Abstract
Neurotrophic factors support the survival of spinal motoneurons (MNs) and have been considered as strong candidates for treating motoneuron diseases. However, it is unclear if the right combination of neurotrophic factor receptors is present in postnatal spinal MNs. In this study, we show that the level of c-ret expression remains relatively stable in embryonic and postnatal spinal MNs. In contrast, the mRNA and protein of GFRα1 and -2 are progressively down-regulated in postnatal life. By 3 and 6 months of age, both receptors are barely detectable in spinal MNs. The down-regulation of GFRα1 appears accelerated in transgenic mice expressing mutant SOD1G93A. Despite the progressive loss of GFRα1 and -2, phosphorylation of c-ret shows no detectable reduction on tyrosine residues or on serine 696. In addition to the GFRα subunits, expression of TrkB also shows a dynamic change. During embryogenesis, there is twice as much full-length TrkB as the truncated TrkB isoform. However, this ratio is reversed in postnatal spinal cord. Expression of the mutant SOD1G93A appears to have no effect on the TrkB receptor ratio. Taken together, our data indicate that the expression of neurotrophic factor receptors, GFRα1, -2, and TrkB, is not static, but undergoes dynamic changes in postnatal spinal MNs. These results provide insights into the use of neurotrophic factors as therapeutic agents for ALS.
Keywords: neurotrophic factors, receptors, spinal moto-neurons, postnatal development
INTRODUCTION
Neurotrophic factors are important regulators of neuronal survival, axon outgrowth, dendritic pruning, and synaptic plasticity (Huang and Reichardt, 2001; Chao, 2003). The classical neurotrophic factors, collectively categorized as neurotrophins, include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4). In general, neurotrophins interact with two receptor systems to achieve their biological functions (Chao, 2003; Huang and Reichardt, 2003). Specifically, NGF activates receptor tyrosine kinase TrkA, BDNF and NT4 activate TrkB, and NT3 activates TrkC. In addition, neurotrophins also interact with p75NTR and play important roles in regulating apoptosis and nerve regeneration (Wang et al., 2002a; Wong et al., 2002; Nykjaer et al., 2004).
Accumulating evidence has indicated that other trophic factors and cytokines also contribute to the development and maintenance of neuronal functions in the nervous system. One notable example is neurotrophic factors of the glial cell-derived neurotrophic factor (GDNF) family ligands or GFLs, which belong to the transforming growth factor β (TGFβ) superfamily and include GDNF, neurturin, artemin, and persephin (Baloh et al., 2000; Airaksinen and Saarma, 2002). Targeted deletion of each of the GFLs reveals their important functions in survival and migration of enteric, sensory, motor, and parasympathetic neurons during development (Heuckeroth et al., 1999; Enomoto et al., 2000; Oppenheim et al., 2000; Airaksinen and Saarma, 2002). It is generally accepted that signal transduction of GFLs involves interaction of GFLs with a specific glycosyl phosphatidylinositol (GPI)-linked GDNF-family receptor-α (GFRα) subunit, which activates the tyrosine kinase receptor c-ret, the signaling component of the receptor complex (Baloh et al., 2000; Airaksinen and Saarma, 2002). Four different GFRα subunits, GFRα1–4, have been characterized and are thought to determine the ligand specificity for the GFL receptor complex. For instance, GDNF interacts with GFRα1, neurturin interacts with GFRα2, artemin interacts with GFRα3, and persephin with GFRα4. Consistent with the proposed model of ligand-receptor interaction, targeted deletions of GDNF, c-ret, and GFRα1 result in remarkably similar deficits in spinal motoneurons (MNs) and kidney organogenesis (Cacalano et al., 1998; Enomoto et al., 1998; Oppenheim et al., 2000, 2002).
Much of our knowledge on the functions of neurotrophins and GFLs in the spinal MNs is derived from in vitro culture experiments and from analyses of mouse mutants with targeted deletions in neurotrophic factors or their receptors. In general, results from these analyses support the notion that neurotrophins, GDNF, and several cytokines are important for survival of spinal MNs (Liu and Jaenisch, 2000; Oppenheim et al., 2000; Holtmann et al., 2005). However, it is unclear if these trophic factors and cytokines regulate distinct subpopulations or overlapping groups of MNs. Furthermore, because most of the analyses have been performed during embryogenesis, it is unclear whether neurotrophic factors and cytokines continue to regulate the survival of MNs in adult spinal cords.
The potent effects of GDNF in promoting the survival of spinal MNs and other neuronal populations strongly suggest that GDNF may have therapeutic potentials in neurodegenerative conditions, such as amyotrophic lateral sclerosis (ALS). Consistent with this notion, several studies have shown that delivery of GDNF using viral vectors improved motor functions and prolonged the survival in mice expressing the mutant human SOD1G93A (Acsadi et al., 2002; Manabe et al., 2002; Wang et al., 2002b; Guillot et al., 2004). However, the modest efficacy of these therapeutic approaches raises several questions regarding the roles of GDNF and other neurotrophic factors in supporting the survival of postnatal spinal MNs. First, although it has been shown that the GDNF receptors are present in the spinal cord of adult mice, the signal intensity of GFRα1 appears quite variable (Glazner et al., 1998; Golden et al., 1998). Thus, it is unclear if the essential components of the GDNF receptor complex are present to regulate neuronal functions in postnatal life. Second, it is possible that the disease process of ALS might negatively impact the signal transduction of neurotrophic factors. This can be achieved either by perturbations to retrograde transport in the motor axons (Zhang et al., 1997; William-son and Cleveland, 1999), distal axonal degeneration (Fischer et al., 2004), or by dys-regulation of the expression of receptors of neurotrophic factors.
To provide answers to these questions, we investigated the expression of receptors for GDNF, neurturin, and BDNF in postnatal spinal MNs. Our results indicate that, while the level of c-ret in the spinal MNs remained relatively stable throughout development and in postnatal life, the expression of GFRα1 and -2 reduced progressively after birth. Compared to the wild-type controls, decline of GFRα1 expression level was more pronounced in mice expressing SOD1G93A. Interestingly, however, the reduction of GFRα1 appears to have no negative impact on the overall phosphorylation of c-ret. The dynamic expression of trophic factor receptors is not limited to GFRα subunits: our results indicate that the full length TrkB receptor is more abundant in the embryonic spinal cord. In contrast, this ratio is reversed in adult spinal cord where the truncated TrkB isoform becomes more abundant than the full length isoform. Taken together, our results indicate that trophic factor receptors undergo dynamic changes in postnatal spinal MNs. Our results also have implications regarding the use of neurotrophic factors in treating motoneuron diseases, such as ALS.
