30-DK56341.
Footnotes
Author Contributions
Conceived and designed the experiments: K.S.C., J.P.H. Performed the experiments: K.S.C., C.S.H., J.R.C. Human specimen collection: A.E.S. Analyzed the data: K.S.C., C.S.H., J.P.H. Wrote the paper: K.S.C., J.P.H.
Competing Financial Interests Statement
The authors declare no competing financial interests.
References
- 1.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devireddy LR, Hart DO, Goetz DH, Green MR. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141:1006–1017. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, Galeva N, Larive CK, Alterman M, Graham DW. Purification and physical-chemical properties of methanobactin: a chalkophore from Methylosinus trichosporium OB3b. Biochemistry. 2005;44:5140–5148. doi: 10.1021/bi047367r. [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, et al. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science. 2004;305:1612–1615. doi: 10.1126/science.1098322. [DOI] [PubMed] [Google Scholar]
- 5.Clarke SE, Stuart J, Sanders-Loehr J. Induction of siderophore activity in Anabaena spp. and its moderation of copper toxicity. Appl Environ Microbiol. 1987;53:917–922. doi: 10.1128/aem.53.5.917-922.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behling LA, et al. NMR, mass spectrometry and chemical evidence reveal a different chemical structure for methanobactin that contains oxazolone rings. J Am Chem Soc. 2008;130:12604–12605. doi: 10.1021/ja804747d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braud A, Hannauer M, Mislin GL, Schalk IJ. The Pseudomonas aeruginosa pyochelin-iron uptake pathway and its metal specificity. J Bacteriol. 2009;191:3517–3525. doi: 10.1128/JB.00010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visca P, et al. Metal regulation of siderophore synthesis in Pseudomonas aeruginosa and functional effects of siderophore-metal complexes. Appl Environ Microbiol. 1992;58:2886–2893. doi: 10.1128/aem.58.9.2886-2893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurz T, Gustafsson B, Brunk UT. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 2006;273:3106–3117. doi: 10.1111/j.1742-4658.2006.05321.x. [DOI] [PubMed] [Google Scholar]
- 10.Luo M, Fadeev EA, Groves JT. Mycobactin-mediated iron acquisition within macrophages. Nat Chem Biol. 2005;1:149–153. doi: 10.1038/nchembio717. [DOI] [PubMed] [Google Scholar]
- 11.Paauw A, Leverstein-van Hall MA, van Kessel KP, Verhoef J, Fluit AC. Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells. PLoS One. 2009;4:e8240. doi: 10.1371/journal.pone.0008240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert M, et al. Effects of the Aspergillus fumigatus siderophore systems on the regulation of macrophage immune effector pathways and iron homeostasis. Immunobiology. 2008;213:767–778. doi: 10.1016/j.imbio.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Thiele DJ, Gitlin JD. Assembling the pieces. Nat Chem Biol. 2008;4:145–147. doi: 10.1038/nchembio0308-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prentice AM, Ghattas H, Cox SE. Host-pathogen interactions: can micronutrients tip the balance? J Nutr. 2007;137:1334–1337. doi: 10.1093/jn/137.5.1334. [DOI] [PubMed] [Google Scholar]
- 15.Brickman TJ, Cummings CA, Liew SY, Relman DA, Armstrong SK. Transcriptional profiling of the iron starvation response in Bordetella pertussis provides new insights into siderophore utilization and virulence gene expression. J Bacteriol. 2011;193:4798–4812. doi: 10.1128/JB.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SL, et al. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A. 2006;103:5977–5982. doi: 10.1073/pnas.0600938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson JP, et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009;5:e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reigstad CS, Hultgren SJ, Gordon JI. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem. 2007;282:21259–21267. doi: 10.1074/jbc.M611502200. [DOI] [PubMed] [Google Scholar]
- 19.Snyder JA, et al. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun. 2004;72:6373–6381. doi: 10.1128/IAI.72.11.6373-6381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer ND, Skaar EP. Molecular Mechanisms of Staphylococcus aureus Iron Acquisition. Annu Rev Microbiol. 2011;65:129–147. doi: 10.1146/annurev-micro-090110-102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachman MA, et al. Klebsiella pneumoniae Yersiniabactin Promotes Respiratory Tract Infection through Evasion of Lipocalin 2. Infect Immun. 2011;79:3309–3316. doi: 10.1128/IAI.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mokracka J, Koczura R, Kaznowski A. Yersiniabactin and other siderophores produced by clinical isolates of Enterobacter spp. and Citrobacter spp. FEMS Immunol Med Microbiol. 2004;40:51–55. doi: 10.1016/S0928-8244(03)00276-1. [DOI] [PubMed] [Google Scholar]
