Abstract
In temperate regions, the shortening day length informs many insect species to prepare for winter by inducing diapause. The adult diapause of the linden bug, Pyrrhocoris apterus, involves a reproductive arrest accompanied by energy storage, reduction of metabolic needs, and preparation to withstand low temperatures. By contrast, nondiapause animals direct nutrient energy to muscle activity and reproduction. The photoperiod-dependent switch from diapause to reproduction is systemically transmitted throughout the organism by juvenile hormone (JH). Here, we show that, at the organ-autonomous level of the insect gut, the decision between reproduction and diapause relies on an interaction between JH signaling and circadian clock genes acting independently of the daily cycle. The JH receptor Methoprene-tolerant and the circadian proteins Clock and Cycle are all required in the gut to activate the Par domain protein 1 gene during reproduction and to simultaneously suppress a mammalian-type cryptochrome 2 gene that promotes the diapause program. A nonperiodic, organ-autonomous feedback between Par domain protein 1 and Cryptochrome 2 then orchestrates expression of downstream genes that mark the diapause vs. reproductive states of the gut. These results show that hormonal signaling through Methoprene-tolerant and circadian proteins controls gut-specific gene activity that is independent of circadian oscillations but differs between reproductive and diapausing animals.
Keywords: reproductive diapause, photoperiodism, basic helix-loop-helix protein, oogenesis
To cope with adverse winter conditions, animals either migrate or minimize their metabolism and hibernate or diapause (1). Animals including insects anticipate these annual rhythms by measuring the changes in night or day length (i.e., photoperiod) through a seasonal clock whose mechanism has yet to be elucidated (2, 3). The hallmarks of diapause in insects such as the linden bug, Pyrrhocoris apterus, and the bean bug, Riptortus pedestris, include cessation of reproduction (4–6) and changes in the physiology of the digestive system (7) and the fat body (8). The arrest is induced by short days and results in small diapause ovaries. Conversely, long days promote ovarian maturation through the action of juvenile hormone (JH), produced by the corpora allata glands (9–11).
JH is an insect sesquiterpenoid that controls reproduction (12) and entry into metamorphosis (13). The connection between JH and reproductive diapause is well documented in various species (14). Application of the JH-mimicking analogue methoprene to diapausing P. apterus or R. pedestris bugs is sufficient to terminate diapause and induce ovarian growth (6, 15). Endogenous JH or added methoprene act through the Methoprene-tolerant (Met) protein to prevent premature metamorphosis in P. apterus juveniles (16). Met is a transcription factor of the basic helix–loop–helix Per-ARNT-Sim (bHLH-PAS) family (17), and it has been characterized as a JH receptor (18, 19). JH-dependent interaction between Met and another bHLH-PAS protein, FISC [synonymous to Taiman (Tai)_in Drosophila melanogaster; FlyBase], have been implicated in oogenesis of Aedes aegypti mosquitoes (20). Recently, A. aegypti Met and the bHLH-PAS circadian clock protein Cycle (Cyc) have been shown to dimerize and activate circadian rhythm-dependent gene expression in response to JH (21).
Whether photoperiodic regulation of seasonal diapause/reproduction timing involves the circadian clock is still debated (2, 22–24). Clinal polymorphism of the circadian gene timeless was observed in D. melanogaster (25, 26), and diapause in another drosophilid fly, Chymomyza costata, is altered by a timeless mutation (27, 28). However, whether these timeless mutations affect a central “photoperiodic clock” in the brain or compromise the execution of diapause in peripheral tissues remains unknown. A systemic RNAi-mediated knockdown of cyc in reproductive R. pedestris under long-day (LD) conditions switched the bugs into a diapause mode, whereas period (per) and cryptochrome (cry) RNAi terminated diapause and induced reproduction in adults experiencing short days (6, 29, 30). These data led the authors to propose that the three circadian genes, cyc, per, and cry, constituted the photoperiodic clock of R. pedestris (6, 29, 30). An alternative explanation is that the circadian clock genes have pleiotropic functions, one of which is to regulate the seasonal physiology by acting downstream of a presently undefined photoperiodic clock (22, 31).
To address the role of circadian genes in the regulation of diapause, we examined expression of circadian genes in the gut of reproductive and diapausing females of P. apterus, a species with robust and well characterized diapause biology (8, 32). We discovered an organ-autonomous regulatory feedback between Cryptochrome 2 (Cry2) and another circadian clock component, a basic leucine-zipper transcription factor, Par domain protein 1, isoform 1 (Pdp1iso1) (33). We show that cry2 represses Pdp1iso1 and triggers a diapause-specific genetic program in the gut, whereas Pdp1iso1 counteracts cry2 and promotes the reproductive state of this organ independently of the daily cycle. Induction of Pdp1iso1 and suppression of cry2 transcription by JH mimic require the JH receptor Met and the circadian proteins Clock (Clk) and Cyc. Therefore, our data indicate organ-autonomous, yet noncircadian, involvement of clock genes and hormonal signaling in diapause regulation.
Results
Differential Response of Circadian Clock Genes cry2 and Pdp1iso1 to Diapause.
Short day-length (SD) conditions induce reproductive diapause in P. apterus females (4), and the ovaries remain small without maturing oocytes (Fig. 1A). To examine whether circadian clock genes are involved in diapause regulation, we isolated selected P. apterus orthologs including cyc, Clk, Pdp1, and cry genes (SI Methods). Our phylogenetic analysis showed that, like some other insects (34), P. apterus possesses type 2 Cryptochrome (Cry2) that is similar to mammalian Cry (35) rather than to the light-sensitive type 1 Cryptochrome of D. melanogaster (Fig. S1). We then compared expression of the circadian clock genes in the gut between reproductive and diapausing adult females. We focused on the gut for two main reasons: (i) the gut is the major organ of nutrient uptake that is prerequisite to oogenesis and (ii) transcripts of clock genes such as cry2 do not show circadian oscillation in P. apterus guts, making comparisons of gene expression levels practical.