METHODS
Antibodies
Antihuman c-ret antibody (R787) was purchased from Immuno-Biological Laboratories (IBL-America, Minneapolis, MN), anti-GDNF family receptor antibodies, anti-GFRα1 and anti-GFRα2, were purchased from Chemicon (Temecula, CA). Epitope-specific rabbit polyclonal antibody to c-ret (phospho S696) was purchased from Abcam (Cambridge, MA) (Fukuda et al., 2002), and antiphosphotyrosine antibody was purchased from Upstate USA, Inc. (Lake Placid, NY). Affinity purified rabbit polyclonal antibody against rat TrkB was generously provided by Dr. L.F. Reichardt and used in Western blot analyses (Farinas et al., 1998; Huang et al., 1999). The TrkB antibody for immunohistochemistry was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and used at 1:200 dilution (TrkB[794], sc-12). Antiactin antibody was purchased from Oncogene Research Products, Inc. (San Diego, CA) and used at 1:40,000 dilution. PCR Master Mix was purchased from QIAGEN (Valencia, CA). Primers for PCR were synthesized by IDT (Coralville, IA).
Animals
Adult ICR mice were purchased from Charles Rivers and transgenic mice over-expressing human SOD1G93A were purchased from the Jackson Laboratory (Gurney et al., 1994). This line of mice usually develops disease at about 90 days of age and they die at about 6 months of age. Paralysis is due to loss of MNs from the spinal cord. Embryos were collected according to the protocol described previously (Huang et al., 1999). Vaginal positive plug was recorded as embryonic day 15.5. Transgenic progeny were identified by PCR using primers specific for human SOD1. The primers used to amplify SOD1 sequences are as follows: forward primers: OIMR0113 – 5′-CAT CAG CCC TAA TCC ATC TGA-3′, OIMR0114 – 5′-CGC GAC TAA CAA TCA AAG TGA-3′; reverse primers: OIMR0042 5′-CTA GGC CAC AGA ATT GAA TCT-3′, OIMR0043 5′-GTA GGT GGA AAT TCT AGC ATC ATC C-3′). The sizes of the PCR products were: the SOD1G93A allele, 236 bp, and the SOD1 wild-type allele, 324 bp. The PCR condition was: 95°C, 30″, 60°C, 30″, 72°C, 45″, for 35 cycles. Animal protocols were approved by the Institution of Animal Care and Use Committee and followed the guidelines established by the National Institutes of Health.
In Situ Hybridization
Probes for in situ hybridization were prepared using plasmid cDNA clones for HB9, c-ret, GFRα1, and -2 (courtesy of Dr. O. DeLapeyriere and Dr. T. Gould) transcribed with T7 or T3 polymerase using digoxigenin (DIG)-labeling reagents and a DIG RNA labeling kit (Roche, Indianapolis, IN) (Garces et al., 2001; Gould and Oppenheim, 2004). Probes were used at a concentration between 200–400 ng/mL. Embryos and postnatal mice were decapitated and the trunks [from postnatal day (P) 0 and P7] or the dissected spinal cords (from 3- and 6-month old) were fixed overnight at room temperature in 4% paraformaldehyde (PFA) in DEPC-treated PBS, cryoprotected in 30% sucrose in DEPC PBS, and embedded in OCT. Routinely, cervical spinal cord from C2–C7 and lumbar spinal cord from L1–L5 were examined. The rostral-caudal boundaries for the cervical and lumbar spinal cord were determined using the neighboring dorsal root ganglia (DRG) as references. The lengths of cervical and lumbar spinal cords examined were 3, 4, 6, 8, and 12 mm at embryonic day (E) 15.5, P0, P7, 3 month old and 6 month old, respectively. Sections were processed at 14 μm thickness using a Leica cryostat, mounted on glass slides, and kept in a freezer until use. Sections were then postfixed for 20 min in 4% PFA (in DEPC-treated PBS)and washed with acetylation solution (73.75 mL DEPC-treated water; 1 mL triethanolamine; 131 μL HCl; 187.5 μL acetic anhydride) and 1% Triton X-100 (in DEPC-treated PBS). Tissue sections were incubated in a humidified chamber with hybridization buffer (Amresco, Cleveland, OH) for 3 to 5 h at room temperature. Hybridization was performed overnight with DIG-labeled riboprobes in a humidified chamber (containing 5X SSC, 50% formamide) at 55°C. The slides were washed with 4X SSC, followed by RNase A (20 μg/mL) treatment at 37°C for 45 min, and subsequent washes with 2X SSC, 1X SSC, and 0.5X SSC at room temperature. For visualizing the in situ hybridization results, the tissue sections were incubated with a 1:2000 dilution of anti-DIG-alkaline phosphatase (AP)–conjugated antibody (DIG Nucleic Acid Detection Kit; Roche), washed with Buffer 3 (100 mM NaCl, 100 mM Tris, pH 9.5, 50 mM MgCl2), and incubated in Buffer 3 containing NBT/BCIP. After rinsing with Buffer 3 to stop the enzymatic reaction, the slides were dried under room temperature and mounted with Crystal Mount (Biomeda, Santa Clara, CA).
Immunoprecipitation
Tissues from cervical and lumbar spinal cord were collected and lysed with 100 μL of ice-cold RIPA buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% deoxycholic acid, 1 mM EDTA, protease inhibitor mixture, 5 mM NaF, 5 mM sodium metavanadate, and 1 mM PMSF). The lysates were homogenized and centrifuged at 14,000 rpm for 20 min at 4°C, and incubated with 5 μg of anti-c-ret antibody in 500 μL LSLD buffer (50 mM HEPES, pH 7.4, 50 mM NaCl, 0.1% Tween 20, 20% glycerol, and 25X protease inhibitor mixture 40 μL/mL) overnight at 4°C. The immune complexes were incubated for 3 h with Protein A/G plus agarose at 4°C (Santa Cruz Biotechnology). The agarose beads bearing the immunoprecipitates were washed three times with ice-cold wash buffer (50 mM HEPES, pH 7.4, 250 mM NaCl, 0.2 mM EDTA, 1% NP-40, and 25X protease inhibitor mixture 40 μL/mL). Samples were separated on an SDS-PAGE gel for Western blot analyses.
