Abstract
Olfactory receptors are G protein-coupled receptors that mediate olfactory chemosensation and serve as chemosensors in other tissues. We find that Olfr78, an olfactory receptor expressed in the kidney, responds to short chain fatty acids (SCFAs). Olfr78 is expressed in the renal juxtaglomerular apparatus, where it mediates renin secretion in response to SCFAs. In addition, both Olfr78 and G protein-coupled receptor 41 (Gpr41), another SCFA receptor, are expressed in smooth muscle cells of small resistance vessels. Propionate, a SCFA shown to induce vasodilation ex vivo, produces an acute hypotensive response in wild-type mice. This effect is differentially modulated by disruption of Olfr78 and Gpr41 expression. SCFAs are end products of fermentation by the gut microbiota and are absorbed into the circulation. Antibiotic treatment reduces the biomass of the gut microbiota and elevates blood pressure in Olfr78 knockout mice. We conclude that SCFAs produced by the gut microbiota modulate blood pressure via Olfr78 and Gpr41.
Keywords: GPCR, MOL2.3, MOR18-2, OR51E2
Olfactory receptors (ORs) are seven transmembrane G protein-coupled receptors (GPCRs) that function as chemosensors in the olfactory epithelium (OE), where they detect exogenous chemical ligands, referred to as odorants (1). ORs also play important roles outside of the OE, serving as specialized chemosensors in a variety of tissues (2, 3). We have recently demonstrated that major components of the olfactory signaling pathway are present in the kidney, where they play important functional roles in the regulation of both glomerular filtration rate (GFR) and renin release (4). In addition to the olfactory G protein Golf and the olfactory adenylate cyclase AC3, we reported that at least six members of the OR gene superfamily are expressed in renal tissue. To explore further the role that OR signaling plays in governing renal and systemic physiological processes, we first determined the ligand profile for one of the renal ORs, olfactory receptor 78 (Olfr78). Olfr78 is a bona fide OR that is expressed in olfactory sensory neurons (5). We find that Olfr78 functions as a receptor for short chain fatty acids (SCFAs) and in particular, for acetate and propionate.
A growing body of evidence indicates that the gut microbiota exerts important influences on the physiology of their mammalian hosts by signaling through metabolic byproducts such as SCFAs, which enter the bloodstream via colonic absorption (6–9). Both adiposity (10) and inflammatory responses (11, 12) are modulated by SCFAs produced by the microbiota. These effects are mediated via SCFA signaling through the G protein-coupled receptors Gpr41 and 43, which are expressed in adipocytes, neutrophils, and sympathetic ganglia (13). Data from ex vivo studies indicate that SCFAs also induce vasodilation in both rodents and humans (14, 15). Furthermore, the presence of acetate in hemodialysis solutions can induce hypotension (16, 17). Intriguingly, a previous study of human populations living in Asia (China and Japan) and Europe (United Kingdom) showed a direct association between urinary formate, a SCFA generated by microbial fermentation of dietary polysaccharides, and blood pressure (18); the signaling pathways and mechanisms underlying this association have not been delineated. In addition, many human studies have examined the effects of various types of dietary fiber on BP reduction (reviewed in ref. 19).
Here, we show that Olfr78 is expressed in smooth muscle cells of the vasculature, including the renal afferent arteriole. The afferent arteriole, part of the juxtaglomerular apparatus (JGA) of the kidney, is responsible for mediating the secretion of renin, an enzyme that plays a key role in the regulation of body fluid volume and blood pressure (BP). We use Olfr78−/− and Gpr41−/− mice and treatment with antibiotics to demonstrate that SCFA receptors exert significant modulatory effects on renin secretion and vascular tone, and that two major determinants of systemic BP are modulated in response to signals generated via gut microbes. The present study extends the list of important physiological processes that are modulated by SCFA receptors, expands the SCFA receptor family to include an OR, and describes a form of cross-talk between the gut microbiota and the renal–cardiovascular system that may be relevant to the pathogenesis and treatment of hypertension.
Results
Localization of Olfr78.
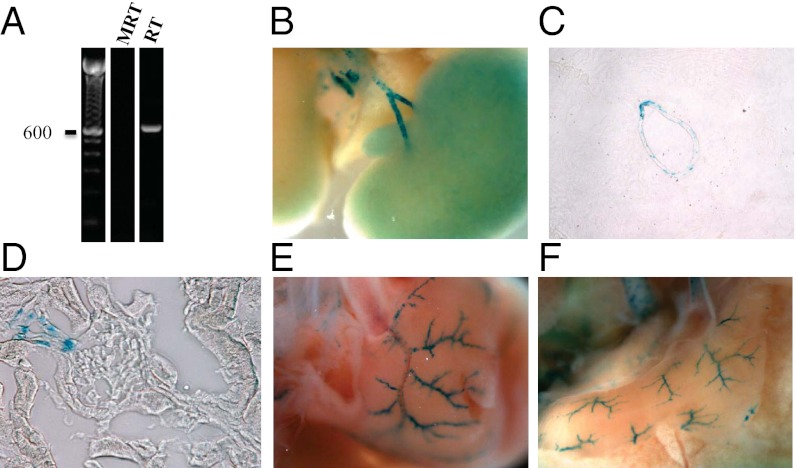
Olfr78 is one of six ORs whose expression we detected in the kidney (4). As shown in Fig. 1A, Olfr78 expression is detectable by RT-PCR analysis of total kidney RNA. We identified the cell types that express Olfr78 within the kidney using a mouse model (5) in which the gene encoding Olfr78 was replaced by β-galactosidase (whose expression is driven by the native Olfr78 promoter). β-Galactosidase staining in the Olfr78−/− mice revealed localization of Olfr78 gene expression to the major branches of the renal artery (Fig. 1 B and C) and the juxtaglomerular afferent arteriole (Fig. 1D). Intriguingly, Olfr78 expression in small resistance vessels in the kidney was restricted to cells of the juxtaglomerular afferent arteriole, which mediate renin secretion. β-Galactosidase staining was never observed in wild-type littermates. Staining in larger vessels colocalized with a marker for smooth muscle cells (smooth muscle actin, SMA), but did not colocalize with the neuronal cell marker tyrosine hydroxylase (Fig. S1 A and B). Some cells were positive for SMA but negative for β-galacatosidase (Fig. S1A), indicating that only a subset of smooth muscle cells express Olfr78.