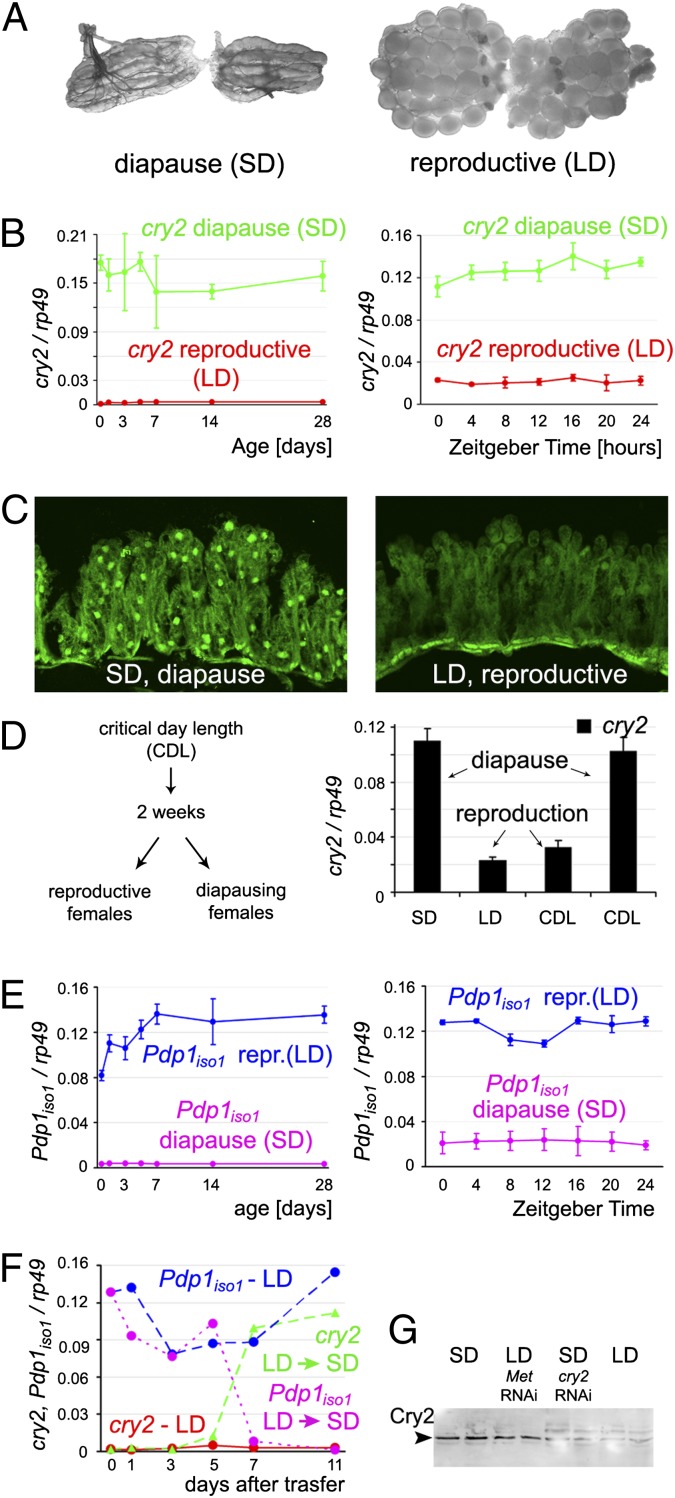
Fig. 1.
The cry2 and Pdp1iso1 genes are inversely regulated under diapause and reproductive conditions in the gut of P. apterus females. (A) Reproductive diapause in P. apterus females. Ovaries are small and contain no maturing oocytes under SD condition (Left) in which females naturally lack JH. Oogenesis commences upon extending the photoperiod (i.e., LD; Right) or after JH mimic treatment. (B) Levels of cry2 mRNA in the gut remain high under diapause and low during the reproductive phase (Left) irrespective of daily fluctuations (Right). (C) The Cry2 protein is detected in cell nuclei of the gut epithelium of diapause females (Left) but not in reproductive females. (D) CDL experiment (Methods) shows that cry2 expression in the gut depends on the reproductive state rather than on the photoperiod. Females that remained nonreproductive (i.e., diapause) after 2 wk of exposure to CDL (16.5 h light, 7.5 h dark) expressed high cry2 levels, whereas those that became reproductive under the same CDL conditions showed down-regulation of cry2. (E) Expression of Pdp1iso1 follows a pattern opposite to that of cry2. (F) Expression of cry2 and Pdp1iso1 transcripts switched to the diapause mode between 5 and 7 d after transfer from LD to SD photoperiod. (G) Immunoblot shows that expression of Cry2 protein in the gut of females experiencing diapause was depleted by cry2 RNAi. Low Cry2 levels occurring in reproductive females increased upon Met RNAi. Levels of mRNAs in B, D, E, and F were determined by using qRT-PCR and were normalized to rp49 expression; data are mean ± SEM from three independent experiments (error bars omitted in F for clarity).
We found, on average, a sixfold enrichment of cry2 mRNA in the guts of diapause females exposed to the SD condition relative to reproductive females experiencing long-day (LD) conditions (Fig. 1B). This difference persisted for at least 4 wk of monitoring and was unaffected by mild daily fluctuations in cry2 expression. Northern hybridization confirmed this result (Fig. S2). Accordingly, the Cry2 protein was abundant in the gut cells of diapause but not of reproductive females (Fig. 1 C and G). Because cry2 expression correlated to the day length and the reproductive status, it was important to determine which of the two factors was causal. We therefore measured cry2 mRNA in females kept under a critical day length (CDL), i.e., conditions allowing approximately 50% of individuals to enter diapause while leaving the other half reproductive. This experiment showed that up-regulation of cry2 in the gut depended on diapause, not on the photoperiod (Fig. 1D).
