Abstract
Onchocerciasis, also known as “river blindness”, is a neglected tropical disease infecting millions of people mainly in Africa and the Middle East but also in South America and Central America. Disease infectivity initiates from the filarial parasitic nematode Onchocerca volvulus, which is transmitted by the blackfly vector Simulium sp. carrying infectious third-stage larvae. Ivermectin has controlled transmission of microfilariae, with an African Program elimination target date of 2025. However, there is currently no point-of-care diagnostic that can distinguish the burden of infection—including active and/or past infection—and enable the elimination program to be effectively monitored. Here, we describe how liquid chromatography-MS–based urine metabolome analysis can be exploited for the identification of a unique biomarker, N-acetyltyramine-O,β-glucuronide (NATOG), a neurotransmitter-derived secretion metabolite from O. volvulus. The regulation of this tyramine neurotransmitter was found to be linked to patient disease infection, including the controversial antibiotic doxycycline treatment that has been shown to both sterilize and kill adult female worms. Further clues to its regulation have been elucidated through biosynthetic pathway determination within the nematode and its human host. Our results demonstrate that NATOG tracks O. volvulus metabolism in both worms and humans, and thus can be considered a host-specific biomarker for onchocerciasis progression. Liquid chromatography-MS–based urine metabolome analysis discovery of NATOG not only has broad implications for a noninvasive host-specific onchocerciasis diagnostic but provides a basis for the metabolome mining of other neglected tropical diseases for the discovery of distinct biomarkers and monitoring of disease progression.
Onchocerciasis, commonly referred to as “river blindness”, is a neglected tropical disease that affects more than 37 million people worldwide. The disease is endemic in many sub-Saharan countries in Africa, with minor foci in Central and South America and in Yemen. The infection is caused by the filarial parasitic nematode Onchocerca volvulus, which is transmitted during a blood meal by a blackfly vector (Simulium sp.) carrying infectious third-stage larvae (1). These larvae develop into adults in the human host, ultimately accumulating in subcutaneous or deep nodules called onchocercomas. In onchocerciasis, microfilariae are born from viviparous females and migrate through nodular tissue to the skin and eyes, resulting in chronic inflammation. Invasion of the cornea by microfilariae leads to blindness, causing the typical pathology associated with this disease.
Treatment options for onchocerciasis include the microfilaricidal drug ivermectin, which has been critical in controlling onchocerciasis transmission (2), and the antibiotic doxycycline, which targets the bacterial endosymbiont Wolbachia, resulting in both death and sterilization of adult nematodes. Recently, concomitant administration of both drugs resulted in promising and improved treatment outcomes (3, 4). Despite this success, challenges still remain, including the development of reliable disease surveillance methods to overcome the need for mass drug administration strategies. Unspecific treatment of whole communities may eventually lead to drug resistance, and thus underscores the importance of both treating and monitoring the disease in a cohesive fashion. Simply stated, the elimination of onchocerciasis, regardless of the form of treatment, will require a method for determining the relationship between infection prevalence, individual infection intensity, and transmission intensity (5).
Several diagnostics are currently used to monitor the disease state in patients. Among these, skin snips are considered the “gold standard,” but this method is becoming increasingly unpopular and is being replaced by PCR assays, ELISAs, enzyme immunoassays, and antigen surveys (6, 7). A shortcoming of these newer assays is their inability to monitor disease progression readily. Recently, an antibody-based assay was described, which allows the parallel detection of four O. volvulus-specific antibodies, and thus discrimination among filarial infections (8). Still, humoral response-based diagnostics carry liabilities with the potential of antibody persistence even after treatments, hence confounding active/past infections. A potential alternative would be the identification of specific metabolites in blood or urine samples because, in theory, this could provide both infection status and a quantitative measure of infection based on metabolite concentration (9, 10). Using liquid chromatography (LC)-MS to analyze O. volvulus-infected serum samples, we demonstrated the promise of metabolomics as an onchocerciasis diagnostic (11). However, technical constraints, such as a need for multiple mass biomarkers and an inability of compound biomarker assignment, led us to investigate whether urine analysis might serve as a better platform for O. volvulus biomarker diagnostics.