Fig. 1.
Olfr78 is expressed in large renal vessels, renal afferent arterioles, and extrarenal vascular beds. Olfr78 mRNA is detectable in whole kidney by PCR (product was sequenced to confirm identity) (A). Olfr78 expression is localized to large renal vessels by β-galactosidase staining in Olfr78−/− mice (B and C, 15× magnification). In addition, β-galactosidase signal is found in renal afferent arterioles (D, 100×) and in small resistance vessels in a variety of other tissues, such as the heart (E) and the diaphragm (F). Also see Fig. S1.
In addition to the kidney, we examined β-galacatosidase staining in a large number of other tissues in Olfr78−/− mice, including OE, vomeronasal organ (VNO), heart, skeletal muscle, brain, testes, large intestine, bladder, lung, liver, pancreas, spleen, and fat. As shown previously, Olfr78 was expressed in the OE and VNO (20). It was also expressed in smooth muscle cells of small blood vessels in a variety of tissues including: heart (Fig. 1E), diaphragm (Fig. 1F), skeletal muscle (Fig. S1C), and skin. In the heart, esophagus, and stomach, we also observed staining in axons of autonomic neurons and neurons of the enteric plexus, respectively (Fig. S1 D and E).
Deorphanization of Olfr78.
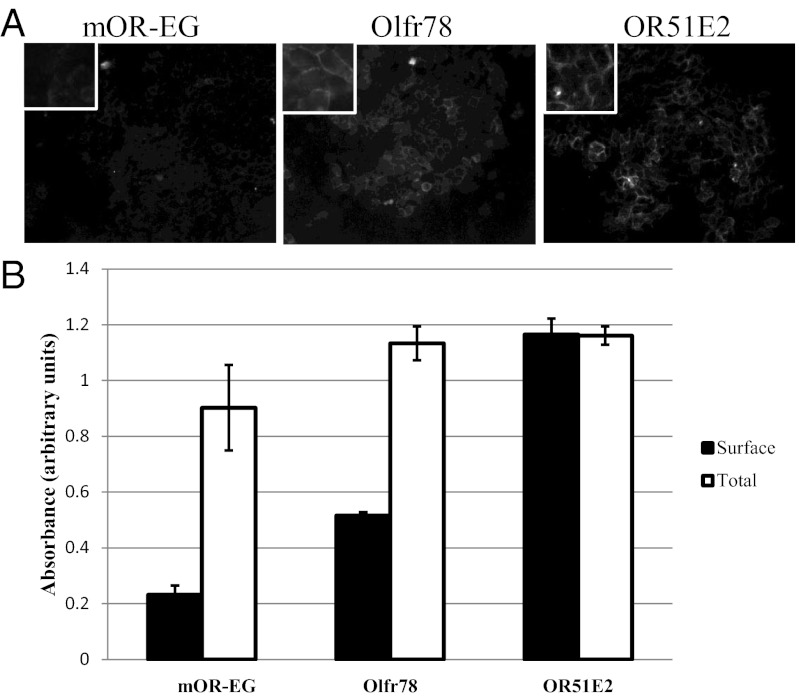
To elucidate the function of Olfr78, we sought to identify the class of ligands to which it responds. For this analysis to succeed, the receptor must traffic to the cell surface when expressed in a cell culture system. Many ORs fail to properly traffic to the cell surface when expressed heterologously, even when coexpressed with a variety of receptor chaperone proteins (21, 22). Therefore, we first assayed the ability of Olfr78 to reach the cell surface in transfected cells using two different methods—surface immunofluorescence and surface ELISA. We prepared Flag-tagged full-length constructs encoding both Olfr78 and its human homolog, OR51E2, and expressed them in HEK 293T cells. mOR-EG (mouse olfactory receptor EG), an OR that has been previously reported to reach the cell surface (23) was tested in parallel. As shown in Fig. 2 A and B, whereas surface expression of mOR-EG (anti-Flag) was comparatively weak, Olfr78 and OR51E2 exhibited strong expression on the surfaces of HEK 293T cells, even when expressed in the absence of receptor chaperone proteins.
Fig. 2.
Olfr78 and its human homolog, OR51E2, traffic to the cell surface when expressed in transfected HEK cells, as shown by surface immunofluorescence (A, 20×; Inset shows higher magnification) and ELISA (B). mOR-EG, which traffics to the surface weakly, is shown for comparison.