In a striking contrast to cry2, expression of isoform 1 of Pdp1 (Pdp1iso1) followed a pattern exactly opposite, being high in the guts of reproductive females and minimal under diapause during the 4-wk testing period (Fig. 1E). A second Pdp1 isoform, originating from an alternative transcriptional start, was not expressed differentially between reproductive and diapause animals (Fig. S3). As was the case with cry2, daily changes in Pdp1iso1 mRNA levels were negligible relative to the major difference associated with the reproductive status (Fig. 1 B and E).
Finally, we tested whether an experimental switch from reproduction to diapause, achieved by shortening the day length, was sufficient to invert the patterns of cry2 and Pdp1iso1 expression in the gut. Remarkably, 5 to 7 d after transferring reproductive females from LD to SD conditions, Pdp1iso1 mRNA declined and cry2 transcription increased to match the diapause pattern (Fig. 1F). These results show that Pdp1 and cry2 in the gut respond to the reproductive/diapause status in opposite manner.
Circadian Genes and JH Signaling Regulate Pdp1 and cry2 Expression.
To explore how Pdp1iso1 and cry2 are regulated, we subjected P. apterus females to RNAi against selected transcription factors that are known to act within the circadian clock or in JH signaling. Fig. S4 shows that RNAi knockdown of each individual transcript in the gut was highly efficient. Genes encoding the bHLH-PAS proteins Clk (36) and Cyc (37) and the JH receptor Met all proved to be important for Pdp1iso1 and cry2 expression. In contrast, RNAi targeting two other members of the bHLH-PAS family, Tai and Tango (Tgo), or the circadian protein Clockwork orange (Cwo), affected neither Pdp1iso1 nor cry2 expression (Fig. 2 A and B). Tai (also known as FISC or steroid receptor coactivator) has been implicated as a partner of Met in JH reception (20, 38) and in JH-dependent oogenesis of A. aegypti mosquitoes (20). Tgo is homologous to the aryl hydrocarbon receptor nuclear translocator and it is currently unrelated to insect circadian clock or JH signaling. dsRNA derived from the heterologous β-gal (lacZ) gene served as a control in all RNAi experiments.
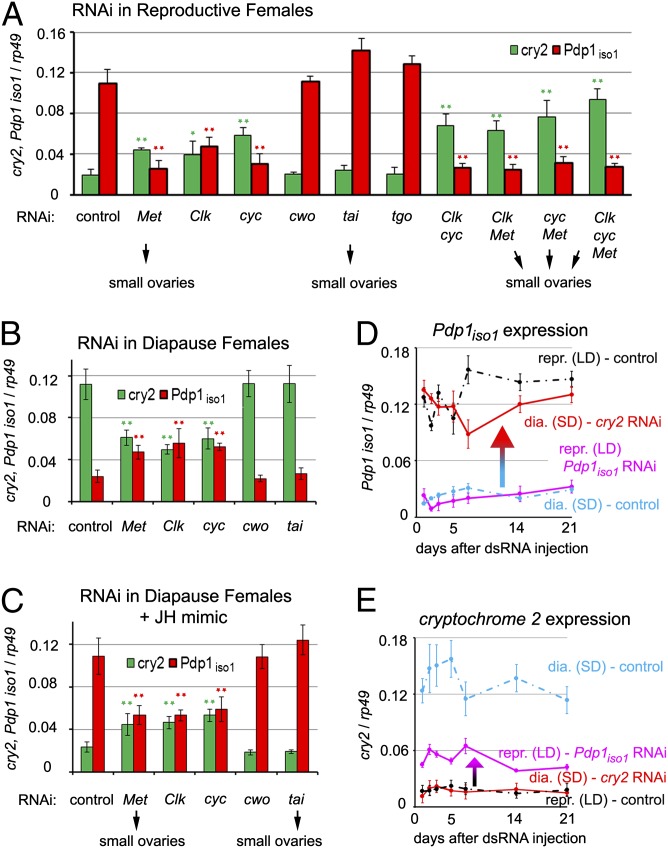
Fig. 2.
Factors required for the reciprocal cry2 and Pdp1iso1 regulation in the gut. (A) Reproductive females that naturally produce JH were injected with dsRNA to silence the indicated genes, and the levels of cry2 and Pdp1iso1 mRNAs in their guts were determined 4 d later, together with ovarian morphology. lacZ dsRNA served as a control. Depletion of Met, Clk, and Cyc (individually or in combinations) altered both transcripts toward their diapause mode; depletion of Met or Tai prevented oogenesis. (B) RNAi depletion of Met, Clk, or Cyc in diapause females partially reduced cry2 and increased Pdp1iso1 mRNAs, equalizing their levels. (C) Met, Clk, and Cyc are necessary for the JH mimic methoprene to revert the levels of cry2 and Pdp1iso1 mRNAs to the reproductive mode, as both transcripts became approximately equalized in the guts of Met, Clk, or cyc RNAi females that were given methoprene. (D and E) Pdp1iso1 and cry2 form a feedback loop of mutual repressors in the gut. dsRNAs were injected to females 1 d after adult ecdysis, and transcript levels in their guts were monitored 1, 2, 3, 5, 7, 14, and 21 d later. Knockdown of either gene remained effective throughout this period. Removal of Cry2 in diapause females caused Pdp1iso1 mRNA to increase near levels normally occurring in guts of reproductive females (D). Conversely, expression of cry2 was enhanced when reproductive females were subjected to Pdp1iso1, although to a lesser extent than in diapause controls. Values are mean ± SEM from three independent experiments. (*P < 0.05 and **P < 0.001 vs. lacZ controls as assessed, Tukey honestly significant difference test).