Results and Discussion
To assess this hypothesis, extraction of nonprotein fractions from urine of African onchocerciasis-positive and -negative samples was undertaken for analysis in global metabolomic profiling. A complete list of all sample origins is detailed in Table S1. Extracts were analyzed by LC-MS, and statistical analysis revealed an MS signal of m/z = 356.1, which was enriched in O. volvulus-positive patients compared with African control samples (12). Extraction of the specific ion chromatogram and the corresponding box-and-whisker plot indicated elevated signal intensities in infected patient samples (Fig. 1A; P = 1.2 × 10−8).
Fig. 1.
Identification of the onchocerciasis-specific biomarker NATOG. (A) Comparison of the extracted ion currents (EIC) in LC-MS experiments of biomarker NATOG in O. volvulus-positive (red, n = 19) and O. volvulus-negative (black, n = 12) samples, and corresponding box and whisker plots. A (a.u.), area (arbitrary units); pos., positive. (B) Specific MS/MS fragmentation pattern of biomarker NATOG in positive mode at three different collision energies: collision-induced dissociation (CID) = 10 V, 20 V, and 40 V. (C) Assignment of MS/MS fragments of biomarker NATOG.
To define the importance of this biomarker better, we sought to determine its structure. High-resolution MS provided a molecular mass of M = 355.3398 g/mol, corresponding to the molecular formula C16H21NO8. To decipher the chemical structure, we enriched the biomarker by HPLC purification, and fragmentation of the specific mass signal in LC-tandem MS (MS/MS) experiments enabled the detection of three major signals originating from the parent ion (m/z = 356.1340; Fig. 1B). These signals permitted decoding of the biomarker structure. The first fragment signal, m/z = 180.1048 (C10H13NO2), suggested neutral loss of glucuronic acid (m/z = 176.0321). At higher fragmentation energies, we observed two further fragmentation signals, m/z = 138.0927 and m/z = 121.0663, which provide evidence for loss of an aliphatic N-acetyl moiety (13). Confirmation of these two fragments allowed us to consider the remaining C8H8O fragment (m/z = 121.0663). Both additional fragments were assigned to the loss of water and ethylene (m/z = 103.0540 and m/z = 93.0701, respectively), indicating a phenolic structure. A systematic combination of all identified fragments allowed assignment to the structure of N-acetyltyramine-O,β-glucuronide (NATOG; Fig. 1C). Further proof of NATOG came via chemical synthesis (Fig. S1). The structure was confirmed by the exact match of the high-resolution mass of synthetic and natural material, together with examination of both the retention time in coinjection experiments and LC-MS/MS fragmentation (Fig. 2 A and B and Fig. S2).
Fig. 2.
Structure confirmation and quantification of biomarker NATOG. (A) Cospiking experiment of the synthetic compound NATOG to a positive urine extract (Top) in comparison to single injections of the positive urine extract (Middle) and the synthetic biomarker NATOG (Bottom). EIC, extracted ion currents. (B) MS/MS fragmentation comparison of the synthetic and natural molecules (collision-induced dissociation = 20 V). (C) Quantitative values of NATOG using isotopic labeled compound D3-NATOG as an internal standard in onchocerciasis-positive (n = 81) and control urine samples. Error bars represent SEM values. Data were analyzed by ANOVA, followed by Tukey's multiple comparison method to determine significance (***, P < 0.001). For placebo (n = 14) and doxycycline (n = 24) sample sets, an unpaired two-tailed Student t test was performed (**P = 0.0072). A detailed overview of the quantitative values, number of analyzed samples, and procedure is provided in Tables S2 and S3. (D) Quantitative values of NATOG for placebo- and doxycycline-treated patients. Columns labeled with x represent sample sets, which were treated 6 mo later with ivermectin (Tables S1 and S2).