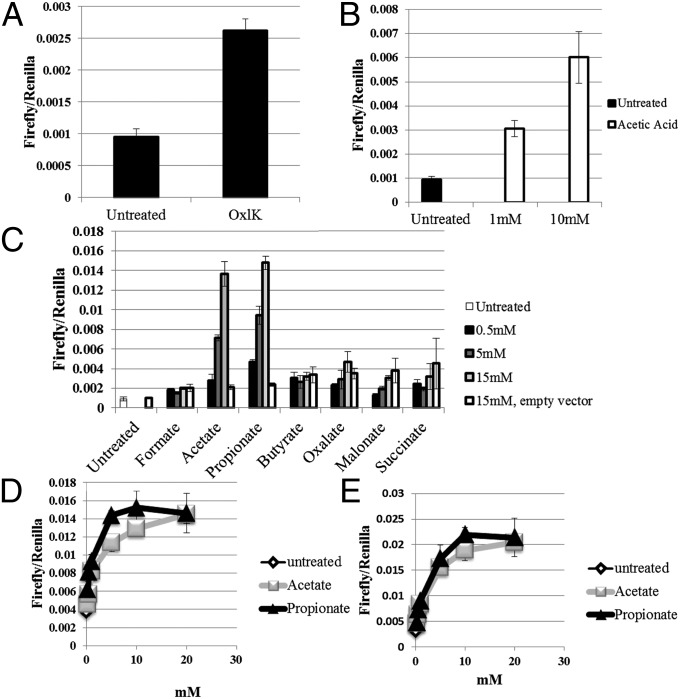
We made use of a luciferase-based reporter assay (24) in which OR-ligand binding produces an increase in cAMP that in turn drives cAMP response element-dependent expression of luciferase. We tested Olfr78 using odorant mixtures designed to cover a wide array of chemical groups and found a response for Olfr78 only to a mixture “OxlK” (Fig. 3A; OxlK is described in Methods). Of the individual components of OxlK, Olfr78 responded only to acetic acid (Fig. 3B). A wide array of compounds with chemical structures similar to acetic acid were then tested, some of which are shown in Fig. 3C. We found that Olfr78 responded only to acetate and propionate. Dose–response curves (Fig. 3 D and E) showed that the human (OR51E2) and mouse orthologs behave similarly (Olfr78: EC50 = 2.35 mM for acetate and 0.92 mM for propionate; OR51E2: EC50 = 2.93 mM for acetate and 2.16 mM for propionate). It is worth noting that previous reports have documented that plasma concentrations of these compounds range between 0.1 and 10 mM (11, 12, 25).
Fig. 3.
Screening for ligands showed that Olfr78 responded only to mix OxlK (A), and that the component of OxlK eliciting a response was acetic acid (B). A screen using chemically similar compounds showed that Olfr78 responded to acetate and propionate, but not to other related compounds (C). Dose–response curves for Olfr78 and OR51E2 are shown in D and E, respectively.
SCFA and Renin Release.
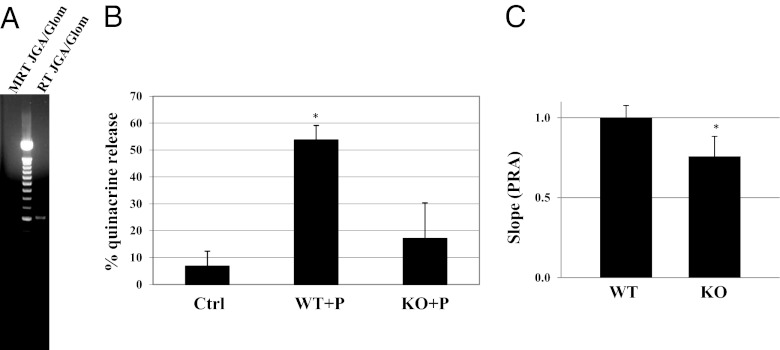
The renin–angiotensin system is a key participant in BP control. We have previously shown that disrupting the olfactory signaling pathway in the kidney causes dysregulation of plasma renin levels (4). The renal JGA, which resides adjacent to the glomerulus, synthesizes and secretes renin. Renal glomeruli with attached JGA were dissected from wild-type mouse kidneys and RNA was extracted. In agreement with the pattern of β-galactosidase staining (Fig. 1D), RT-PCR demonstrated that Olfr78 is expressed in JGA/glomeruli (Fig. 4A). Additional PCR reactions (Fig. S2) demonstrated expression of mRNAs encoding AC3 and Golf, which are downstream signal transducing components of the olfactory signaling machinery, in cells present in the isolated JGA. We have previously shown that AC3 and Golf are present in macula densa cells, an epithelial cell type that is a component of the JGA (4).
Fig. 4.
Olfr78 expression is detectable by PCR (A) in a preparation of microdissected glomeruli/JGAs (expected size: 614 nt; product was sequenced to confirm identity). Propionate (P) induces quinacrine release (which is a surrogate for renin release) in wild-type but not Olfr78−/− animals (B). PRA levels (plasma renin activity, normalized ex vivo to wild-type and determined as the slope of the time course of fluorescent product production in a kinetic assay) (27, 28) are significantly reduced in Olfr78−/− mice (C). * indicates statistical significance.