- 23.Mabbett AN, et al. Virulence properties of asymptomatic bacteriuria Escherichia coli. Int J Med Microbiol. 2009;299:53–63. doi: 10.1016/j.ijmm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.The CRC Handbook of Chemistry and Physics. 71. 11. CRC Press; 1991. pp. 44–45. [Google Scholar]
- 25.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark HW, Gage SD. On the Bactericidal Action of Copper. Public Health Pap Rep. 1905;31:175–204. [PMC free article] [PubMed] [Google Scholar]
- 27.Grass G, et al. Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J Bacteriol. 2004;186:5826–5833. doi: 10.1128/JB.186.17.5826-5833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beswick PH, et al. Copper toxicity: evidence for the conversion of cupric to cuprous copper in vivo under anaerobic conditions. Chem Biol Interact. 1976;14:347–356. doi: 10.1016/0009-2797(76)90113-7. [DOI] [PubMed] [Google Scholar]
- 29.Arceneaux JE, Boutwell ME, Byers BR. Enhancement of copper toxicity by siderophores in Bacillus megaterium. Antimicrob Agents Chemother. 1984;25:650–652. doi: 10.1128/aac.25.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKnight DM, Morel FMM. Copper complexation by siderophores from filamentous blue green algae. Limnol Oceanogr. 1980;25:62–71. [Google Scholar]
- 31.Hakemian AS, Rosenzweig AC. The biochemistry of methane oxidation. Annu Rev Biochem. 2007;76:223–241. doi: 10.1146/annurev.biochem.76.061505.175355. [DOI] [PubMed] [Google Scholar]
- 32.Balasubramanian R, Rosenzweig AC. Copper methanobactin: a molecule whose time has come. Curr Opin Chem Biol. 2008;12:245–249. doi: 10.1016/j.cbpa.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker J, Sengupta M, Jayaswal RK, Morrissey JA. The Staphylococcus aureus CsoR regulates both chromosomal and plasmid-encoded copper resistance mechanisms. Environ Microbiol. 2011;13:2495–2507. doi: 10.1111/j.1462-2920.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- 34.Nandakumar R, Espirito Santo C, Madayiputhiya N, Grass G. Quantitative proteomic profiling of the Escherichia coli response to metallic copper surfaces. Biometals. 2011;24:429–444. doi: 10.1007/s10534-011-9434-5. [DOI] [PubMed] [Google Scholar]
- 35.Shafeeq S, et al. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol Microbiol. 2011;81:1255–1270. doi: 10.1111/j.1365-2958.2011.07758.x. [DOI] [PubMed] [Google Scholar]
- 36.Wolschendorf F, et al. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2011;108:1621–1626. doi: 10.1073/pnas.1009261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leary SC, Winge DR. The Janus face of copper: its expanding roles in biology and the pathophysiology of disease. Meeting on Copper and Related Metals in Biology. EMBO Rep. 2007;8:224–227. doi: 10.1038/sj.embor.7400915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caza M, Lepine F, Dozois CM. Secretion, but not overall synthesis, of catecholate siderophores contributes to virulence of extraintestinal pathogenic Escherichia coli. Molecular microbiology. 2011;80:266–282. doi: 10.1111/j.1365-2958.2011.07570.x. [DOI] [PubMed] [Google Scholar]
- 40.Caza M, Lepine F, Milot S, Dozois CM. Specific roles of the iroBCDEN genes in virulence of an avian pathogenic Escherichia coli O78 strain and in production of salmochelins. Infect Immun. 2008;76:3539–3549. doi: 10.1128/IAI.00455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crouch ML, Castor M, Karlinsey JE, Kalhorn T, Fang FC. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Molecular microbiology. 2008;67:971–983. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- 42.Hellman NE, et al. Mechanisms of copper incorporation into human ceruloplasmin. J Biol Chem. 2002;277:46632–46638. doi: 10.1074/jbc.M206246200. [DOI] [PubMed] [Google Scholar]
- 43.Kenney GE, Rosenzweig AC. Chemistry and Biology of the Copper Chelator Methanobactin. ACS Chem Biol. 2011;17:260–268. doi: 10.1021/cb2003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms. Chem Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 45.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Versalovic J, editor. Manual of Clinical Microbiology. 10. ASM Press; Washington, DC: 2011. [Google Scholar]
- 47.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa S, Ichiki R, Abo M, Yoshimura E. Revision of analytical conditions for determining ligand molecules specific to soft metal ions using dequenching of copper(I)-bathocuproine disulfonate as a detection system. Anal Chem. 2009;81:9199–9200. doi: 10.1021/ac901782d. [DOI] [PubMed] [Google Scholar]
- 49.Wagner P, Heinecke JW. Copper ions promote peroxidation of low density lipoprotein lipid by binding to histidine residues of apolipoprotein B100, but they are reduced at other sites on LDL. Arterioscler Thromb Vasc Biol. 1997;17:3338–3346. doi: 10.1161/01.atv.17.11.3338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.