Individual or combinatorial RNAi silencing of Clk, cyc, or Met reduced the Pdp1iso1 transcript in the guts of reproductive (LD condition) females (Fig. 2A), in which it was normally abundant (Fig. 1E). Unexpectedly, reduction in Pdp1iso1 mRNA levels upon depletion of Clk, cyc, or Met coincided with up-regulation of the cry2 transcript (Fig. 2A) and, at least in the case of Met RNAi, of the Cry2 protein (Fig. 1G). This reciprocal Pdp1iso1 and cry2 regulation was even more prominent in the double and triple knockdown experiments combining Clk, cyc, and Met dsRNAs (Fig. 2A). Silencing of Met or tai reduced the ovaries in reproductive females to a size found during diapause (SD condition; Fig. 1A), confirming the expected necessity of JH signaling for oogenesis (6, 20). Therefore, high expression of Pdp1iso1 in the gut of reproductive females required Clk, Cyc, and Met, whereas oogenesis itself required the JH/Met/Tai signaling but was independent of Clk and Cyc. Conversely to the situation in reproductive females, removal of Clk, cyc, or Met increased Pdp1iso1 and reduced cry2 mRNA levels in the gut of diapause females (Fig. 2B). All three genes (Clk, cyc, and Met) that were important for the differential regulation of Pdp1iso1 and cry2 were themselves expressed in the guts of reproductive and diapausing females, with levels slightly higher in the latter (Fig. S5). Therefore, the switch from the reproductive to the diapause mode was not caused by the absence of Cyc, Clk, or the JH receptor Met.
The requirement of Met for Pdp1iso1 expression suggested a role for JH in regulating the circadian genes. Indeed, topical application of the JH mimic methoprene on diapausing females, which causes them to exit diapause, resulted in up-regulation of Pdp1iso1 and a simultaneous decrease of cry2 mRNA (Fig. 2C) to levels seen in the guts of reproductive females (compare Fig. 2 A vs. C). Therefore, the exogenous JH mimic was able to override the gut diapause status, imposed on the animals by SD conditions, by acting upon circadian gene expression. Importantly, application of methoprene could no longer enhance Pdp1iso1 or reduce cry2 mRNA levels in diapause females deficient for Met, Clk, or cyc function (Fig. 2C), indicating that the JH receptor Met as well as the two circadian proteins were required for JH-dependent regulation of Pdp1iso1 and cry2 in the gut. Consistent with the lack of effect of cwo and tai RNAi on Pdp1iso1 and cry2 expression (Fig. 2 A and B), methoprene still induced Pdp1iso1 and suppressed cry2 when applied to diapause females upon cwo or tai silencing (Fig. 2C).
Our next goal was to verify whether JH signaling acted autonomously in the gut. As expected, methoprene turned on Pdp1iso1 and suppressed cry2 even in guts that had been removed from diapause females and cultured in vitro (Fig. 3B and Fig. S6). However, subjecting animals to Met RNAi before gut dissection prevented this effect of the JH mimic on both Pdp1iso1 and cry2 expression (Fig. 3B). Taken together, these experiments show that the function of Clk, cyc, and Met in the gut is necessary for JH-dependent regulation of Pdp1iso1 and cry2 in this organ.
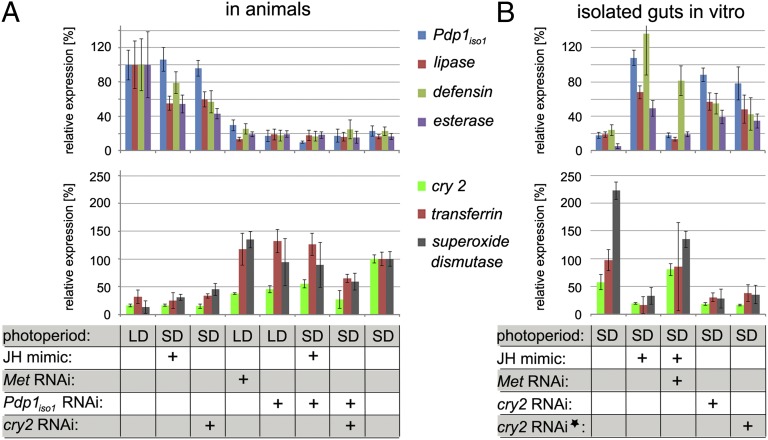
Fig. 3.
Regulation of genes downstream of Cry2, Pdp1iso1, and JH/Met in the gut. (A) Reproductive (i.e., LD) and diapause (i.e., SD) females were injected with dsRNAs and, after 4 d, treated with methoprene as indicated below the columns. Four days later, mRNA levels were measured in their guts for Pdp1iso1, lip, def, and est genes, whose expression characterizes the reproductive state (Upper), and for cry2, tf, and sod genes, which are active under diapause (Lower). (B) Expression of the reproduction and diapause downstream genes in isolated guts. Guts were dissected from control or from diapause (i.e., SD) females 2 d after dsRNA injection, and were cultured for 2 d with or without methoprene as indicated. Alternatively, isolated guts were exposed to cry2 dsRNA for 2 d in culture (asterisk). All data have been normalized to rp49 expression and are shown relative to the levels of reproduction downstream genes in untreated LD females (Upper) and to the levels of diapause downstream genes in untreated SD females (Lower), respectively, that were set to 100%. Statistical significance of the differences is shown in Table S1.
Reciprocal Regulation Between Pdp1iso1 and cry2.
High expression of Pdp1iso1 coincides with suppression of cry2 and vice versa, depending on whether females experience reproduction or diapause, respectively (Fig. 1). This inverse relationship suggests a negative feedback between the two circadian genes. Indeed, experimental depletion of cry2 itself led to elevated expression of Pdp1iso1 in the guts of diapause females (Figs. 2D and 3A, Upper). Conversely, low levels of cry2 mRNA in the guts of reproductive females increased upon Pdp1iso1 RNAi, albeit to lower levels than normally observed under diapause (Figs. 2E and 3A, Lower). These changes then persisted for at least 3 wk (Fig. 2 D and E), suggesting that manipulation of cry2 or Pdp1iso1 reprogrammed expression of the other gene for as long as the RNAi was effective. Nevertheless, as cry2 RNAi females in SD conditions still retained small diapause ovaries, the loss of cry2 and the resulting ectopic expression of Pdp1iso1 alone were not sufficient to render the entire animal reproductive.
