We reported that persistent cannabis use was associated with neuropsychological decline, from adolescence to midlife (1). Two commentators suggested alternative explanations; we tested these and report the results here.
Rogeberg (2) wonders whether socioeconomic differences explain the association between cannabis and neuropsychological decline. His argument is based on his assumption that cannabis use is more common in youngsters of low socioeconomic status (SES). He also believes that the intelligence quotients (IQs) of low-SES children are temporarily boosted by schooling but that when they leave school and choose their own niches, their IQs rebound to their former low baseline. If many cannabis users were low-SES children, Rogeberg says this coincidence would create the false impression that cannabis use was responsible for their IQ drop in adulthood.
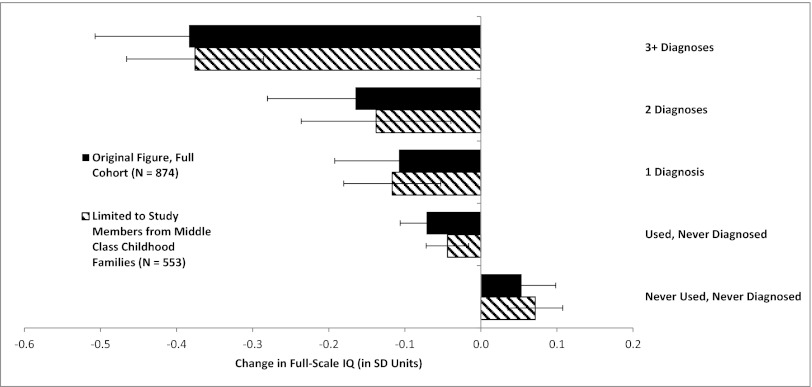
Rogeberg’s (2) idea and simulated data are interesting, but actual data exclude the possibility that the IQ drop we observed was attributable to SES differences. First, adolescent cannabis users are not concentrated in the lower classes; cannabis is used by young people from all social strata. In the Dunedin cohort, low SES did not significantly predict adolescent-onset cannabis dependence (χ2 = 1.15; P = 0.56); only 23% of the adolescent cannabis users were from low-SES families (whose breadwinners had low-skill occupations such as foodpacker), making it unlikely that low SES explains why adolescent-onset cannabis users’ IQs decline. Second, as previously reported (3), the IQ scores of children from low-SES families did not change from the beginning of schooling to adolescence, nor did they change from adolescence to adulthood (Fig. 1); in a critical test, low SES was unrelated to adolescent-to-adult IQ decline (r = −0.006; P = 0.86). These findings do not support the claim that low-SES children’s IQs are temporarily boosted while in school and subsequently decline to baseline. Third, unsurprisingly, when we statistically controlled for SES, the association between persistent cannabis use and IQ decline that we reported (β = −0.152; t = −4.45; P < 0.0001) remained unaltered (β = −0.158; t = −4.58; P < 0.0001). We further restricted our analysis to study members who grew up in middle class families (whose breadwinners had occupations such as building inspector, aircraft mechanic), excluding low-SES families, as well as high-SES families (professional occupations such as dentist), thus precluding potential for low-SES confounding. The association between persistent cannabis use and IQ decline remained unaltered (β = −0.155; t = −3.61; P = 0.0003) (Figs. 2 and 3). Finally, we reported many different mental functions, including executive function, memory, processing speed, perceptual reasoning, and verbal comprehension (1). Some of these abilities are more susceptible to SES-related effects than others. If decline were attributable to SES, we would expect to see worse decline in those tests. The data did not fit this pattern, further excluding the possibility that the IQ drop is attributable to SES alone.
Fig. 1.
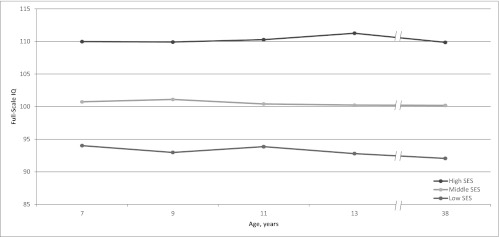
Mean IQ scores for children from low, as well as middle and high, SES backgrounds in the Dunedin cohort. The figure does not support the claim that their IQs were boosted by schooling and dropped thereafter.
Fig. 2.
Change in full-scale IQ (in SD units) from childhood to adulthood as a function of the number of study waves between ages 18–38 y for which a study member met criteria for cannabis dependence. The black bars show the results for the full cohort, from our original report (see figure 1 ref. 1). The striped bars show the same pattern of association when the same analysis was restricted to study members from middle class SES backgrounds.
Fig. 3.
