ADP-ribosylation was one of the first molecular mechanisms described to be used by bacterial protein toxins to target eukaryotic cells. Most potent and devastating toxins belong to this group, including diphtheria toxin and Pseudomonas exotoxins A, which block protein synthesis by ADP-ribosylation of elongation factor 2. Cholera toxin from Vibrio cholerae, which causes several thousand cases of death worldwide every year, is another ADP-ribosylating toxin, of which ADP-ribosylate Gs proteins is involved in receptor signaling (1). All of these toxins modify host proteins by transferring ADP ribose from NAD+ onto specific eukaryotic target proteins, thereby altering their physiological functions. Although ADP-ribosylating toxins share only limited sequence similarity, crystal structures available from various toxins are amazingly similar (2). However, so far the precise molecular reaction by which these toxins catalyze the ADP-ribosylation of their target proteins has remained largely enigmatic. In an exciting study published in PNAS (3), Tsurumura et al. describe several crystal structures of Clostridium perfringens iota toxin, in complex with its protein substrate actin, which are obtained during different phases of the ADP-ribosylation process. The authors’ findings are groundbreaking not only for the understanding of toxin-induced ADP-ribosylation but also for comprehension of the molecular reaction induced by an increasing number of endogenous ADP ribosyltransferases.
C. perfringens iota toxin is exclusively produced by type E strains of C. perfringens and involved in enterotoxemia and diarrhea in mammals. The toxin belongs to the family of actin ADP-ribosylating toxins (4). Other members of this toxin family are Clostridium difficile transferase CDT, Clostridium spiroforme toxin CST, Clostridium botulinum C2 toxin, and Bacillus cereus/sphaericus vegetative insecticidal proteins. All of these actin ADP-ribosylating toxins are binary toxins, which consist of an enzyme component with ADP-ribosyltransferase activity and a separated binding component, which is responsible for transport of the enzyme component into target cells (Fig. 1A).
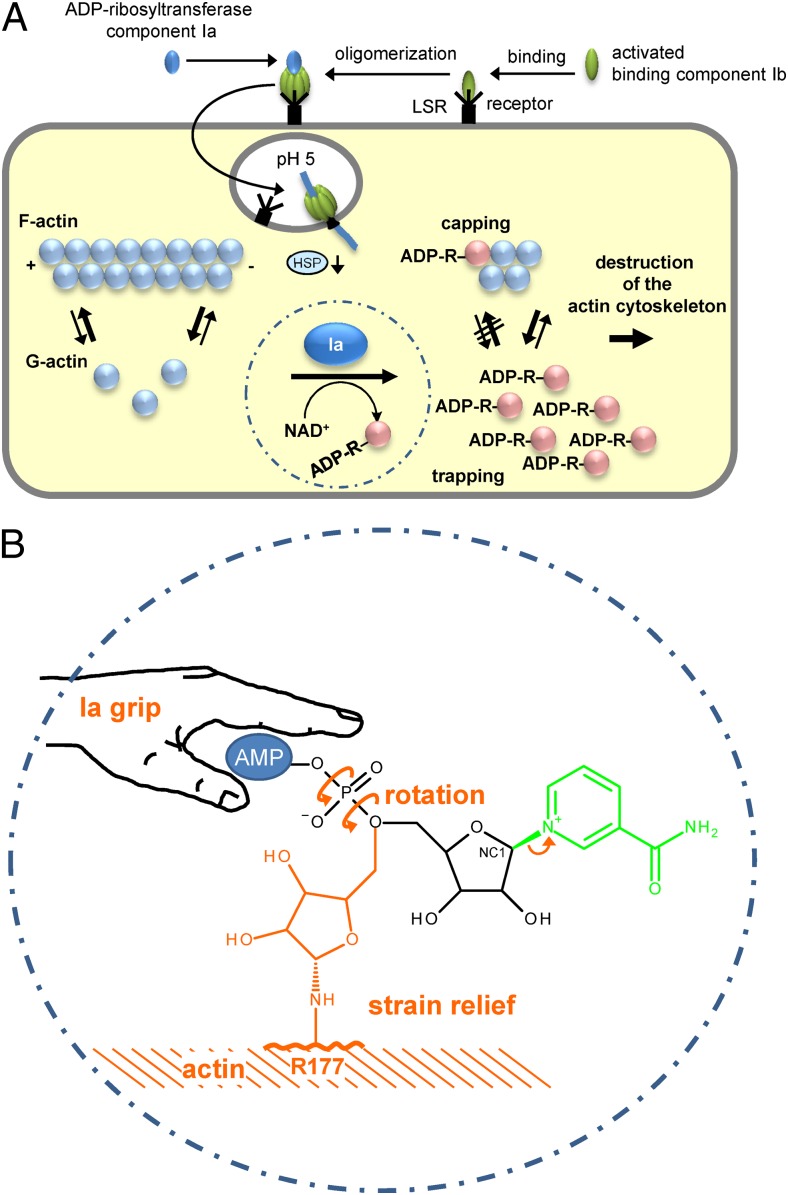
Fig. 1.