After confirmation of the molecular structure of NATOG, we sought to quantify it in urine samples, which would reinforce its potential as a biomarker for diagnostic use. Toward this goal, we analyzed a series of O. volvulus-positive and -negative samples and extended our study with four sample sets to evaluate the specificity of this biomarker. To permit precise quantification, the D3-labeled analog of NATOG (D3-NATOG) was prepared (Fig. S3). Importantly, D3-NATOG has the same physical properties as NATOG but can be distinguished due to the molecular mass difference that allows parallel analysis (14). Calibration curves were constructed for precise quantification (Fig. S4), and D3-NATOG was spiked into each analyzed urine sample at a known concentration before extraction of the nonprotein fractions to allow unbiased analysis.
The obtained quantitative values are summarized in Fig. 2C. As anticipated, analysis of a larger sample set confirmed our observed qualitative differences of onchocerciasis-positive samples compared with African control samples that unequivocally revealed elevated values of biomarker NATOG in O. volvulus-infected individuals, with an average value of 36.9 ± 4.0 μM (±SEM). This represents approximately a sixfold increase compared with African control samples (7.0 ± 2.7 μM; P < 0.001). As a second O. volvulus-negative control group, we examined North American samples, which were found to have an average value of 1.1 ± 0.2 μM.
Filarial nematodes contain the endosymbiotic bacteria Wolbachia, and apart from many animal filarial species, these endobacteria are present in several human filariae, such as Wuchereria bancrofti, Brugia malayi, and O. volvulus. In 2000, a landmark study provided evidence that doxycycline cleared Wolbachia from the endodermis and uteri of adult female O. volvulus, leading to extensive worm sterility (15). Thus, monitoring the influence such treatment would have on NATOG could provide an additional link between NATOG and worm metabolism. We analyzed urine samples from patients with O. volvulus who received either doxycycline or placebo over a 4- to 6-wk period. Sample sets were collected 20 mo after treatment and were shown both to kill the endosymbiont Wolbachia and to sterilize the nematode (16, 17). The quantification experiments revealed a significantly reduced concentration of NATOG in doxycycline-treated patients (9.5 ± 1.7 μM) compared with untreated O. volvulus-positive patients (P < 0.001) and placebo-treated patients (33.5 ± 10.7 μM; P = 0.0072). As shown in Fig. 2D, remarkably diminished levels of NATOG were seen in patients treated with doxycycline. These results are consistent with an increasing body of data demonstrating doxycycline’s antifilaricidal capacity, and they further establish the value of biomarker NATOG as a means for monitoring onchocerciasis progression.
Having established the importance of NATOG in West African samples, we then analyzed the presence of NATOG in Central American samples. In this case, we observed a low abundance of biomarker NATOG in Guatemalan O. volvulus-positive samples (8.4 ± 1.6 μM; P < 0.001). This finding can be rationalized based on the presence of genetically different O. volvulus species (18). This result is also in line with previous observations for blood biomarkers specific for African O. volvulus infections, including a report detailing nonoverlapping polypeptide composition as well as IgG4 response differences seen between Central American and West African samples (11, 19). Due to the close phylogenetic relationship between O. volvulus and other filarial species, we examined samples of patients with lymphatic filariasis infections. A remarkably diminished difference in NATOG concentration with high statistical significance (4.2 ± 0.7 μM; P < 0.001) was observed. Although these data suggest a metabolic specificity of NATOG for O. volvulus and the host, future sampling of other human filarial nematode species, such as Loa loa and Mansonella perstans, will help to establish a more complete picture of the metabolic regulation of NATOG and these other parasitic disease states (20).