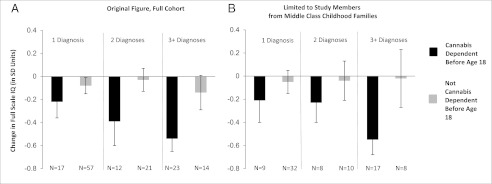
Change in full-scale IQ (in SD units) from childhood to adulthood among study members with one, two, or three or more diagnoses of cannabis dependence as a function of age of onset of cannabis dependence. Panel A reproduced from ref. 1. (A) Results for the full cohort, from our original report (see figure 2 in ref. 1). (B) Results when the analysis is restricted to study members from middle class SES backgrounds. Panel B shows the same pattern of association as panel A.
In a parallel fashion, Daly (4) suggests that conscientiousness, one of the Five-Factor Model personality traits (5), may explain the cannabis–IQ decline association. Daly’s idea is interesting, although his own test in the National Child Development Study disconfirmed his thesis about conscientiousness. Daly’s test was hampered by two shortcomings in his data. His cannabis-exposure variable does not ascertain the essential features of exposure in our study, young age of onset or persistence. His predictor, personality, was measured after cannabis use (at age 50 y), placing putative cause after effect, in the wrong time sequence. Fortunately, the Dunedin Study is better able to test Daly’s idea. Our previously published measure of childhood self-control (6), a precursor of conscientiousness, was assessed before cannabis use began. It was unrelated to IQ decline (r = −0.034; P = 0.31). We evaluated the association between cannabis use and IQ decline, controlling for childhood self-control. The association between persistent cannabis use and IQ decline that we reported (β = −0.152; t = −4.45; P < 0.0001) remained unaltered (β = −0.151; t = −4.38; P = 0.0001). These tests exclude the possibility that the IQ drop is attributable to initial differences in conscientious personality.
Observational studies like ours cannot prove causation, and yet many important research questions, including whether cannabis alters cognitive function, are intractable to experimentation. It is unethical to randomly assign youngsters to use cannabis for years, to test whether such use impairs mentation. However, even if experimentation were ethical, it could merely show that cannabis has potential capacity to impair cognition. Only a population-representative observational study tests whether cannabis actually is impairing cognition in the real world and how much (7). Certain methodological strategies can help observational studies examine causal hypotheses (8). None of these strategies is sufficiently persuasive, but each augments inference. By incorporating them, we aimed to push the Dunedin Study as far toward causal inference as possible, while acknowledging in our paper the inherent limits of its correlational design. First, our prospective design established temporal sequence: the putative cause, cannabis, preceded the putative outcome, cognitive function. Second, we tested for dose–response contingency; the younger the cannabis user and the more years of use, the worse the cognitive outcome. Third, we compared each participant’s cognitive scores at age 38 y to their former scores on the same tests taken up to age 13 y, a within-individual change design that controls for any and all influences occurring during childhood. Scores decreased from childhood testing to repeat testing at age 38 y among young-onset persistent cannabis users but not among age peers. Fourth, we checked that the finding was not limited to the way we measured cognitive outcomes; persistent cannabis users’ friends and family also noticed the cognitive problems. Fifth, we ruled out plausible artifactual explanations; the effect of cannabis on cognition was not an artifact of other substances abused, schizophrenia, or current cannabis use. Finally, we attempted to rule out rival causal explanations, i.e., factors occurring after age 13 y that might account for the IQ drop. Many young cannabis users opted out of education, but that did not account for their IQ drop. It is imperative to rule out alternative explanations in observational studies, but doing so must be justified by a theoretical rationale; in fact, statistical control for confounders “is not, contrary to the usual belief, ‘playing it safe’, since under several plausible assumptions such control will generate misleading results” (9). In any event, SES and conscientiousness were raised as potential explanations (2, 4), but here we show they did not account for the finding.
Footnotes
The authors declare no conflict of interest.
References
- 1.Meier MH, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109(40):E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogeberg O. Correlations between cannabis use and IQ change in the Dunedin cohort are consistent with confounding from socioeconomic status. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1215678110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moffitt TE, Caspi A, Harkness AR, Silva PA. The natural history of change in intellectual performance: Who changes? How much? Is it meaningful? J Child Psychol Psychiatry. 1993;34(4):455–506. doi: 10.1111/j.1469-7610.1993.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 4.Daly M. Personality may explain the association between cannabis use and neuropsychological impairment. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1218571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspi A, Roberts BW, Shiner RL. Personality development: Stability and change. Annu Rev Psychol. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- 6.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Academy of Medical Sciences Identifying the Environmental Causes of Disease: How Should We Decide What to Believe and When to Take Action? 2007. (Academy of Medical Sciences, London). Available at www.acmedsci.ac.uk/p48prid50.html. Accessed January 14, 2013.
- 8.Susser E, Schwartz S, Morabia A, Bromet EJ. Psychiatric Epidemiology. Oxford: Oxford Univ Press; 2006. [Google Scholar]
- 9.Meehl PE. High school yearbooks: A reply to Schwartz. J Abnorm Psych. 1971;77(2):143–148. doi: 10.1037/h0031999. [DOI] [PubMed] [Google Scholar]