To determine if propionate modulates renin release, renin-containing granules of the afferent arteriole were labeled with quinacrine in dissected JGA/glomeruli. The disappearance of quinacrine over time was measured as an index of renin release (26, 27). Addition of 10 mM propionate to the bathing solution of JGA/glomeruli from wild-type mice resulted in a decrease in cell-associated quinacrine fluorescence. This effect was absent in JGA/glomeruli from Olfr78−/− mice (Fig. 4B). Consistent with this result, plasma renin measurements (27, 28) revealed that Olfr78−/− mice fed a standard chow diet rich in plant polysaccharides have significantly lower plasma renin levels than wild-type mice fed the same diet (Fig. 4C, P < 0.05). To assess whether these decreased renin levels are correlated with reduced baseline blood pressure, we measured mean arterial pressure (MAP) by carotid artery cannulation in anesthetized animals. The results indicated that Olfr78-KO mice had a MAP of 81.4 ± 2.8 mmHg (n = 9), whereas wild-type littermates had MAP of 94.5 ± 2.4 mmHg (n = 7) (P < 0.005).
SCFA and Blood Pressure.
The effects of propionate on renin release suggest that this SCFA exerts a chronic hypertensive effect on the complex array of pathways that contribute to the regulation of blood pressure. We next examined whether propionate administration also exerts acute effects on BP. All mice were maintained on a plant polysaccharide-rich chow.
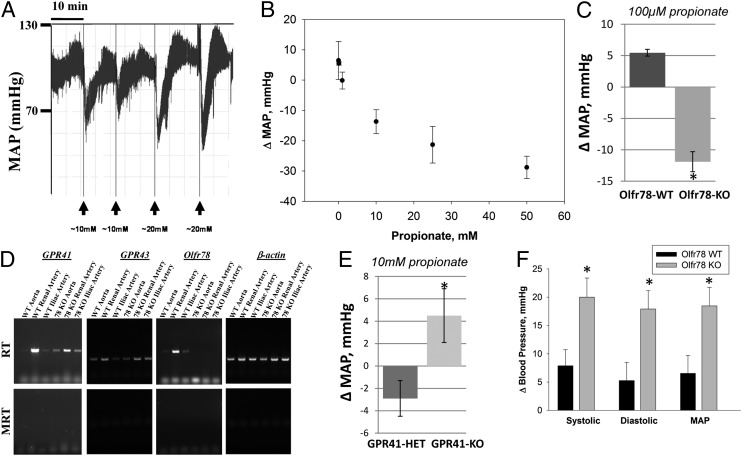
Propionate administration caused a large, rapid, and reproducible drop in BP (Fig. 5A)—a decrease of ∼20 mmHg that occurred over 1–2 min and recovered within ∼5 min. Fig. 5B demonstrates that this response is dose dependent. Olfr78−/− mice also manifest a hypotensive response to propionate, indicating that one or more other receptors likely mediate this response. As illustrated in Fig. 5C, Olfr78−/− mice are considerably more sensitive to the hypotensive effects of propionate than are their wild-type littermates; whereas 100 μM propionate produced a 5.5 ± 0.5 mmHg increase in the blood pressures of wild-type animals (Fig. 5 B and C) (n = 4), it induced a fall of 11.9 ± 1.6 mmHg in Olfr78−/− mice (n = 7) (P < 0.000013).
Fig. 5.
Propionate causes a drop in BP in wild-type animals that is both reproducible (A) and dose dependent (B). In Olfr78−/− mice, this response is accentuated at low propionate doses (C). In addition to Olfr78, SCFA receptors Gpr41 and Gpr43 are also expressed in the vasculature, as revealed by RT-PCR analysis of mRNA prepared from iliac arteries, renal arteries, and aortas of wild-type and Olfr78−/− mice (D). In Gpr41−/− mice, the response to propionate is blunted. Whereas 10 mM propionate produces a hypotensive effect in Gpr41+/− mice, no such hypotensive effect is detected in the Gpr41−/− mice (E). Treatment with orally administered antibiotics produced a marked reduction in microbial biomass in the gut (Figs. S4 and S5). This reduction was associated with significantly increased systolic (sys), diastolic (dias), and mean arterial blood pressure (MAP) in Olfr78−/− animals, but not in wild-type animals (F) (P < 0.04 vs. wild type). * indicates statistical significance.
Two other SCFA GPCR receptors exhibit ligand response profiles similar to that of Olfr78: Gpr41 and Gpr43 (29). Therefore, we screened several vessels (renal artery, aorta, and iliac artery) from both wild-type and Olfr78−/− mice by RT-PCR to assay for Gpr41, Gpr43, and Olfr78 expression. All three G protein-coupled receptors were expressed in all three arteries examined, with Gpr41 and Olfr78 appearing to be relatively enriched in renal artery (Fig. 5D). We also found that AC3 and Golf expression was detectable in aorta and renal artery but not iliac artery by RT-PCR (Fig. S3).
We next assessed the effects of propionate on BP in Gpr41−/− and heterozygous Gpr41+/− littermates (10) that had been maintained on a plant polysaccharide-rich diet. Whereas 10 mM propionate (a concentration at the high end of the physiologically relevant range) (12) produced a slight 2.9 ± 1.6 mmHg hypotensive response in Gpr41+/− heterozygotes (n = 8), the same propionate dose produced a modest 4.5 ± 2.4 mmHg hypertensive response (not seen with an equal volume of normal saline) in Gpr41−/− animals (Fig. 5E) (n = 6) (P < 0.035), although a hypotensive response to propionate was observed in these animals at higher, supraphysiological doses of propionate. Taken together, these data indicate that Gpr41 contributes to the hypotensive effects of propionate, whereas Olfr78 functions to raise BP and to antagonize the hypotensive effects of propionate.