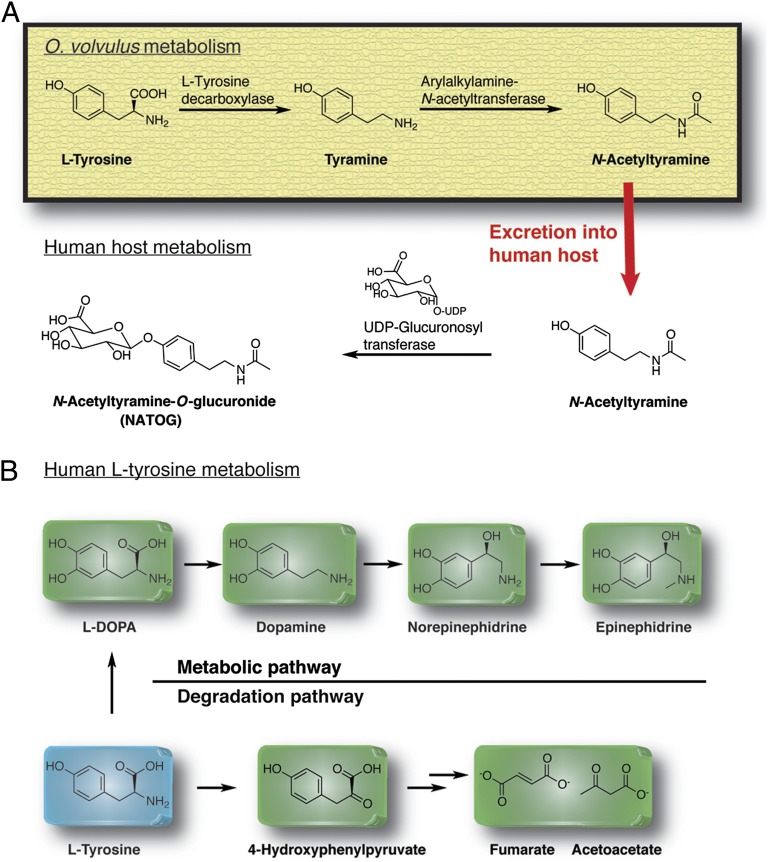
The metabolic pathway of biomarker NATOG reveals an intimate relationship between O. volvulus and its human host (Fig. 3A). The “core” moiety of NATOG is tyramine and represents a structural counterpart of vertebrate neurotransmitters (21). The path to tyramine requires L-tyrosine decarboxylation by L-tyrosine decarboxylase and represents the major conversion of L-tyrosine in invertebrates. The discovery of a specific tyramine receptor in Caenorhabditis elegans infers an importance of this neurotransmitter in nematodes (22). Accordingly, a high level of activity of this decarboxylase was identified in the third-stage larvae of O. volvulus, suggesting a critical role for this neurotransmitter in the worm’s development (23), a point in fact that readily justifies the reduced concentration of biomarker NATOG in doxycycline-treated patients, which leads to the sterilization of adult worms (16, 17). Based on literature precedents, we concluded that N-acetylation of tyramine by arylalkylamine N-acetyltransferase (AANAT) is the second metabolic step (24, 25) in the biosynthesis of NATOG. Importantly, N-acetylation and monoamine oxidation by monoamine oxidases (MAOs) are the two deactivation mechanisms of excess neurotransmitters that nematodes use before excretion. To support this notion further, an AANAT has been isolated from O. volvulus, which has a high degree of specificity for arylalkylamines like tyramine, tryptamine, or octopamine over arylamines or polyamines (25).
Fig. 3.
Proposed biosynthesis of NATOG. (A) Biosynthetic pathways of NATOG in O. volvulus and the human host. (B) Metabolic and degradation pathway of L-tyrosine in humans.
On excretion of the deactivated nematode neurotransmitter N-acetyltyramine into the human host, it is undoubtedly transported into different tissues, including the liver, kidney, or spleen. In these tissues, glucuronidation of the phenol moiety by a glucuronosyltransferase yields NATOG before excretion (Fig. 3A). Mammals use this metabolic pathway to convert exogenous phenols into more hydrophilic compounds that possess better excretion properties (26).