Western Blot Analyses
Immunoblotting was performed as descried previously (Wiggins et al., 2004). Briefly, protein extracts were prepared in RIPA buffers from mouse cervical and lumber spinal cord, resolved on SDS-polyacrylamide gels, and transferred to polyvinylidene difluoride (PVDF) membranes (Amersham, Arlington Heights, IL). The membrane was incubated with anti-phosphotyrosine (p-Tyr) polyclonal antibody (1:2000, mouse monoclonal IgG; Calbiochem, San Diego, CA), rabbit polyclonal to c-ret (phospho S696, 1:2500), antihuman c-ret (R787) rabbit IgG affinity purify (0.5 μg/mL), rabbit anti-GFRα1 polyclonal antibody (1:1000), rabbit anti-GFRα2 polyclonal antibody (1:1500), or rabbit anti-TrkB polyclonal antibody (1:2000) overnight at 4°C, followed by incubation with the appropriate second antibody for 1 h at room temperature. The proteins were visualized on Hyperfilm ECL (Amersham) by Chemiluminescence Reagent (ECL Plus Western Blotting Detection Reagent; Amersham). The molecular sizes of the developed proteins were estimated by comparison with rainbow molecular weight protein markers (Amersham).
Image Capture and Quantification
Quantification of the protein levels in Western blots was performed using the ImageJ Software v. 1.34s (Image Processing and Analysis in Java). Images of the Western blots were scanned, saved as JPEG files in Photoshop CS, and imported into ImageJ for quantification purposes. The relative intensity of specific protein signal was quantified by assigning a bracket around each band and normalizing to the signal intensity of the endogenous level of actin. For the dynamic changes of c-ret, GFRα1, and -2 during development and in postnatal life (Fig. 2), the relative signal intensity of each protein was normalized to the level of signal at E15.5. Results are presented as mean ± SEM using samples from at least three different animals. Statistical analyses of the differences in the levels of GFRα1 and TrkB isoforms between wild-type and SOD1G93A transgenic mice were performed using Student’s t test.
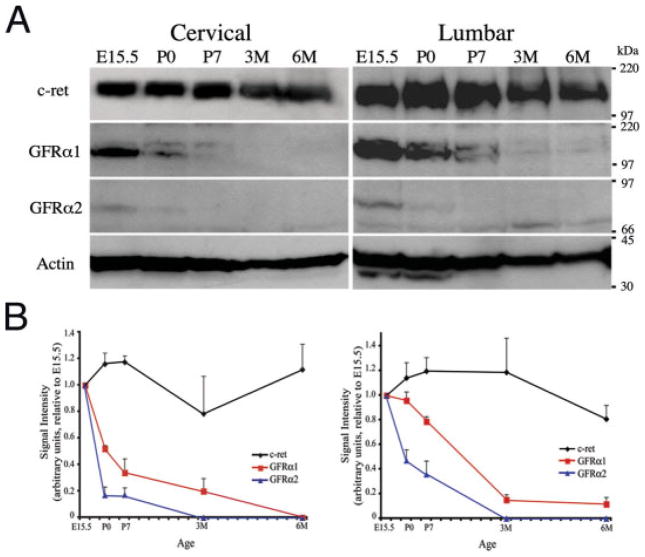
Figure 2.
Quantitative analyses of the expression of neurotrophic factor receptors in developing and postnatal spinal cords. (A) Western blot analyses of c-ret, GFRα1, and -2 in cervical and lumbar spinal cord during embryogenesis and in postnatal development. Protein extracts were prepared from spinal cords of E15.5, P0, P7, 3- and 6-month-old mice, separated in SDS-PAGE, transferred to PVDF membranes, and probed with antibodies specific for c-ret, GFRα1, or -2. The level of actin is used as internal control for loading. Consistent with the in situ hybridization results, there is a progressive down-regulation of GFRα1 and -2 in postnatal spinal cords. (B) The levels of c-ret, GFRα1, and -2 were quantified according to that of each protein in E15.5 spinal cords and represented as relative signal intensities. Whereas the level of c-ret protein remains relatively constant at all the stages, the levels of GFRα1 and -2 decline progressively in postnatal life. By 3 months of age, GFRα1 is reduced to less than 80% of the level at E15.5, whereas GFRα2 is undetectable.
RESULTS
Progressive Down-Regulation of GFRα1 and -2 in Postnatal Spinal MNs
To investigate the expression of GDNF-family ligand (GFL) receptors in the spinal MNs, we used in situ hybridization to detect c-ret, GFRα1, and -2 mRNA during embryogenesis and in postnatal life (Garces et al., 2001; Gould and Oppenheim, 2004). To provide a thorough examination of these receptors in spinal MNs, both cervical and lumbar spinal cords were collected from E15.5, P0, P7, 3-, and 6-month-old mice, and serially sectioned at 14 μm in thickness. For each stage, at least 1200 μm of cervical (from C2 to C7) and lumbar (from L1 to L5) spinal cords were subjected to examination using riboprobes specific for c-ret, GFRα1, and -2. The homeodomain transcription factor HB9 was used as a marker for spinal MNs (Arber et al., 1999; Thaler et al., 1999). To ensure that similar levels of spinal cord were examined, sections were arranged in series such that the immediately adjacent sections were hybridized with different probes.
Consistent with the reported results (Arber et al., 1999; Thaler et al., 1999; Garces et al., 2001; Gould and Oppenheim, 2004), we found that c-ret and GFRα1 were expressed at a very high level in essentially all MNs at E15.5, whereas GFRα2 mRNA was more restricted to a smaller number of MNs in cervical and lumbar spinal cords (unpublished observations). While the levels of HB9 and c-ret mRNA remained consistently high in individual MNs throughout all entire postnatal stages examined, the expression level of GFRα1 was significantly reduced in postnatal life (Fig. 1). At P0 and P7, a low signal intensity of GFRα1 was present in both the cervical and lumbar spinal cords [Fig. 1(Ad,j), (Bd,j)]. By 3- and 6-months, GFRα1 mRNA was barely detectable [Fig. 1(Ap), (Bp)]. The level of GFRα2 showed an even more pronounced reduction in postnatal spinal MNs, such that its signal intensity was barely detectable above the background level at both cervical and lumbar levels [panels (e,k,q), in Fig. 1(A,B)]. In contrast to the dynamic changes in the expression of GFRα subunits, the level of TrkB expression, shown by immunohistochemistry, remained relatively stable in spinal motoneurons at P0, P7, 3-, and 6-months. Consistent with previous reports, TrkB receptors were detected in motoneurons, as well as in glial cells and white matter tracts [Fig. 1(A,B), panels (f,l,r)].