To exclude the possibility that removal of cry2 function might have induced Pdp1iso1 by increasing JH production in diapause females, we examined transcript levels of the Krüppel-homolog 1 gene (Kr-h1), whose expression strictly depends on the presence of JH and Met in P. apterus (16). As expected, Kr-h1 mRNA was low during diapause (a JH-free state), and application of methoprene induced it near the levels occurring in the guts of reproductive females (Fig. S7). The expression of Kr-h1 was also low in diapause females subjected to cry2 RNAi (Fig. S7), indicating that JH remained low in these animals. Therefore, although methoprene can induce Pdp1iso1 (Fig. 2C), endogenous JH is likely not required for Pdp1iso1 up-regulation that results from cry2 removal.
To test the autonomy of the feedback between Pdp1iso1 and cry2, we cultured guts isolated from diapause females with cry2 dsRNA in the absence of methoprene. After 48 h, we detected depletion of cry2 mRNA and up-regulation of Pdp1iso1 in these cultured organs (Fig. 3B), confirming that the mutual repression between cry2 and Pdp1iso1 can operate independently of the systemic JH signal.
Genes Downstream of Pdp1iso1 and cry2.
Our next question was whether the expression of Pdp1iso1 or cry2 was important for the gut function. Twenty-four P. apterus genes expected to function in the gut were cloned (SI Methods), and corresponding mRNA levels were compared between diapause and reproductive female guts. Five of these transcripts were identified as dependent on the reproductive status. These encode digestive enzymes [lipase (lip) and esterase (est)], an antimicrobial peptide [defensin (def)], an oxidative stress response enzyme [superoxide dismutase (sod)], and transferrin (tf) whose relationship to diapause has been previously reported (6). Three of these transcripts, lip, est, and def, were preferentially expressed in the gut of reproductive females (we refer to them as reproduction downstream genes), whereas sod and tf were highly active in diapause guts (diapause downstream genes).
First, we established that application of methoprene to diapause females turned on expression of the reproduction downstream genes while suppressing the diapause downstream genes in the gut (Fig. 3A; Table S1 shows statistical analysis). Both the induction and the suppression required Met, because all changes were abolished when animals were previously injected with Met dsRNA (Fig. 3A and Table S1). Importantly, expression of the reproduction downstream genes was also reduced in the guts of reproductive females exposed to RNAi against Pdp1iso1, in which lip, est, and def remained weakly expressed despite the presence of endogenous JH (in reproductive females) or exogenous JH mimic (applied to diapause females; Fig. 3A and Table S1). Therefore, expression of the reproduction-associated genes lip, est, and def in the gut depends on Pdp1iso1 downstream of JH signaling.
The low level of Pdp1iso1 expression, either natural under diapause or upon RNAi in reproductive females, resulted in activation of cry2 and the diapause downstream genes sod and tf (Fig. 3A and Table S1). To discriminate whether it was the absence of Pdp1iso1 or the presence of cry2 activity that induced the diapause downstream genes sod and tf, we exposed diapause females under SD conditions to cry2 and Pdp1iso1 double RNAi. Guts of these females expressed low levels of sod and tf (Fig. 3A and Table S1), indicating that the function of cry2 was required for expression of the diapause-specific transcripts.
Finally, we examined regulation of the downstream genes by JH/Met and Cry2 in cultured guts isolated from diapause females. The levels of the reproduction and diapause downstream genes switched to the reproductive pattern after methoprene application in vitro, and the JH mimic had no such effect on guts obtained from diapausing females that were subjected to Met RNAi (Fig. 3B and Table S1). Depletion of Cry2 in the gut, whether deployed before dissection in intact diapause females or in culture, resulted in up-regulation of Pdp1iso1 and the reproduction downstream genes, whereas the diapause downstream transcripts decreased (Fig. 3B and Table S1).
Discussion
In many insects, including P. apterus, the seasonal decision between reproduction and diapause involves JH (12). Under extended day length, signals from neurosecretory cells in the pars intercerebralis of the brain stimulate JH secretion from the corpora allata gland (Fig. 4). JH then acts on target organs to induce exit from diapause and to promote oogenesis. By using the gut of P. apterus females, we show that, at the organ level, JH achieves the switch from a diapause mode to a reproduction mode through its receptor Met. In the gut of reproductive females, the function of Met is necessary for enhanced expression of Pdp1iso1 and downstream genes that are active during reproduction and for simultaneous suppression of cry2 and other genes that characterize diapause (Fig. 4). When supplied to diapausing females experiencing SD conditions, methoprene overcomes the lack of endogenous JH and terminates diapause. Also in this scenario, Met is needed in the gut for the JH mimic to activate Pdp1iso1, suppress cry2, and switch expression of the respective downstream genes toward the reproductive mode (Fig. 3B).
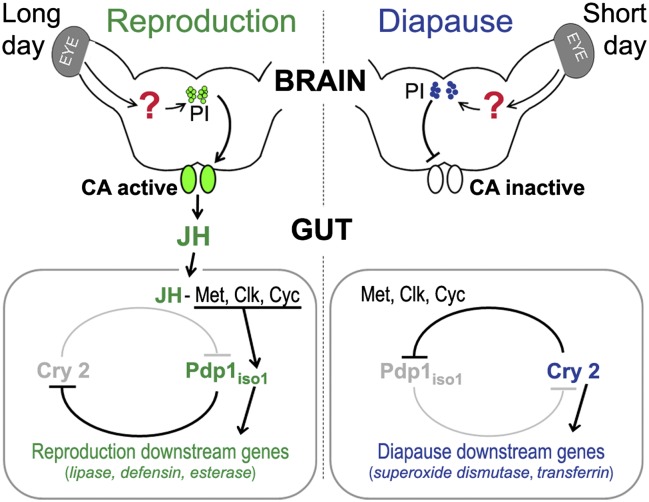
Fig. 4.