Understanding the lack of NATOG in healthy donors is also critical. The simplest interpretation is that tyramine is not a major metabolite of L-tyrosine conversion in humans, and is thus considered a trace amine. Diverse metabolic pathways are known for L-tyrosine with hydroxylation to form L-3,4-dihydroxyphenylalanine (L-DOPA) by the L-tyrosine hydroxylase as the major pathway (Fig. 3B), wherein L-DOPA is decarboxylated to dopamine, followed by hydroxylation and methylation to epinephrine. Furthermore, tyramine is not a product in the degradation process of L-tyrosine because the initial metabolic step is the conversion of the amine to 4-hydroxyphenylpyruvate by L-tyrosine transaminase (Fig. 3B). Indeed, the major pathway of tyramine metabolism in humans is the oxidative deamination by MAO to yield p-hydroxyphenylacetic acid, while N-acetyltyramine is formed as a minor metabolite (27). AANATs in humans regulate acetylation of arylalkylamines also using tyramine as a substrate for this enzyme class (28). In a side context, it has been shown in rats that inhibition of MAO leads to increased formation of N-acetyltyramine as an alternative metabolic pathway (27). This anomaly of L-tyrosine metabolism has also been described for CNS dysfunctions (29). It is interesting to note that the so-called “nodding disease” has been linked to onchocerciasis and whether N-acetyltyramine has an impact on the pathology seen may merit additional research (30).
In summary, we have identified an exogenous human urine metabolite NATOG that can be traced to an O. volvulus biosynthetic pathway. The unique structure of NATOG, coupled with its origin as a worm metabolite, demonstrates an intimate link with the O. volvulus life cycle. It is noteworthy that doxycycline treatment for O. volvulus infections greatly reduces the concentration of NATOG, and this outcome argues that endosymbiotic targeting appears to be therapeutically efficacious. The discovery of NATOG as a biomarker for O. volvulus should prove valuable in the development of a diagnostic, which will assist in facilitating disease elimination (31).
Materials and Methods
Sample Preparation and Metabolite Extraction.
Solvents used were of HPLC grade. A methanol precipitation of proteins was conducted by adding 400 mL of ice-cold methanol to 100-mL aliquots of urine samples spiked with a known concentration of isotopic-labeled internal standard D3-NATOG. The samples were vigorously shaken for 30 s and allowed to rest on ice for 30 min. After centrifugation at 13,780 × g for 5 min, the metabolite-containing supernatant was removed from the precipitated protein pellet and transferred to fresh tubes. The supernatant samples were dried in a GeneVac EX-2 Evaporation System (GeneVac, Inc.) at ambient temperature and then resuspended to a volume of 50 mL in water/acetonitrile (95:5 ratio), vigorously shaken for 30 s, and centrifuged again at 13,780 × g for 5 min. After being transferred to prelabeled LC vials, samples were stored at 4 °C and transferred to the LC-MS thermostated autosampler cooled to 8 °C.
LC-Electrospray Ionization-TOF-MS Analysis.
The samples (3-μL injection volume) were analyzed with electrospray ionization (ESI) TOF-MS (Agilent TOF 6210; Agilent Technologies) and chromatographic separation by HPLC (Agilent 1200 LC; Agilent Technologies) with a flow rate of 70 μL/min using a T3 Atlantis column (3 mm, 1.0 mm × 150 mm; Waters Corporation). The column temperature was maintained at 30 °C. Eluting buffers were buffer A (0.1% HCOOH in H2O) and buffer B (0.1% HCOOH in MeOH). Gradients were as follows: 0 → 4 min, 2% → 2% buffer B; 4 → 25 min, 2% → 95% buffer B; 25 → 30 min, 95% → 2% buffer B; 30 → 33 min, 2% buffer B; and 33 → 58 min, and 2% → 2% buffer B. The chromatographic eluent was directly injected into the ion source without prior splitting. Data were collected using positive ESI mode scanning in the centroid mode from 100 to 500 m/z with a scan rate of 1.0 spectrum per second in a 2-GHz extended dynamic range. The capillary voltage was 3,500 V; the nebulizer pressure, drying gas flow, and gas temperature were set to 20 psig, 7 L/min, and 350 °C, respectively. Parameters of the mass spectrometer were tuned prior to each sample set with Agilent ESI tune mix for TOF systems (Agilent Technologies). LC-MS/MS fragmentation patterns were obtained on an Accurate Mass quadrupole TOF LC/MS 6520 (Agilent Technologies) at the indicated collision-induced dissociation energy.