Figure 1.
Expression of c-ret, GFRα1, -2, and TrkB in postnatal spinal MNs of wild-type mice. (A) In situ hybridization using probes specific for c-ret (c,i,o), GFRα1 (d,j,p), and -2 (e,k,q) was performed on adjacent sections of cervical spinal cords (C3–5) to characterize the expression level of neurotrophic factor receptors in postnatal mice. Nissl stain (a,g,m) and in situ hybridization for homeodomain transcription factor HB9 (b,h,n) were used to demonstrate the general architecture of the spinal cord and as a marker for motoneurons, respectively. The results indicate that, while the expression level of c-ret remains consistently high in postnatal spinal MNs, the expression of GFRα1 and -2 shows progressive down-regulation from postnatal day 0 (P0). By 6 months of age, GFRα1 and -2 are barely detectable in spinal MNs. In contrast, TrkB receptor expression, shown by immunohistochemistry, appears stable in spinal motoneurons, glial cells, and white matter tracts in the spinal cord (f,l,r). (B) Similar to the results in cervical spinal cord, GFRα1 and -2 also show progressive down-regulation in lumbar spinal cord (L3–5), whereas TrkB expression can be diffusely detected in the spinal motoneurons, glia, and white matter tracts. Scale bars in the two (r) panels = 200 μm.
To provide more a quantitative analysis of the changes in the expression of GFRα1 and-2, we prepared protein extracts from spinal cords at the same ages and from the same level as those used for in situ hybridization, and performed Western blots analyses using antibodies specific for c-ret, GFRα1, and -2 [Fig. 2(A)]. To compare the expression level of these receptors during embryogenesis and in postnatal life, the amount of each receptor was standardized to the endogenous level of actin and their levels at a given age were expressed as relative signal intensity to the level at E15.5 [Fig. 2(B)]. Consistent with the results from in situ hybridization (Fig. 1), Western blot analyses showed that the c-ret protein level remained relatively constant from E15.5 to 6 months old (Fig. 2). However, the level of GFRα1 in cervical spinal cord showed a marked reduction in postnatal life. By 6 months of age, there was no detectable GFRα1 in cervical spinal cord [Fig. 2(B)]. The level of GFRα1 in lumbar spinal cord also reduced in postnatal life, though the rate of decline was not as dramatic compared to that in cervical spinal cord. By 3 months old, the level of GFRα1 reduced to less than 20% of the level at E15.5 [Fig. 2(B)].
Similar to the results from previous studies (Garces et al., 2001), we found that the level of GFRα2 mRNA was much lower than that of c-ret and GFRα1 in both cervical and lumbar spinal cords in all the stages examined (Fig. 1). Consistent with these results, we found that GFRα2 protein was very low at E15.5, followed by a progressive decline in postnatal life [Fig. 2(B)]. At 3 months of age, there was barely any detectable amount of GFRα2 in either cervical or lumbar spinal cord [Fig. 2(B)]. Taken together, our results from in situ hybridization and Western blot analyses provided convincing evidence that the levels of GFRα1 and -2 reduce progressively in postnatal life. In contrast, the level of c-ret in spinal MNs remained relatively stable throughout embryonic and postnatal stages examined.
Accelerated Down-Regulation of GFRα1 in Mouse Model of ALS
Our observations that the expression of GFRα1 underwent dramatic down-regulation in postnatal spinal MNs provided valuable information regarding how these neurons might respond to neurotrophic factor GDNF. To investigate if the process of motor neuron disease further influenced the expression of GDNF receptors, we took a similar approach to determine the level of c-ret and GFRα1 in postnatal cervical spinal MNs. To compare the relative levels of GFRα1 in both wild-type and transgenic mice expressing the human SOD1G93A gene (SOD1G93A/+) (Gurney et al., 1994), we standardized the amount of GFRα1 to the endogenous actin. Using this approach, we found that the level of GFRα1 was similar in wild-type and SOD1G93A/+ mice at E15.5. However, the relative level of GFRα1 protein showed a significant reduction in SOD1G93A/+ mice throughout the entire postnatal stages [Fig. 3(A)]. The most dramatic difference was present between P0 and 1 month of age, when the level of GFRα1 in SOD1G93A/+ mutants was reduced by about 40% compared to the wild-type littermates [Fig. 3(B)]. In contrast, there was no difference in the level of c-ret between wild-type and SOD1G93A/+ mice [Fig. 3(A)]. The progressive down-regulation of GFRα1 was observed in both male and female mice with no detectable differences (unpublished observations). In fact, results presented in Figure 3 represent a combination of tissues from both males and females. There were no detectable differences when results were analyzed separately based on gender.
Figure 3.
Accelerated down-regulation of GFRα1 in the spinal cord of SOD1G93A/+ mutant mice. (A) Western blot analyses of c-ret and GFRα1 expression in SOD1G93A/+ mutant mice and wild-type littermates show an accelerated decline in GFRα1 in the cervical spinal cord of SOD1G93A/+ mutants. (B) Quantitative analyses in SOD1G93A/+ mutant mice and wild-type littermates show that the down-regulation of GFRα1 is more pronounced in the mutants at P0, 1, 2, and 4 months of age. At least three animals were analyzed for each age group for wild-type and SOD1G93A/+ mutants. Student’s t test, *p < 0.05.
Phosphorylation of c-ret on Tyrosines and Serine 696 in Postnatal Spinal Cord
The progressive down-regulation of GFRα1 in wild-type spinal MNs and in SOD1G93A/+ mutants raises the possibility that absence of GFRα1 and -2 may influence signaling of the GDNF receptor c-ret. To address this issue, we collected cell lysates from cervical spinal cord of wild-type mice at E15.5, P7, 3-, and 6 months, immunoprecipitated the endogenous c-ret receptor using anti-c-ret antibody, and detected phosphorylation of tyrosine residues in c-ret using anti-phosphotyrosine (p-Tyr) specific antibody. To ensure that similar amounts of c-ret protein were loaded in each lane, we stripped the membrane and reprobed it with anti-c-ret antibody. Using cell lysates collected from these samples, we found that, despite the progressive loss of GFRα1 and -2 in wild-type postnatal spinal MNs (Fig. 2), phosphorylation on tyrosine residues in c-ret appeared to be similar in all ages tested [Fig. 4(A)], suggesting that loss of GFRα1 and -2 may not significantly affect the overall phosphorylation on tyrosine residues in c-ret. Moreover, detection of c-ret phosphorylation on serine residue 686 (S686), important for c-ret-regulated Rac activity and lamellipodia formation (Fukuda et al., 2002), using an epitope-specific antibody, also revealed no difference in any of the samples [Fig. 4(B)]. To further test this hypothesis, we performed similar experiments using cell lysates from P0 and P7 cervical spinal cords collected from wild-type and SOD1G93A/+ mutants. Similar to the results in Figure 4(A), our data showed that there was no detectable difference in tyrosine phosphorylation in c-ret between wild-type and SOD1G93A/+ mutants at P0 and P7 [Fig. 4(C,D)]. Taken together, these results indicated that the down-regulation of GFRα1 in spinal MNs had no detectable effect on phosphorylation on tyrosines and S686.