Regulation of the reproductive/diapause status of the insect gut. Under LD conditions, which favor reproduction (Left), the corpora allata (CA) secrete JH in response to signals from the pars intercerebralis (PI) of the brain. In the gut, JH acts through its receptor Met, Clk, and Cyc to stimulate expression of Pdp1iso1 and other genes that characterize the reproductive state, whereas expression of cry2 and diapause downstream genes is suppressed. Although the regulation involves circadian clock genes, it is independent of daily oscillations of the circadian gene expression. In the absence of endogenous JH under short photoperiod (Right), expression of cry2 prevails over Pdp1iso1, favoring the diapause-specific program. By acting through Met, Clk, and Cyc, exogenous JH mimic can induce the reproductive program under SD condition.
Unlike Met, its partner Tai was not required for the reciprocal Pdp1iso1 and cry2 regulation in the guts of reproductive and diapause females (Fig. 2). Similarly, tai RNAi did not prevent methoprene from inducing Pdp1iso1 and repressing cry2 during diapause. Therefore, Tai is unlikely to be part of a JH receptor complex that contains Met and mediates the effect of methoprene on the diapause gut. Nonetheless, Met and Tai were necessary for ovarian growth at the entire organism level, because either Met or tai RNAi blocked oogenesis in reproductive females or in methoprene-treated females under SD conditions (Fig. 2 A and C). These results indicate that, in P. apterus, JH stimulates oogenesis through Met and Tai and regulates gene expression in the gut through Met, Cyc, and Clk.
The inverse correlation of Pdp1iso1 and cry2 activity with diapause and with the presence of JH suggests that Pdp1iso1 and Cry2 direct the gut toward the reproductive or the diapause state by acting as mutual repressors (Fig. 4). Expression of Pdp1iso1 in the guts of diapause females increased not only following methoprene treatment but also upon cry2 RNAi, even when isolated guts were cultured without methoprene. Therefore, although the Pdp1iso1/cry2 regulatory circuit responds to the systemic JH signal, it operates organ-autonomously. Interestingly, although cry2 RNAi in diapause females enhanced Pdp1iso1 mRNA near levels observed during reproduction, cry2 expression upon Pdp1iso1 knockdown reached less than half its diapause level (Figs. 2 D and E and 3A). The difference could be caused by residual Pdp1iso1 protein or an additional signal from the brain (9, 10).
RNAi knockdown of Met, cyc, or Clk in reproductive females and in methoprene-treated diapause females prevented expression of Pdp1iso1 to prevail over cry2 in the presence of endogenous JH or its added mimic, and combined silencing of two or all three of these genes in reproductive females inverted the Pdp1iso1/cry2 expression ratio to the diapause mode (Fig. 2A). These results show that signaling through the JH receptor Met cooperates with the bHLH-PAS transcription factors Clk and Cyc at the level of regulating the Pdp1iso1 and cry2 genes to promote the reproductive program in the gut (Fig. 4). In agreement with our data, the Clk-Cyc dimer is known to activate the Pdp1 gene in D. melanogaster (33). However, in contrast to the cyclic expression pattern of the fly Pdp1 orthologue (33) or of the mammalian cry gene (35), neither Pdp1iso1 nor cry2 mRNAs displayed daily fluctuations in the gut of P. apterus. Instead, expression of both genes depended on whether females experienced oogenesis or diapause, and our critical photoperiod experiments further showed that the reproductive status, rather than the day length, was the decisive cue.
Taken together, our data show a pathway in which JH conveys upstream photoperiodic information to downstream target tissues. Intriguingly, the key players mediating the diapause/reproductive state of the gut involve the circadian genes Clock, cycle, Pdp1, and cry2. Previous work in R. pedestris (6, 39) showed that Cyc, Per, and Cry2 orthologues were all required for a circadian cycle of cuticle deposition. In addition, knockdown of per or cry2 stimulated oogenesis even under diapause-inducing conditions, whereas cyc RNAi always prevented it. Based on altered expression of JH-response genes, the authors implicated Per and Cyc in a photoperiodic clock operating upstream of JH secretion, and excluded their involvement in the pleiotropic regulation of ovarian development itself (6). In our P. apterus model, oogenesis under long photoperiod could be blocked upon depletion of the JH receptor Met or its partner Tai, but not by RNAi against Cyc, Clk, or Pdp1iso1. Likewise, cry2 RNAi was insufficient to induce oogenesis in diapause females. Consistently, JH-dependent expression of the Kr-h1 gene did not significantly increase in the gut of these Cry2-deficient females (Fig. S7). Although we cannot rule out effects of systemic RNAi on the brain, we do not suspect that the circadian proteins control the seasonal status of the gut by acting upstream of JH secretion. Instead, our data show that Clock, Cyc, Pdp1, and Cry2 engage in an organ-autonomous regulatory mechanism that is independent of circadian oscillations and that responds to the centrally released hormonal signal. Our results therefore support the idea that circadian clock components can operate pleiotropically in peripheral tissues to execute the reproductive/diapause program without necessarily being connected to their other function as part of the canonical circadian clock (3, 22, 31).
Methods
Animal Rearing Conditions.
P. apterus bugs (short-winged form) were reared at 25 °C as described previously (40), and were maintained from hatching either under LD conditions (18 h light, 6 h dark) that permit reproduction or under diapause-inducing short photoperiod (12 h light, 12 h dark). For CDL experiments, developing bugs were kept in an intermediate photoperiod of 16.5 h of light and 7.5 h of dark. Females were dissected 2 wk after adult ecdysis, and their diapause phenotype was assessed according to ovarian morphology.
mRNA Quantification.