Data Preprocessing, Pattern Determination, and Statistical Analysis.
The initial screening for the identification of potent biomarker mass signals was analyzed using the statistical analysis program XCMS online (11). Different sample sets of onchocerciasis-positive and African-negative control samples were analyzed using the standard parameters for an ESI-TOF system. An example output for this initial screening analysis is shown in Fig. 1A. Statistical comparison of the intensity data was conducted using the statistical analysis program XCMS online built in Welch’s t test. Quantitative data were obtained with manual mass peak integration using Mass Hunter Qualitative Analysis software, version B.03.01 (Agilent Technologies). ANOVA and an unpaired two-tailed Student t test were performed using GraphPad Prism, version 5.0b for Mac OS X (GraphPad Software).
A complete description of materials and methods, including synthetic procedures, an ethics statement, and detailed quantification procedures, can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sara Lustigman, Peter Enyong, Nidia Rizzo, Nancy Cruz-Ortiz, Mauricio Sauerbrey, Eduardo Catú, and Frank O. Richards for their assistance with sample collection. This study was supported by the Worm Institute for Research and Medicine at The Scripps Research Institute and by a postdoctoral fellowship from the German Academic Exchange Service (to D.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221969110/-/DCSupplemental.
References
- 1.Mackenzie CD, Homeida MM, Hopkins AD, Lawrence JC. Elimination of onchocerciasis from Africa: Possible? Trends Parasitol. 2012;28(1):16–22. doi: 10.1016/j.pt.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Gardon J, et al. Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: A randomised controlled trial. Lancet. 2002;360(9328):203–210. doi: 10.1016/S0140-6736(02)09456-4. [DOI] [PubMed] [Google Scholar]
- 3.Tamarozzi F, et al. Long term impact of large scale community-directed delivery of doxycycline for the treatment of onchocerciasis. Parasit Vectors. 2012;5:53. doi: 10.1186/1756-3305-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saint André Av, et al. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295(5561):1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- 5.Babalola OE. Ocular onchocerciasis: Current management and future prospects. Clin Ophthalmol. 2011;5:1479–1491. doi: 10.2147/OPTH.S8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayong LS, et al. Development and evaluation of an antigen detection dipstick assay for the diagnosis of human onchocerciasis. Trop Med Int Health. 2005;10(3):228–233. doi: 10.1111/j.1365-3156.2004.01384.x. [DOI] [PubMed] [Google Scholar]
- 7.Gopal H, et al. Oligonucleotide based magnetic bead capture of Onchocerca volvulus DNA for PCR pool screening of vector black flies. PLoS Negl Trop Dis. 2012;6(6):e1712. doi: 10.1371/journal.pntd.0001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burbelo PD, Leahy HP, Iadarola MJ, Nutman TB. A four-antigen mixture for rapid assessment of Onchocerca volvulus infection. PLoS Negl Trop Dis. 2009;3(5):e438. doi: 10.1371/journal.pntd.0000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007;26(1):51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinayavekhin N, Homan EA, Saghatelian A. Exploring disease through metabolomics. ACS Chem Biol. 2010;5(1):91–103. doi: 10.1021/cb900271r. [DOI] [PubMed] [Google Scholar]