(A) Up-take and action of C. perfringens iota toxin. Iota toxin consists of the ADP-ribosyltransferase component Ia and the receptor binding component Ib. Both are activated by proteolytic cleavage (not shown). Ib binds to its cell surface receptor LSR (lipolysis-stimulated lipoprotein receptor) (5) and forms heptamers to which Ia binds. The toxin-LSR complex is endocytosed. At low pH of endosomes the toxin heptamers insert into the vesicle membrane and translocate Ia into the cytosol with the help of cellular chaperons (e.g., heat-shock protein 90, HSP). In the cytosol, Ia ADP-ribosylates monomeric G-actin in Arg177. ADP-ribosylated actin is not able to polymerize and is trapped in the monomeric form (trapping). Moreover, ADP-ribosylated actin acts as a capping protein to block polymerization of nonmodified actin. (B) Mechanism of ADP ribosylation of actin by Ia suggested by Tsurumura et al. (3). The ADP ribosyltransferase component Ia binds the AMP moiety of NAD+ with a tight grip in a prereaction state. ADP ribosylation reaction starts with release of nicotinamide and formation of a first oxocarbenium cation intermediate (small arrow). Thereafter, a rotation around the axes of the phosphodiester bond forms a second oxocarbenium cation, which results in strain relief and brings the C1 (NC1) of the ribosyl moiety near to arginine177 (R177) of actin to complete the transfer of ADP ribose.
Actin, the toxins’ target, is one of the most abundant and conserved eukaryotic proteins involved in numerous pivotal cellular functions. Actin is an essential part of the cytoskeleton and participates in cellular motility and migration, cytokinesis, phagocytosis, endocytosis, and secretion. All of these functions depend on the dynamic polymerization and depolymerization of monomeric G-actin to form F-actin filaments. The binary actin ADP-ribosylating toxins modify actin at arginine-177 (Arg177), and thereby sterically prevent the polymerization to actin filaments. The only localization where ADP-ribosylated actin can bind to F-actin is the plus (barbed) end of the filaments. Here, ADP-ribosylated actin has a capping function and prevents growth of nonmodified actin. In contrast, the minus or pointed ends of F-actin are free: there depolymerization occurs. Released G-actin is further on ADP-ribosylated by the toxin. Toxin-induced depolymerization of actin has dramatic effects on target cells, with destruction of the actin cytoskeleton and subsequent apoptosis or remodeling of microtubules and increase in adherence and colonization of bacteria (5).
The enzyme component (Ia) of iota toxin with actin in the presence of a stable NAD+ analog (β-TAD) was crystallized by Tsuge et al. previously (6). In the present study Tsurumura et al. were able to obtain crystal complexes of Ia with actin, revealing “structural snapshots” along the reaction coordinate of ADP-ribosylation. These structures confirm and improve a “strain-alleviation model” of ADP-ribosylation, which was previously proposed from the same group (6). Using crystal soaking experiments with the apo-Ia-actin complex, they obtained an NAD+-Ia-actin complex and an Ia-ADP ribosyl-actin product complex at 1.75 and 2.2 Å, respectively. By chance, the authors obtained the complex NAD+-Ia-actin as a prereaction state by using the cryoprotectant ethylene glycol, which blocked the ADP ribosylation reaction. All arginine-modifying ADP-ribosylating toxins are characterized by an EXE motif (378Glu-X-Glu380 in Ia). This motif is part of ADP ribosylation turn-turn loop (7) and plays a pivotal role in catalysis and protein substrate recognition. Although the second Glu is essential for ADP-ribosylation and NADase activity, the first Glu (Glu378) is needed for the ADP-ribosyltransferase reaction but not for NAD+ hydrolysis. Therefore,
Tsurumura et al. were able to obtain crystal complexes of Ia with actin, revealing ‘structural snapshots’ along the reaction coordinate of ADP-ribosylation.