Total RNA was isolated from guts of adult P. apterus females with the TRIzol reagent (Invitrogen). After TURBO DNase (Ambion) treatment, 1 µg of total RNA was used for cDNA synthesis with SuperScript III reverse transcriptase (Invitrogen). Relative transcript levels were measured by quantitative RT-PCR (qRT-PCR) by using the iQ SYBR Green Supermix kit and the C1000 Thermal Cycler (Bio-Rad). All data were normalized to the relative levels of ribosomal protein (Rp49) mRNA as described previously (41). Primer sequences used for qRT-PCR are listed in Table S2.
Antibodies and Immunodetection.
A mixture of synthetic peptides, ESSTERTKKTEESIYC and CFAPPASTFRGSLNKK, corresponding to the C-terminal region of P. apterus Cry2 was used to generate polyclonal antibodies in guinea pigs (Eurogentec). Western blots were performed with the anti-Cry2 antibody diluted 1:200. For tissue staining, guts were dissected in cold Ringer solution and then fixed for 4 h in 4% paraformaldehyde in PBS solution with 0.03% Triton X-100. After incubating overnight in 30% (wt/vol) sucrose in PBS solution at 4 °C, the tissue was embedded in a Tissue-Tek system (Sakura Finetek). Cryosections 15 µm thick were successively incubated with the Cry2 antibody (1:100) overnight at room temperature and with a FITC-conjugated guinea pig secondary antibody (1:1,000; Jackson Immunoresearch) for 2 h at room temperature. The washed and mounted tissue (Aqua Polymount; Polysciences) was observed with a FluoView 1000 confocal microscope (Olympus).
RNAi.
dsRNA was synthesized by using the T3 and T7 MEGAscript kit (Ambion) from plasmids containing the appropriate gene fragments (Table S3 shows primer sequences). Before cloning, these fragments were cross-examined by using dot-plot analysis to avoid interference of the individual dsRNAs with multiple target genes. Experimental animals were injected with 4 µL of 4 µg/µL dsRNA solution. For double and triple knockdown experiments, individual dsRNA were mixed in equal ratio and diluted to the final concentration of 4 µg/µL. The efficiency of RNAi-mediated depletion of each targeted mRNA was verified by qRT-PCR.
Organ Culture Experiments.
Guts of intact or RNAi-treated females were dissected and washed twice in Grace medium (G8142; Sigma), supplemented with a twice-concentrated antibiotic/antimycotic mix (A5955; Sigma). Individual guts were incubated in 96-well plates (50 µL media per well) at 22 °C to 23 °C in the dark. For in vitro RNAi experiments, 5 µL of cry2 dsRNA (4 µg/µL) was added to each well.
JH Mimic Treatments.
Five microliters of 0.3 mM methoprene (VUOS) in acetone (or acetone alone for control) were topically applied to the dorsal side of CO2-anesthetized females. The animals were then kept under SD conditions for 4 d. Guts were then dissected and subjected directly to qRT-PCR or first cultured as described earlier. Five microliters of 0.3 mM methoprene (or acetone alone for control) was applied on individual guts, cultured in 50 µL of Grace media.
Supplementary Material
Acknowledgments
We thank Jana Mikešová for insect rearing, Ivan Fiala for phylogenetic analysis, and Ivo Sauman for permanent support. This work was supported by Academy of Sciences Grants IAA500960802 and Z50070508, Grant Agency of the Czech Republic Projects 204/08/P579 and P502-10-1612, and Marie Curie Fellowship Award 276569 from the European Union (to M.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. 1563012 (Pdp1iso1), 1563013 (Pdp1iso2), 1562940 (cyc), 1562939 (Clk), 1563010 (cwo), 1563014 (tgo), 1562945 (tai), 1563016 (lip), 1563019 (def), 1563020 (est), 1563024 (sod), and 1563021 (tf)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217060110/-/DCSupplemental.
References
- 1.Hahn DA, Denlinger DL. Energetics of insect diapause. Annu Rev Entomol. 2011;56:103–121. doi: 10.1146/annurev-ento-112408-085436. [DOI] [PubMed] [Google Scholar]
- 2.Koštál V. Insect photoperiodic calendar and circadian clock: Independence, cooperation, or unity? J Insect Physiol. 2011;57(5):538–556. doi: 10.1016/j.jinsphys.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw WE, Holzapfel CM. What season is it anyway? Circadian tracking vs. photoperiodic anticipation in insects. J Biol Rhythms. 2010;25(3):155–165. doi: 10.1177/0748730410365656. [DOI] [PubMed] [Google Scholar]
- 4.Hodek I. Diapause in females of Pyrrhocoris apterus L (Heteroptera) Acta Entomol Bohemoslov. 1968;65:422–435. [Google Scholar]
- 5.Slama K. Hormonal control of respiratory metabolism during growth, reproduction, and diapause in female adults of Pyrrhocoris apterus L (Hemiptera) J Insect Physiol. 1964;10:283–303. [Google Scholar]
- 6.Ikeno T, Tanaka SI, Numata H, Goto SG. Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol. 2010;8:116. doi: 10.1186/1741-7007-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Socha R, Sula J, Zemek R. Feeding, drinking and digestive enzyme activities in long- and short-day females of Pyrrhocoris apterus (Heteroptera) Physiol Entomol. 1997;22:161–169. [Google Scholar]
- 8.Kostál V, Tollarová M, Dolezel D. Dynamism in physiology and gene transcription during reproductive diapause in a heteropteran bug, Pyrrhocoris apterus. J Insect Physiol. 2008;54(1):77–88. doi: 10.1016/j.jinsphys.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Hodková M. Nervous inhibition of corpora allata by photoperoid in Pyrrhocoris apterus. Nature. 1976;263(5577):521–523. doi: 10.1038/263521a0. [DOI] [PubMed] [Google Scholar]
- 10.Hodková M, Okuda T, Wagner RM. Regulation of corpora allata in females of Pyrrhocoris apterus (Heteroptera) (a mini-review) In Vitro Cell Dev Biol Anim. 2001;37(9):560–563. doi: 10.1290/1071-2690(2001)037<0560:ROCAIF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Shimokawa K, Numata H, Shiga S. Neurons important for the photoperiodic control of diapause in the bean bug, Riptortus pedestris. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194(8):751–762. doi: 10.1007/s00359-008-0346-y. [DOI] [PubMed] [Google Scholar]