Figure 4.

No difference in c-ret phosphorylation on tyrosine residues and on serine 696 (S696) in postnatal cervical spinal cord. (A,B) Phosphorylation of tyrosine residues (A) and serine 696 (S696) (B) was determined in cervical spinal cords from E15.5, P7, 3-, and 6-month-old wild-type mice, and showed no detectable difference. (C,D) Despite a more significant down-regulation of GFRα1 in SOD1G93A/+ mutant spinal cord, there was no detectable difference in the phosphorylation on tyrosine residues in c-ret between wild-type and SOD1G93A/+ mutant mice at P0 and P7. Protein extracts were prepared from SOD1G93A/+ mutants and wild-type littermates, immunoprecipitated with c-ret antibody, and probed with either anti-phosphotyrosine or c-ret antibody. The relative level of c-ret phosphorylated on the tyrosine residues was used to compare the difference between wild-type and SOD1G93A/+ mutants.
Reversal of Full-Length and Truncated TrkB Receptor Ratio in Postnatal Spinal Cord
The neurotrophins, BDNF, NT3, and -4/5, are collectively required for the survival of a certain population of spinal MNs in vivo and have been shown to promote motor neuron survival and functional recovery in injury paradigms (Liu and Jaenisch, 2000; Yuan et al., 2000; Aszmann et al., 2002). In particular, BDNF, when used in combination with GDNF, increased motor neuron survival and improved motor functions in the neonatal rats with crush injury to the brachial plexus (Aszmann et al., 2002). In vivo, the BDNF receptor TrkB exists in two major isoforms, the full length TrkB with tyrosine kinase domain and at least two truncated TrkBs that lack the kinase domain due to alternative splicing. Several lines of evidence have indicated that the truncated TrkB functions as a dominant negative receptor in modulating responses to BDNF or intracellular signaling pathways (Eide et al., 1996; Baxter et al., 1997; Saarelainen et al., 2000). To further understand the role of TrkB receptors in supporting the survival of MNs, we investigated the expression of TrkB receptor isoforms in the postnatal spinal cords. Using an antibody that recognized the extracellular domain of rat TrkBs (Farinas et al., 1998; Huang et al., 1999), we showed that the predominant isoform in both cervical and lumbar spinal cord during embryogenesis was the full length TrkB [Fig. 5(A)]. Specifically, the amount of full length TrkB in E15.5 cervical and lumbar spinal cord was about 1.7- and 2.3-fold that of the truncated isoform, respectively [Fig. 5(B)]. However, the level of full length TrkB reduced and the truncated TrkB increased progressively in postnatal life. By P7, the ratio of full length TrkB to truncated isoform was about equal in the cervical spinal cord, and by 6 months old there was twice as much truncated TrkB compared to the full length isoform [Fig. 5(B)]. A similar trend was also observed in the lumbar spinal cord, though the ratio of full length to truncated isoform appeared to level off slightly earlier, at 3 months of age [Fig. 5(B)].
Figure 5.
Reversal of full length TrkB and truncated TrkB receptor ratio in postnatal spinal cords. (A) The majority of TrkB receptor in cervical and lumbar spinal cords is the full length isoform (TrkB-FL) at E15.5 and P0. However, the ratio undergoes a reversal during postnatal development such that by 3 to 6 months of age, there is twice as much truncated TrkB isoform (TrkB-T) in the spinal cord. Protein extracts were prepared from three different animals at each age group. (B) Quantifications of the ratio of full length versus truncated isoforms of TrkB are represented as mean ± SEM (n = 3).
Because our results in GDNF receptors showed that the down-regulation of GFRα1 was accelerated in transgenic mice carrying the mutant SOD1G93A gene, we asked if the reversal of the ratio of TrkB receptor isoforms was also affected in these transgenic mice. To test this hypothesis, we first determined TrkB expression in cervical spinal cord using immunohistochemistry. Our results showed that, despite the significant loss of spinal MNs at 4 months of age, TrkB could be detected in the remaining neurons, glial cells, and white matter tracts in SOD1G93A/+ mutants [Fig. 6(A)]. Furthermore, Western blot analyses using cell lysates from cervical spinal cord showed no detectable difference in the ratio of full length to truncated TrkB isoforms between wild-type and SOD1G93A/+ mice at P0, 1, 2, and 4 months of age [Fig. 6(B,C)].
Figure 6.
Persistent expression of TrkB in spinal motoneurons in wild-type and SOD1G93A/+. (A) Histopathological examinations of cervical spinal cord from 4-month-old wild-type and SOD1G93A/+ show a significant loss of spinal motor neurons in SOD1G93A/+ mutants [c.f. panels (a) and (c) with (b) and (d), respectively]. However, TrkB can still be detected in the remaining neurons, glial cells, and white matter tracts in SOD1G93A/+ mutants [c.f. panels (c) and (d)]. Scale bar in (d) = 200 μm. (B) The reversal of full length and truncated TrkB ratio shows no difference between wild-type and SOD1G93A/+ mutant mice. Due to the progression of disease in SOD1G93A/+ mutants, protein extracts were analyzed from the cervical spinal cords from wild-type and SOD1G93A/+ mutants at E15.5, P0, 1, 2, and 4 months of age. (C) Quantifications of the ratio of TrkB receptor isoforms at different postnatal stages are represented as mean ± SEM (n = 3).