Blood Pressure Regulation After Antibiotic Treatment.
To investigate whether the role of Olfr78 in BP regulation is dependent on the gut microbiota, wild-type and Olfr78−/− mice were treated with antibiotics for 3 wk (30) and BP was measured by tail cuff plethysmography 5 d per week both before and after antibiotic treatment. Mice were weighed 5 d per week throughout the experiment, and no significant differences were seen (either before and after antibiotics or between WT and KO). Fecal pellets were collected before (−11 d, −7 d, and −5 d) and during antibiotic administration (17 d, 20 d, and 21 d). We quantified fecal DNA content—a biomarker of microbial productivity (biomass) and sequenced the variable region 4 (V4) of bacterial 16S rRNA genes present in each fecal sample. 16S rRNA sequence datasets were analyzed using UniFrac, a tool that measures phylogenetic similarity between microbial communities based on the degree to which their taxa share branch length on a 16S rRNA-based bacterial tree of life (31). Treatment with an orally administered antibiotic mixture composed of vancomycin, ampicillin, and neomycin for 3 wk resulted in a dramatic reduction in fecal microbial biomass and major changes in the structure of the microbiota (Figs. S4 and S5). 16S rRNA sequencing analysis revealed no significant differences in fecal microbiota composition between wild-type and Olfr78−/− mice before or at the end of the antibiotic treatment (Figs. S4 and S5). However, antibiotic treatment resulted in a significant increase in BP in Olfr78−/− animals as measured by tail cuff plethysmography, but had no effect in wild-type mice (MAP rose from 86 to 104 mmHg (P < 0.007; t test), systolic BP from 99 to 119 mmHg (P < 0.004), and diastolic BP rose from 79 to 97 mmHg (P < 0.01); values for wild-type mice were: MAP: 107 vs. 115 mmHg, P = 0.23; systolic BP 92 vs. 98 mmHg, P = 0.10; and diastolic: 84 vs. 89 mmHg, P = 0.35) (Fig. 5F). In addition, no significant changes in heart rate were observed in both groups of mice in response to antibiotic treatment. These data led us to conclude that products of the microbiota, likely acetate and propionate, influence BP and that this effect is mediated in part by Olfr78.
Discussion
ORs play important roles as specialized chemosensors outside the OE (2). We have recently demonstrated that components of the olfactory signaling pathway are present in the kidney and that they participate in regulating GFR and renin release (4). To better understand the role of olfactory signaling in the kidney, we examined the role of Olfr78, an OR expressed in renal tissue, by investigating this receptor’s ligand profile, localization, and physiological function.
Although most ORs fail to traffic beyond the endoplasmic reticulum when heterologously expressed in cultured cell lines, Olfr78 and its human ortholog (OR51E2) both traffic to the plasmalemma, allowing us to examine its odorant-binding profiles. We found that two SCFAs, acetate and propionate, are ligands for Olfr78 and OR51E2. Remarkably, Olfr78 is unresponsive to other SCFAs, indicating that it is highly specific for these two compounds.
Neuhaus et al. previously reported that OR51E2 is activated by several androgens and by β-ionone (32). We were unable to detect a response to β-ionone for Olfr78 or OR51E2 in our luciferase-based reporter assay, and β-ionone also failed to elicit changes in BP when delivered intravenously. It is possible that this difference stems from the different methods used; Neuhaus et al. (32) used a calcium imaging method to detect odorant responses, whereas we assayed cAMP (via luciferase). We did, however, confirm in a separate assay that activation of Olfr78 and OR51E2 by acetate or propionate produces a signal detectable by calcium imaging. Our results agree with those of Saito et al. (33) who recently reported that among 93 odorants tested, OR51E2 responded only to propionate (using a luciferase-based cAMP reporter assay).
We localized Olfr78 to the renal juxtaglomerular afferent arteriole as well as to smooth muscle cells of other arteries and to a subset of nerves in the heart and in the gut. Neuronal expression of Olfr78 has been reported previously (34, 35), and in both our study and in this previous work, a “patchy” pattern of Olfr78 expression in blood vessels was seen. This staining pattern was previously attributed to nerve endings (34). Although Olfr78 is clearly expressed in autonomic nerves elsewhere (34, 35) (see, for example, Fig. S1D), the vascular distribution of Olfr78 did not correspond to that of the neuronal marker tyrosine hydroxylase (TH), but instead colocalized with SMA. Thus, Olfr78 is expressed both in nerves and in smooth muscle cells lining vessels. The localization of Olfr78 to the JGA, to both large and small blood vessels, and to autonomic nerves in the heart makes it well positioned to play a role in BP regulation.
We find that propionate causes renin release from isolated juxtaglomerular apparati ex vivo and that this response is absent in Olfr78−/− mice (Fig. 4B). We also show that at baseline, Olfr78 KO animals tend to manifest lower blood pressure, an effect that is consistent with the lower plasma renin levels which we detect in Olfr78 null mice (Fig. 4C). These observations indicate that Olfr78 plays a unique role in mediating secretion of renin in response to SCFAs. Renin release from JGA cells is stimulated by production of cAMP and inhibited by increases in cytosolic calcium levels (36, 37). Thus, the capacity of Olfr78 to induce elevations of cytosolic cAMP in response to SCFAs (Fig. 3), taken together with the fact that ORs natively signal via adenylate cyclase in the OE (38), is consistent with the possibility that activation of Olfr78 leads to renin release by stimulating cAMP production in juxtaglomerular cells.