- 12.Raikhel AS, Brown MR, Bellés X. Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Insect Science. Amsterdam: Elsevier; 2005. pp. 433–491. [Google Scholar]
- 13.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 14.Denlinger DL, Yocum GD, Rinehart JP. Hormonal control of diapause. In: Gilbert LI, editor. Insect Endocrinology. Amsterdam: Elsevier; 2012. pp. 430–463. [Google Scholar]
- 15.Slama K. Physiological and biochemical effects of juvenoids. In: Slama K, Romanuk M, Sorm F, editors. Insect Hormones and Bioanalogues. New York: Springer; 1974. pp. 236–243. [Google Scholar]
- 16.Konopova B, Smykal V, Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE. 2011;6(12):e28728. doi: 10.1371/journal.pone.0028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashok M, Turner C, Wilson TG. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA. 1998;95(6):2761–2766. doi: 10.1073/pnas.95.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura K, Oda M, Makita S, Chinzei Y. Characterization of the Drosophila Methoprene -tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 2005;272(5):1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 19.Charles JP, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108(52):21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Mead EA, Zhu JS. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci USA. 2011;108(2):638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin SW, Zou Z, Saha TT, Raikhel AS. bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc Natl Acad Sci USA. 2012;109(41):16576–16581. doi: 10.1073/pnas.1214209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradshaw WE, Holzapfel CM. Circadian clock genes, ovarian development and diapause. BMC Biol. 2010;8:115. doi: 10.1186/1741-7007-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders DS, Bertossa RC. Deciphering time measurement: The role of circadian ‘clock’ genes and formal experimentation in insect photoperiodism. J Insect Physiol. 2011;57(5):557–566. doi: 10.1016/j.jinsphys.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Schiesari L, Kyriacou CP, Costa R. The hormonal and circadian basis for insect photoperiodic timing. FEBS Lett. 2011;585(10):1450–1460. doi: 10.1016/j.febslet.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Sandrelli F, et al. A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science. 2007;316(5833):1898–1900. doi: 10.1126/science.1138426. [DOI] [PubMed] [Google Scholar]
- 26.Tauber E, et al. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science. 2007;316(5833):1895–1898. doi: 10.1126/science.1138412. [DOI] [PubMed] [Google Scholar]
- 27.Stehlík J, Závodská R, Shimada K, Sauman I, Kostál V. Photoperiodic induction of diapause requires regulated transcription of timeless in the larval brain of Chymomyza costata. J Biol Rhythms. 2008;23(2):129–139. doi: 10.1177/0748730407313364. [DOI] [PubMed] [Google Scholar]
- 28.Kobelková A, Bajgar A, Dolezel D. Functional molecular analysis of a circadian clock gene timeless promoter from the Drosophilid fly Chymomyza costata. J Biol Rhythms. 2010;25(6):399–409. doi: 10.1177/0748730410385283. [DOI] [PubMed] [Google Scholar]
- 29.Ikeno T, Numata H, Goto SG. Circadian clock genes period and cycle regulate photoperiodic diapause in the bean bug Riptortus pedestris males. J Insect Physiol. 2011;57(7):935–938. doi: 10.1016/j.jinsphys.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Ikeno T, Numata H, Goto SG. Photoperiodic response requires mammalian-type cryptochrome in the bean bug Riptortus pedestris. Biochem Biophys Res Commun. 2011;410(3):394–397. doi: 10.1016/j.bbrc.2011.05.142. [DOI] [PubMed] [Google Scholar]
- 31.Emerson KJ, Bradshaw WE, Holzapfel CM. Complications of complexity: Integrating environmental, genetic and hormonal control of insect diapause. Trends Genet. 2009;25(5):217–225. doi: 10.1016/j.tig.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Socha R. Pyrrhocoris apterus (Heteroptera) - an experimental model species: A review. Eur J Entomol. 1993;90:241–286. [Google Scholar]
- 33.Cyran SA, et al. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112(3):329–341. doi: 10.1016/s0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 34.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24(4):948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 35.Kume K, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98(2):193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 36.Allada R, White NE, So WV, Hall JC, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93(5):791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 37.Rutila JE, et al. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell. 1998;93(5):805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 38.Zhang ZL, Xu JJ, Sheng ZT, Sui YP, Palli SR. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J Biol Chem. 2011;286(10):8437–8447. doi: 10.1074/jbc.M110.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikeno T, Katagiri C, Numata H, Goto SG. Causal involvement of mammalian-type cryptochrome in the circadian cuticle deposition rhythm in the bean bug Riptortus pedestris. Insect Mol Biol. 2011;20(3):409–415. doi: 10.1111/j.1365-2583.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 40.Dolezel D, Sauman I, Kost’ál V, Hodkova M. Photoperiodic and food signals control expression pattern of the clock gene, period, in the linden bug, Pyrrhocoris apterus. J Biol Rhythms. 2007;22(4):335–342. doi: 10.1177/0748730407303624. [DOI] [PubMed] [Google Scholar]
- 41.Dolezel D, Zdechovanova L, Sauman I, Hodkova M. Endocrine-dependent expression of circadian clock genes in insects. Cell Mol Life Sci. 2008;65(6):964–969. doi: 10.1007/s00018-008-7506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.