DISCUSSION
In this study, we investigated the expression profiles of receptors for GDNF, neurturin, and BDNF in the postnatal spinal MNs. We chose to study these neurotrophic factors because of their documented roles in supporting the survival of MNs. Our results indicated that the mRNA and protein levels of GFRα1 and -2 declined progressively in postnatal spinal MNs (Figs. 1 and 2). In contrast, the level of c-ret remained relatively stable throughout embryogenesis and in post-natal stages (Figs. 1 and 2). The level of GFRα1 was further reduced in the transgenic mice expressing one of the mutant human SOD1 genes, SOD1G93A, before the onset of disease (Fig. 3). Interestingly, the progressive loss of GFRα1 and -2 in postnatal spinal MNs appeared to have no detectable effects on the phosphorylation of c-ret on tyrosines or S686 (Fig. 4). Further reduction of GFRα1 in the spinal MNs of SOD1G93A/+ mutants also showed no negative effects on c-ret phosphorylation on tyrosines (Fig. 4). In contrast to the changes in GFR subunits, the total amount of TrkB receptors remained relatively stable in postnatal spinal cords. However, the ratio of TrkB receptor isoforms showed significant change in spinal MNs during embryogenesis and in postnatal life (Fig. 5). Taken together, our results support the notion that the expression of neurotrophic factor receptors is dynamic in postnatal spinal MNs. These results provide insights into the signaling mechanisms of these neurotrophic factors in postnatal MNs and into the use of trophic factors as therapeutic agents for motor neuron diseases, such as ALS.
Dynamic Expression of c-ret and TrkB in Postnatal Spinal MNs: Implications for Signal Transduction
GFRα subunits and tyrosine kinase receptor c-ret are essential components in transducing signaling from GFLs (Baloh et al., 2000; Airaksinen and Saarma, 2002). It is generally accepted that the functions of GFRα subunits are carried out by two modes of action. One is the “cis-signaling” mode in which GFRα subunits and c-ret are expressed in the same cells and binding of GFLs to the GFRα subunits assists the activation of c-ret, and the other is the “trans-signaling” or “RET-independent signaling” mode whereby GFRα subunits from the neighboring cells activate signal transduction in an non-cell-autonomous manner (Airaksinen and Saarma, 2002; Enomoto et al., 2004). Results from previous studies and our current study indicate that in embryonic spinal MNs both c-ret and GFRα subunits (GFRα1 and to a lesser extent GFRα2) are coexpressed in the same cells (Garces et al., 2001; Gould and Oppenheim, 2004). The observations that spinal MN deficits in Gdnf, c-ret, or Gfrα1 mutants are quite similar in severity and timing support the notion that the cis-signaling mode is operative in MNs during development (Cacalano et al., 1998; Enomoto et al., 1998; Oppenheim et al., 2000, 2002).
Despite these results, it is unclear if the cis-signaling continues to function in postnatal spinal MNs. Although two prior studies have investigated the expression of c-ret and GFRα subunits in adult central nervous system, there are significant variations in the results (Glazner et al., 1998; Golden et al., 1998). Using more quantitative approaches covering a wider range of time, we show that even within the same group of spinal MNs, expression of GFRα subunits undergoes dynamic changes in postnatal life (Figs. 1 and 2). Furthermore, the reduction of GFRα1 expression is even more pronounced in transgenic mice expressing SOD1G93A, occurring even before the onset of disease (Fig. 3). These results raise the possibility that progressive loss of GFRα subunits in post-natal spinal MNs may negatively influence the cis-signaling mode of activating c-ret. Although our initial characterizations of the basal phosphorylation on tyrosine and S696 residues in c-ret do not support such a hypothesis, we cannot completely rule out the possibility that phosphorylation of specific tyrosine residue(s) may be defective or the kinetics of activating the kinase activity of c-ret may alter in the absence of GFRα subunits in adult MNs. Future experiments using more epitope-specific c-ret antibodies and conditional mouse mutants that selectively remove GFRα1 from postnatal spinal MNs will help address this issue.
Similar to our findings in postnatal spinal MNs, previous studies have shown that, in the adult CNS, c-ret is expressed in many regions with no or very low levels of GFRα1 (Glazner et al., 1998; Golden et al., 1998, 1999). How might signal transduction through c-ret be sustained in the presence of low or no GFRα subunits? One possibility is that c-ret, when bound to low levels of GFRα1 and -2, may be sufficient to maintain a steady state level of autophosphorylation, recruit adaptor proteins, and activate downstream pathways, such as PI3 kinase and MAPKs (Airaksinen and Saarma, 2002). Alternatively, it is possible that, in the absence of GFRα subunits, cross talk among receptor tyrosine kinases, cytokine receptors, and ion channels may promote and maintain signal transduction through c-ret (Chuang et al., 2001; Tsui-Pierchala et al., 2002).
The neurotrophin receptor TrkB interacts with several neurotrophins, including BDNF, NT3, and -4, and has been considered as an important signaling molecule mediating survival signals in spinal MNs. Consistent with these results, mice lacking the kinase domain of TrkB show ≈35% reduction in the lumbar spinal MNs (Klein et al., 1993), whereas loss of BDNF, NT3, and -4 collectively leads to a reduction of ≈20% of MNs (Liu and Jaenisch, 2000). The cause for the slightly more severe deficit in TrkB kinase mutants is unclear, but one possibility is that the truncated TrkB may have dominant negative effects (more discussions below). Although mice with targeted mutation in the full length TrkB or both TrkB and TrkC have been generated (Minichiello and Klein, 1996; Xu et al., 2000), the cellular deficits in spinal MNs have not been fully analyzed. Our results indicate that, during development, there is twice as much full length TrkB compared to truncated TrkB. However, as development proceeds into peri- and postnatal stages, the ratio of full length to truncated TrkB becomes reversed. Several lines of evidence have indicated that truncated TrkB functions as a dominant negative receptor (Eide et al., 1996; Baxter et al., 1997; Saarelainen et al., 2000). Thus, the progressive increase in this isoform may create an unfavorable milieu for postnatal spinal MNs. It will be interesting to investigate if removal of this truncated isoform using a conditional gene targeting approach will promote the prosurvival effects of BDNF.