We have previously shown that mice that do not express the AC3 isoform of adenylate cyclase also manifest reduced plasma renin levels, and that AC3 localizes to the macula densa (4). We find that AC3 expression can be detected in dissected juxtaglomerular apparatus preparations, which include JG cells, glomeruli, and macula densa cells (Fig. S2). However, although both Olfr78 and AC3/Golf are detected by PCR in dissected JGA preparations, they localize to separate cell populations [JG cells (Fig. 1D) and macula densa cells (4), respectively]. Because renin release by JGA cells appears to be dependent upon the calcium-inhibitable AC5 and/or -6 isoforms of adenylate cyclase (39), we conclude that the effects of AC3 knockout on plasma renin levels are not likely to be attributable to direct effects on JG cell cAMP levels. Rather, AC3 likely acts within macula densa cells to participate in the initiation of the paracrine signals that stimulate JG cell renin secretion (4).
We found that propionate administration lowers BP in a rapid, reproducible, and dose-dependent manner. Previous reports have documented plasma concentrations between 0.1 and 10 mM (11, 12, 25). Thus, the propionate dose responses that we observed in vivo (Fig. 5B) and in the Olfr78 luciferase assay (Fig. 3 D and E) correspond to physiological concentrations. At the higher end of the physiological range (10 mM), we see a hypotensive response in WT mice of −13.7 mmHg (±3.9). Although this response is transient, its magnitude is large enough to ensure that it would be physiologically relevant. Furthermore, it has been shown that transient changes in BP have the potential to “reset” baseline BP (40, 41) and thus to exert physiologically significant effects even after the acute effect has resolved. Whereas the effect of propionate on renin release is absent in Olfr78-deficient mice, the acute hypotensive effect of propionate is accentuated at low physiological doses in these animals, indicating that Olfr78 activation antagonizes the acute hypotensive effects of this SCFA. We believe that these data, together with the localization of Olfr78 to vascular smooth muscle in resistance beds, establish that the influence of Olfr78 on the acute blood pressure changes in response to propionate is likely due to its expression in the smooth muscle cells of resistance vessels. This implies that, both in the case of renin release and smooth muscle cell responses, propionate may stimulate Olfr78 to support BP and to counter the powerful hypotensive effects of propionate mediated through other receptors or pathways. A likely candidate for these other receptors may be Gpr41 and/or Gpr43, two previously characterized SCFA receptors (10–12) that we find to be expressed in the kidney and major arteries (Fig. S3). In mice lacking Gpr41, administration of propionate in a dose sufficient to produce a calculated serum concentration of 10 mM does not produce a hypotensive response, implying that at least some portion of the hypotensive effect of propionate is mediated by Gpr41. Thus, the data indicate that activating Olfr78 raises blood pressure, and activating Gpr41 lowers blood pressure. Importantly, Gpr41 responds in vitro to much lower doses of propionate (EC50 = ∼11 μM) (11, 29) than does Olfr78 (EC50 = ∼0.9 mM; Fig. 3). Thus, in the absence of Olfr78 the response to propionate that is driven by Gpr41 predominates and there is an exaggerated hypotension, even at doses as low as 100 μM. Conversely, in the absence of Gpr41, a dose at the high end of the physiological range that should maximally activate Olfr78 (10mM) results in modest hypertension.
It is worth noting that Olfr78 and Gpr41 appear to signal through different G protein α-subunits and different second messenger systems. The data presented here, and those of Saito et al. (33), demonstrate that Olfr78 can activate Gαs to induce cAMP production. Gpr41 and Gpr43 appear to activate Gαi (and/or Gαo) to decrease cAMP and to produce elevations in cytosolic calcium and reductions in cAMP (42). The fact that these receptors couple to distinct second messenger pathways may explain, at least in part, their apparently opposing effects on blood pressure in response to SCFA stimulation. Thus, the net physiological effects of SCFAs may be complex, as multiple SCFAs receptors are found in many of the same tissues and activate a variety of signaling pathways. Although the effect of propionate on blood pressure was observed in every animal tested, the precise time course of the response was subject to significant variability among animals. Thus, we were not able to determine whether, in addition to the concentration dependence of the blood pressure response, the time course of the response also differed in wild-type vs. null mice.
SCFAs are produced by fermentation of polysaccharides by the gut microbiota and enter the bloodstream primarily via the portal vein circulation (43–45). Given that gut microbes are the primary source of SCFAs in the plasma (25), we also assayed whether reducing the biomass of the gut microbiota with antibiotics modulates BP in Olfr78−/− mice. Addition of antibiotics to the drinking water caused a significant increase in systolic, diastolic, and mean BP in Olfr78-deficient mice, but did not significantly affect BP in wild-type littermates. Taken together, these data suggest that propionate and possibly acetate generated by the gut microbiota are able to modulate blood pressure through their effects on multiple receptors and pathways. Propionate- and perhaps acetate-mediated stimulation of Olfr78 increases BP, whereas these compounds act through Gpr41 to decrease BP. These opposing responses may produce a “buffering” effect to guard against wide swings in blood pressure as a consequence of normal, physiological variations in SCFA concentration. Consistent with this idea, Olfr78−/− mice appear to be more susceptible to the hypotensive effects of propionate. According to our model, when the ligand for both Olfr78 and Gpr41 is removed (via antibiotic treatment), this has little effect in a wild-type animal because the mutually antagonistic actions of both receptors are similarly inhibited, and these effects essentially cancel out. However, in an Olfr78−/− animal, propionate would be acting solely on Gpr41 to affect BP; therefore removing the source of this ligand would be expected to block the unopposed hypotensive effect of propionate and thus produce a substantial increase in BP.