by exchanging Glu380 to serine NAD+ hydrolysis was blocked and a NAD+-Ia-actin complex could be crystallized.
In this prereaction state, the ADP moiety of NAD+ is in a tight grip of Gln300, Asn335, and Arg352 of Ia (Fig. 1B). The nicotinamide mononucleotide (NMN) phosphate is coordinated by Arg295, whereas the NMN ribose interacts with the EXE motif Glu380 and Glu378. Finally, Arg296 of Ia stabilizes with the carboxyl amide group of the nicotinic acid moiety, resulting in a distorted and strained form of the NMN part of NAD+, which is typical for all ADP-ribosyltransferases known. The first step in the SN1 ADP-ribosylation reaction is cleavage of the glycosidic bond between nicotinamide and ribose by development of a first-transition state oxocarbenium cation, which is stabilized by Glu380. Tsurumura et al. (3) suggest that this intermediate is also stabilized by Tyr251 via cation-pi-interaction. At this point of the reaction, the electrophile (NC1 of N-ribose) is still ∼8 Å apart from the actin acceptor amino acid Arg177. To manage this distance, the authors suspect a central rotation step. This rotation includes mainly the β-phosphate of ADP ribose, resulting in strain relief and the formation of a second oxocarbenium cation, which is able to reach Arg177 of actin. Indeed, the proposed postreaction state could be resolved in the crystals of Tsurumura et al. (3). There are still some open questions. The role of Tyr251 of Ia in stabilizing the oxocarbenium ion transition-state has not been experimentally shown. In addition, the function of Asp179 of actin should be clarified by mutational analysis.
The described mechanism might be relevant not only for ADP-ribosylating toxins but also for the whole family of ADP-ribosyltransferases. Here, the authors present a model for the ADP-ribosylation of arginine residues. However, is this congruent with the modification of cysteine, asparagine, threonine, glutamine, or diphthamide by other types of ADP-ribosylating enzymes? Of course, supporting experiments for other ART-families need to follow.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4267.
References
- 1.Krueger KM, Barbieri JT. The family of bacterial ADP-ribosylating exotoxins. Clin Microbiol Rev. 1995;8(1):34–47. doi: 10.1128/cmr.8.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fieldhouse RJ, Merrill AR. Needle in the haystack: Structure-based toxin discovery. Trends Biochem Sci. 2008;33(11):546–556. doi: 10.1016/j.tibs.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Tsurumura T, et al. Arginine ADP-ribosylation mechanism based on structural snapshots of iota-toxin and actin complex. Proc Natl Acad Sci USA. 2013;110:4267–4272. doi: 10.1073/pnas.1217227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth H, Aktories K, Popoff MR, Stiles BG. Binary bacterial toxins: Biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol Mol Biol Rev. 2004;68(3):373–402. doi: 10.1128/MMBR.68.3.373-402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aktories K, Lang AE, Schwan C, Mannherz HG. Actin as target for modification by bacterial protein toxins. FEBS J. 2011;278(23):4526–4543. doi: 10.1111/j.1742-4658.2011.08113.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsuge H, et al. Structural basis of actin recognition and arginine ADP-ribosylation by Clostridium perfringens iota-toxin. Proc Natl Acad Sci USA. 2008;105(21):7399–7404. doi: 10.1073/pnas.0801215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S, Arvai AS, Clancy SB, Tainer JA. Crystal structure and novel recognition motif of rho ADP-ribosylating C3 exoenzyme from Clostridium botulinum: Structural insights for recognition specificity and catalysis. J Mol Biol. 2001;305(1):95–107. doi: 10.1006/jmbi.2000.4292. [DOI] [PubMed] [Google Scholar]