Stage-Specific Dependence of Multiple Trophic Factors in Spinal MNs
A number of neurotrophic factors and cytokines have been shown to support the survival of spinal MNs in either dissociated neurons or slice cultures (Arce et al., 1998; Rakowicz et al., 2002). However, the effects of these factors are in general more potent under in vitro conditions than those revealed by genetic studies, suggesting that there is significant functional redundancy, as well as complexity, in supporting the survival of spinal MNs. For instance, although GFRα1 and c-ret are expressed in almost all spinal MNs during embryogenesis, targeted deletion of c-ret, Gfrα1, or Gdnf results in a modest loss of MNs (Garces et al., 2000; Oppenheim et al., 2000, 2002), suggesting that spinal MNs are intrinsically heterogeneous in trophic factor dependence. Thus, it is likely that an individual neuron may depend on more than one trophic factor to achieve a sustainable survival. Several lines of evidence support this notion. For instance, a recent study suggests that only very few motoneurons (i.e., the fusimotor neurons) are dependent on GDNF (Whitehead et al., 2005). Consistent with this notion, administration of multiple, but not individual, neurotrophins rescues specific pools of avian spinal cord MNs from programmed cell death (Gould and Oppenheim, 2004). Furthermore, whereas loss of BDNF alone has no effects on the survival of spinal MNs, removal of BDNF, NT3, and -4 leads to a significant, albeit modest, loss of MNs in spinal cord (Jones et al., 1994; Liu and Jaenisch, 2000). Similarly, targeted deletion of cntf, lif, and cardiotrophin-1 results in a collective loss of about 20–30% of spinal MNs (Holtmann et al., 2005). Future detailed topographic mapping of neuronal loss in these mutants is required to determine if the three distinct groups of trophic factors, including neurotrophins, GFLs, and CNTF family, support a nonoverlapping group of MNs.
In addition to the dependence on multiple neurotrophic factors, the dynamic change of trophic factor receptor expression in postnatal spinal MNs (Figs. 1 and 2) raises the possibility that trophic factor dependence of spinal MNs may be stage-specific. In other words, spinal MNs may undergo switches of neurotrophic factor dependence at different developmental and postnatal stages. Consistent with this notion, neuronal deficits in Gdnf−/− and bdnf−/− nt3−/− nt4/5−/− mutants occur primarily during embryogenesis (Liu and Jaenisch, 2000; Oppenheim et al., 2000). However, due to the perinatal lethality of these mutants, it has not been possible to study the in vivo functions of these trophic factors in postnatal spinal MNs. Thus, GDNF, BDNF, NT3, and -4 may continue to support survival of spinal MNs in postnatal life. In contrast to the neurotrophin mutants, neuronal deficits due to loss of CNTF, LIF, and Cardiotrophin are detected in perinatal and early postnatal mice (Holtmann et al., 2005), suggesting that, at least for a subset of spinal MNs, trophic factor dependence occurs in a stage-dependent manner.
The switch in neurotrophin dependence is well documented in somatic sensory neurons. This phenomenon was first revealed by in vitro cultures showing that sensory neurons switch their dependence from NT3 and BDNF at E11.5–13.5 to NGF at E15.5–P0 (Buchman and Davies, 1993). Detailed examinations of the expression of neurotrophin receptors and analyses of sensory neuron deficits in mutants lacking neurotrophin indicate that such a switch in trophic factor dependence is most likely due to the dynamic change in Trk receptor expression during the early generation of sensory neurons (Huang et al., 1999). Interestingly, at later stages of development (e.g., after E15.5) and in postnatal life, a significant number of sensory neurons in DRG and TG switch on the expression of c-ret and GFRα subunits (Molliver et al., 1997; Huang et al., 1999), suggesting that the dependence of neurotrophins and GFLs in sensory neurons is a dynamic process during neurogenesis and in postnatal life. While results in this study provide the first evidence that a switch in neurotrophic factor dependence may also occur in spinal MNs, detailed characterizations of a wider spectrum of neurotrophic factor receptors in MNs will be needed to fully reveal the underlying mechanisms. Supporting this notion, a recent study shows that, compared to GDNF, insulin growth factor 1 (IGF-1) is much more potent in supporting survival of spinal MNs and improving survival of SOD1G93A/+ mice (Kaspar et al., 2003).
Perspectives on Gene Therapy Using Neurotrophic Factors in Motor Neuron Diseases
One important goal in studying neurotrophic factors is to determine their therapeutic potentials in neurodegenerative conditions. Indeed, the significant level of GDNF in the Schwann cells and skeletal muscles under physiological conditions has inspired a multitude of approaches to deliver GDNF to spinal MNs in the experimental model of ALS (Acsadi et al., 2002; Manabe et al., 2002; Wang et al., 2002b; Guillot et al., 2004). However, regardless of the means of delivery, exogenously delivered GDNF only modestly prolonged the survival of SOD1G93A/+ mutants. It is unclear if the inefficiency of GDNF is due to the progressive loss of GFRα subunits in adult spinal MNs (Josephson et al., 2001), or due to the fact that GDNF is effective only in a subset of spinal MNs (Whitehead et al., 2005). It is also possible that postnatal spinal MNs may also depend on mechanisms independent of trophic factors, such as activation of cAMP, the ability to metabolize radical oxygen species (ROS) through glia, or increase in local blood/oxygen supply (Meyer-Franke et al., 1998; Clement et al., 2003). Consistent with this notion, recent studies using either viral vectors to retrogradely deliver VEGF to MNs or by direct intracerebroventricularly injection of recombinant VEGF show significant improvement in survival and motor functions in the mouse model of ALS (Azzouz et al., 2004; Storkebaum et al., 2005). It is unclear, however, if VEGF receptors are present in spinal MNs per se and/or in the adjacent blood vessels.
In summary, results from the current study support the notion that the expression of certain trophic factor receptors, such as GFRα1, -2, and TrkB, undergoes dynamic change in postnatal spinal MNs. Such a dynamic process raises many interesting questions regarding the signaling mechanisms and the therapeutic efficacy of neurotrophic factors in postnatal spinal MNs. Our results, and those from another study, also bring up the important caveat that there may be substantial variations in the expression of neurotrophin factor receptors in postnatal spinal motoneurons in different species (Josephson et al., 2001). Future studies in humans should provide further insights into the design of more selective and effective treatment.
Acknowledgments
We would like to thank Dr. O. DeLapeyriere and Dr. T. Gould for the in situ probes for c-ret, GFRα1, and -2, Dr. L.F. Reichardt for TrkB antibody, Amy A. Tang for help with animal husbandry, and Dr. Stephen DeArmond and members of the Huang laboratory for comments on this manuscript. J. Zhang was supported in part by the ALSA Challenge Grant (#332). Work in the Huang laboratory has been supported by a VA Merit Review Award. E.J.H. is a recipient of the Presidential Early Career Award for Scientists and Engineers (PECASE) and the Independent Scientist Award from NINDS.
Contract grant sponsor: NIH/NINDS; contract grant numbers: NS48393 and NS44223.