The effects of the antibiotic treatment on BP in the Olfr78−/− mice are modest. However, when viewed in light of the extensive network of mechanisms that intersect to maintain BP within a very narrow range (46), it is remarkable that a chronic and significant perturbation in the BP set point can be achieved by perturbing the microbiota with antibiotics. It is interesting to note in this context that in Olfr78−/− mice treated with antibiotics, BP rose to values exceeding those measured in the wild-type mice at baseline. This observation suggests that other compensatory mechanisms are induced to maintain BP in the face of the unopposed hypotensive effects of SCFAs in Olfr78−/− mice, and that the effects of these SCFA-independent mechanisms are unmasked to produce hypertension when the SCFA source is removed. Because propionate did not modulate renin release from Olfr78−/− JGAs, the hypertension unmasked by antibiotics in these mice is likely to be mediated by changes in vascular resistance or cardiac output rather than by changes in renin secretion.
SCFA receptors, responding to signals from the microbiota, participate in many important physiological pathways in the host, including nutrient metabolism, adiposity, and inflammatory responses (10–12). The present study extends the list of important physiological processes that are modulated by SCFA receptors to include BP regulation and also expands the SCFA receptor family to include an OR. This cross-talk between the gut microbiota and the renal–cardiovascular system constitutes a unique pathway that may be relevant to the pathogenesis and treatment of hypertension.
Materials and Methods
RT-PCR.
RT-PCR was performed using standard protocols. Details of studies are provided in SI Materials and Methods.
β-Galactosidase Staining.
Cryosections were prepared from mouse kidneys that had been perfusion fixed in 4% (vol/vol) periodate-lysine-paraformaldehyde (PLP) (42). β-Galactosidase staining was performed using standard protocols (47). For whole-mount X-gal staining tissues were fixed in 4% (vol/vol) paraformaldehyde for 1 h, and then stained using standard protocols. When immunostaining was performed in concert with β-galactosidase staining, the chromagen stain was developed first, and immunostaining was then performed as previously described (4).
Surface Localization.
Surface immunofluorescence (nonpermeablized stain) and surface ELISA were performed as previously described (24, 48). Wells for surface ELISA were assayed in quadruplicate.
Luciferase Assay.
Luciferase assay was performed as previously described (24), with all treatments performed in triplicate. Odorant mixtures for initial testing of ORs were developed to cover a wide amount of odorant space. All mixes contained each compound at a final concentration of 0.3 mM. Three of the mixes were based, in part, on mixes used by Bozza et al. (49) and Ma and Shepherd (50) and were termed BzB (n-valeraldehyde, heptaldehyde, nonyl aldehyde), BzC (L-carvone, eugenol, and cinnamaldehyde), and MA (amyl acetate, 3-octanone, and acetophenone). These three mixes are expected to activate 26% of olfactory sensory neurons. Two additional custom mixes were used: Thi-Di (1,6-hexanedithiol, 1,2-ethanedithiol, 1-methyl-1propanethiol, 1,4-butanedithiol, and 2,3-butanedithiol) and OxlK (2,3-butanedione, pyruvaldehyde, acetic acid, 1,2-ethanedithiol, and 2-butanone).
In Vivo Studies.
All experiments were performed in accordance with the policies and procedures of the Yale Institutional Animal Care and Use Committee, the University of Southern California Institutional Animal Care and Use Committee, and the Johns Hopkins University Animal Care and Use Committee, as well as the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Olfr78−/− mice, initially generated by Bozza et al. (5), were purchased from Jackson Laboratories; Gpr41−/− mice have been previously described (10). Mice were housed with food and water ad libitum. Details of in vivo studies can be found in SI Materials and Methods.
Multiplex Sequencing of Amplicons Generated from Bacterial 16S rRNA Genes.
These studies are described in SI Materials and Methods.
Other Analyses.
The statistical significance of differences of measurements of various aspects of host physiology and ex vivo assays was determined by Student t test (P < 0.05 considered significant). EC50 values were calculated using Systat software (SigmaPlot).
Supplementary Material
Acknowledgments
The authors thank Dr. Kazushige Touhara (University of Toyko) for providing the mOR-EG construct, Dr. Hannah Chapin (University of Washington) for helpful advice regarding the ELISA assays, Daniel Gergen (Johns Hopkins University) for technical assistance with the tail-cuff blood pressure measurements, and Dr. Cynthia Sears (Johns Hopkins University) and Marty Meier (Washington University in St. Louis) for assistance with quantitation of fecal bacteria and V4-16S rRNA gene amplification. This work was supported by funding from the National Institutes of Health Grants DK081610 (to J.L.P.), DK64324 (to J.P.-P.), and DK17433 (to M.J.C.); and the Leducq Foundation (M.J.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215927110/-/DCSupplemental.
References
- 1.Buck L, Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 2.Spehr M, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299(5615):2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 3.Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell. 2009;17(5):649–661. doi: 10.1016/j.devcel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pluznick JL, et al. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA. 2009;106(6):2059–2064. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozza T, et al. Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron. 2009;61(2):220–233. doi: 10.1016/j.neuron.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arumugam M, et al. MetaHIT Consortium Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel BS, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 12.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura I, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci USA. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nutting CW, Islam S, Daugirdas JT. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am J Physiol. 1991;261(2 Pt 2):H561–H567. doi: 10.1152/ajpheart.1991.261.2.H561. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut. 1990;31(12):1391–1394. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keshaviah PR. The role of acetate in the etiology of symptomatic hypotension. Artif Organs. 1982;6(4):378–387. doi: 10.1111/j.1525-1594.1982.tb04130.x. [DOI] [PubMed] [Google Scholar]
- 17.Pagel MD, Ahmad S, Vizzo JE, Scribner BH. Acetate and bicarbonate fluctuations and acetate intolerance during dialysis. Kidney Int. 1982;21(3):513–518. doi: 10.1038/ki.1982.54. [DOI] [PubMed] [Google Scholar]
- 18.Holmes E, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453(7193):396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelton SP, et al. Effect of dietary fiber intake on blood pressure: A meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23(3):475–481. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 20.Lévai O, Feistel T, Breer H, Strotmann J. Cells in the vomeronasal organ express odorant receptors but project to the accessory olfactory bulb. J Comp Neurol. 2006;498(4):476–490. doi: 10.1002/cne.21067. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Echeverri F, Moyer BD. Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic. 2003;4(6):416–433. doi: 10.1034/j.1600-0854.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 22.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119(5):679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Von Dannecker LE, Mercadante AF, Malnic B. Ric-8B promotes functional expression of odorant receptors. Proc Natl Acad Sci USA. 2006;103(24):9310–9314. doi: 10.1073/pnas.0600697103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang H, Matsunami H. Evaluating cell-surface expression and measuring activation of mammalian odorant receptors in heterologous cells. Nat Protoc. 2008;3(9):1402–1413. doi: 10.1038/nprot.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B. 1987;86(3):439–472. doi: 10.1016/0305-0491(87)90433-0. [DOI] [PubMed] [Google Scholar]
- 26.Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol. 2009;20(5):1002–1011. doi: 10.1681/ASN.2008070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH. Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol. 2004;287(2):F329–F335. doi: 10.1152/ajprenal.00420.2003. [DOI] [PubMed] [Google Scholar]
- 28.Kang JJ, et al. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51(6):1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 30.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Lozupone C, Knight R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuhaus EM, et al. Activation of an olfactory receptor inhibits proliferation of prostate cancer cells. J Biol Chem. 2009;284(24):16218–16225. doi: 10.1074/jbc.M109.012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a Mammalian receptor repertoire. Sci Signal. 2009;2(60):ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber M, Pehl U, Breer H, Strotmann J. Olfactory receptor expressed in ganglia of the autonomic nervous system. J Neurosci Res. 2002;68(2):176–184. doi: 10.1002/jnr.10164. [DOI] [PubMed] [Google Scholar]
- 35.Rafalzik S, et al. Cholinergic signal transduction in the mouse sphenopalatine ganglion. Brain Res. 2008;1241:42–55. doi: 10.1016/j.brainres.2008.08.095. [DOI] [PubMed] [Google Scholar]
- 36.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70(4):1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, et al. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292(1):F27–F37. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- 38.Wong ST, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27(3):487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension. 2007;49(1):162–169. doi: 10.1161/01.HYP.0000250708.04205.d4. [DOI] [PubMed] [Google Scholar]
- 40.Tokarev D, Jezová D. Effect of nitric oxide inhibition on blood pressure and corticosterone responses in adult rats neonatally treated with glutamate. Physiol Res. 2000;49(Suppl 1):S87–S94. [PubMed] [Google Scholar]
- 41.Johnson RA, Freeman RH. Sustained hypertension in the rat induced by chronic blockade of nitric oxide production. Am J Hypertens. 1992;5(12 Pt 1):919–922. doi: 10.1093/ajh/5.12.919. [DOI] [PubMed] [Google Scholar]
- 42.Brown D, Sorscher EJ, Ausiello DA, Benos DJ. Immunocytochemical localization of Na+ channels in rat kidney medulla. Am J Physiol. 1989;256(2 Pt 2):F366–F369. doi: 10.1152/ajprenal.1989.256.2.F366. [DOI] [PubMed] [Google Scholar]
- 43.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters SG, Pomare EW, Fisher CA. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut. 1992;33(9):1249–1252. doi: 10.1136/gut.33.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78(6):1500–1507. [PubMed] [Google Scholar]
- 46.Guyton AC, Coleman TG, Granger HJ. Circulation: Overall regulation. Annu Rev Physiol. 1972;34:13–46. doi: 10.1146/annurev.ph.34.030172.000305. [DOI] [PubMed] [Google Scholar]
- 47.Nishio S, et al. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010;21(2):295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chapin HC, Rajendran V, Capasso A, Caplan MJ. Detecting the surface localization and cytoplasmic cleavage of membrane-bound proteins. Methods Cell Biol. 2009;94:223–239. doi: 10.1016/S0091-679X(08)94011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22(8):3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma M, Shepherd GM. Functional mosaic organization of mouse olfactory receptor neurons. Proc Natl Acad Sci USA. 2000;97(23):12869–12874. doi: 10.1073/pnas.220301797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.