References
- Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, Wang Y, et al. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13:1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Arce V, Pollock RA, Philippe JM, Pennica D, Henderson CE, deLapeyriere O. Synergistic effects of schwann- and muscle-derived factors on motoneuron survival involve GDNF and cardiotrophin-1 (CT-1) J Neurosci. 1998;18:1440–1448. doi: 10.1523/JNEUROSCI.18-04-01440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszmann OC, Korak KJ, Kropf N, Fine E, Aebischer P, Frey M. Simultaneous GDNF and BDNF application leads to increased motoneuron survival and improved functional outcome in an experimental model for obstetric brachial plexus lesions. Plast Reconstr Surg. 2002;110:1066–1072. doi: 10.1097/01.PRS.0000020990.82332.43. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors—implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- Baxter GT, Radeke MJ, Kuo RC, Makrides V, Hinkle B, Hoang R, Medina-Selby A, et al. Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2. J Neurosci. 1997;17:2683–2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, et al. Wild-type non-neuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Heuckeroth RO, Golden JP, Johnson EM, Milbrandt J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development. 2000;127:4877–4889. doi: 10.1242/dev.127.22.4877. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Hughes I, Golden J, Baloh RH, Yonemura S, Heuckeroth RO, Johnson EM, Jr, et al. GFRalpha1 expression in cells lacking RET is dispensable for organogenesis and nerve regeneration. Neuron. 2004;44:623–636. doi: 10.1016/j.neuron.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Farinas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A. Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron. 1998;21:325–334. doi: 10.1016/s0896-6273(00)80542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kiuchi K, Takahashi M. Novel mechanism of regulation of Rac activity and lamellipodia formation by RET tyrosine kinase. J Biol Chem. 2002;277:19114–19121. doi: 10.1074/jbc.M200643200. [DOI] [PubMed] [Google Scholar]
- Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, deLapeyriere O. GFRalpha 1 is required for development of distinct subpopulations of motoneuron. J Neurosci. 2000;20:4992–5000. doi: 10.1523/JNEUROSCI.20-13-04992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garces A, Livet J, Grillet N, Henderson CE, Delapeyriere O. Responsiveness to neurturin of subpopulations of embryonic rat spinal motoneuron does not correlate with expression of GFR alpha 1 or GFR alpha 2. Dev Dyn. 2001;220:189–197. doi: 10.1002/1097-0177(20010301)220:3<189::AID-DVDY1106>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Gould TW, Oppenheim RW. The function of neurotrophic factor receptors expressed by the developing adductor motor pool in vivo. J Neurosci. 2004;24:4668–4682. doi: 10.1523/JNEUROSCI.0580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot S, Azzouz M, Deglon N, Zurn A, Aebischer P. Local GDNF expression mediated by lentiviral vector protects facial nerve motoneurons but not spinal motoneurons in SOD1(G93A) transgenic mice. Neurobiol Dis. 2004;16:139–149. doi: 10.1016/j.nbd.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, et al. Motor neuron degeneration in mice that express a human Cu,Zn super-oxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Holtmann B, Wiese S, Samsam M, Grohmann K, Pennica D, Martini R, Sendtner M. Triple knock-out of CNTF, LIF, and CT-1 defines cooperative and distinct roles of these neurotrophic factors for motoneuron maintenance and function. J Neurosci. 2005;25:1778–1787. doi: 10.1523/JNEUROSCI.4249-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Wilkinson GA, Fariñas I, Backus C, Zang K, Wong SL, Reichardt LF. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development. 1999;126:2191–2203. doi: 10.1242/dev.126.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson A, Widenfalk J, Trifunovski A, Widmer HR, Olson L, Spenger C. GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia. J Comp Neurol. 2001;440:204–217. doi: 10.1002/cne.1380. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Liu X, Jaenisch R. Severe peripheral sensory neuron loss and modest motor neuron reduction in mice with combined deficiency of brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5. Dev Dyn. 2000;218:94–101. doi: 10.1002/(SICI)1097-0177(200005)218:1<94::AID-DVDY8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Nagano I, Gazi MS, Murakami T, Shiote M, Shoji M, Kitagawa H, et al. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Apoptosis. 2002;7:329–334. doi: 10.1023/a:1016123413038. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, Reichardt LF, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L, Klein R. TrkB and TrkC neurotrophin receptors cooperate in promoting survival of hippocampal and cerebellar granule neurons. Genes Dev. 1996;10:2849–2858. doi: 10.1101/gad.10.22.2849. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, et al. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Parsadanian AS, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW, Prevette DM, Gould T, Enomoto H, Milbrandt J. Neuronal development and survival in mice deficient in the GDNF co-receptor Ret. Program No. 428.16. Abstracts, Society for Neuroscience Annual Meeting; Washington, DC. 2002. [Google Scholar]
- Rakowicz WP, Staples CS, Milbrandt J, Brunstrom JE, Johnson EM., Jr Glial cell line-derived neurotrophic factor promotes the survival of early postnatal spinal motor neurons in the lateral and medial motor columns in slice culture. J Neurosci. 2002;22:3953–3962. doi: 10.1523/JNEUROSCI.22-10-03953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T, Pussinen R, Koponen E, Alhonen L, Wong G, Sirvio J, Castren E. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons have impaired long-term spatial memory but normal hippocampal LTP. Synapse. 2000;38:102–104. doi: 10.1002/1098-2396(200010)38:1<102::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Milbrandt J, Johnson EM., Jr NGF utilizes c-Ret via a novel GFL-independent, inter-RTK signaling mechanism to maintain the trophic status of mature sympathetic neurons. Neuron. 2002;33:261–273. doi: 10.1016/s0896-6273(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002b;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead J, Keller-Peck C, Kucera J, Tourtellotte WG. Glial cell-line derived neurotrophic factor-dependent fusimotor neuron survival during development. Mech Dev. 2005;122:27–41. doi: 10.1016/j.mod.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Wiggins AK, Wei G, Doxakis E, Wong C, Tang AA, Zang K, Luo EJ, et al. Interaction of Brn3a and HIPK2 mediates transcriptional repression of sensory neuron survival. J Cell Biol. 2004;167:257–267. doi: 10.1083/jcb.200406131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, Reichardt LF. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 2000;26:233–245. doi: 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Wu W, So KF, Cheung AL, Prevette DM, Oppenheim RW. Effects of neurotrophic factors on motoneuron survival following axonal injury in newborn rats. NeuroReport. 2000;11:2237–2241. doi: 10.1097/00001756-200007140-00035. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]