- 11.Denery JR, Nunes AAK, Hixon MS, Dickerson TJ, Janda KD. Metabolomics-based discovery of diagnostic biomarkers for onchocerciasis. PLoS Negl Trop Dis. 2010;4(10):e834. doi: 10.1371/journal.pntd.0000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: A web-based platform to process untargeted metabolomic data. Anal Chem. 2012;84(11):5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couch MW, Williams CM. Mass spectrometry of tryptamines and acetylated tryptamine derivatives. Anal Biochem. 1972;50(2):612–622. doi: 10.1016/0003-2697(72)90073-5. [DOI] [PubMed] [Google Scholar]
- 14.Brückl T, Globisch D, Wagner M, Müller M, Carell T. Parallel isotope-based quantification of modified tRNA nucleosides. Angew Chem Int Ed Engl. 2009;48(42):7932–7934. doi: 10.1002/anie.200902740. [DOI] [PubMed] [Google Scholar]
- 15.Hoerauf A, et al. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355(9211):1242–1243. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
- 16.Hoerauf A, et al. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: A randomized placebo-controlled study. Med Microbiol Immunol (Berl) 2008;197(3):295–311. doi: 10.1007/s00430-007-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoerauf A, et al. Efficacy of 5-week doxycycline treatment on adult Onchocerca volvulus. Parasitol Res. 2009;104(2):437–447. doi: 10.1007/s00436-008-1217-8. [DOI] [PubMed] [Google Scholar]
- 18.Unnasch TR, Williams SA. The genomes of Onchocerca volvulus. Int J Parasitol. 2000;30(4):543–552. doi: 10.1016/s0020-7519(99)00184-8. [DOI] [PubMed] [Google Scholar]
- 19.Guzmán GE, Akuffo HO, Lavebratt C, Luján R. Differential immune response to Onchocerca volvulus: IgG4 antibody responses differ in onchocerciasis patients from Guatemala and Ghana. Acta Trop. 1997;63(1):15–31. doi: 10.1016/s0001-706x(96)00613-4. [DOI] [PubMed] [Google Scholar]
- 20.Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop. 2011;120(Suppl 1):S109–S120. doi: 10.1016/j.actatropica.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Roeder T. Tyramine and octopamine: Ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 22.Rex E, Komuniecki RW. Characterization of a tyramine receptor from Caenorhabditis elegans. J Neurochem. 2002;82(6):1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- 23.Tang L, Frank G. Identification and characterization of an aromatic amino acid decarboxylase from the filarial nematode, Dirofilaria immitis. Biol Chem. 2001;382(1):115–122. doi: 10.1515/BC.2001.017. [DOI] [PubMed] [Google Scholar]
- 24.Isaac RE, MacGregor D, Coates D. Metabolism and inactivation of neurotransmitters in nematodes. Parasitology. 1996;113(Suppl):S157–S173. doi: 10.1017/s0031182000077957. [DOI] [PubMed] [Google Scholar]
- 25.Aisien SO, Hellmund C, Walter RD. Characterization of the arylalkylamine N-acetyltransferase in Onchocerca volvulus. Parasitol Res. 1996;82(4):369–371. doi: 10.1007/s004360050128. [DOI] [PubMed] [Google Scholar]
- 26.King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab. 2000;1(2):143–161. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- 27.Tacker M, McIsaac WM, Creaven PJ. Effect of tranylcypromine sulphate on the metabolism of (14 C)tyramine in vivo in the rat. J Pharm Pharmacol. 1972;24(3):245–246. doi: 10.1111/j.2042-7158.1972.tb08973.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferry G, et al. Substrate specificity and inhibition studies of human serotonin N-acetyltransferase. J Biol Chem. 2000;275(12):8794–8805. doi: 10.1074/jbc.275.12.8794. [DOI] [PubMed] [Google Scholar]
- 29.Von Studnitz W, Hanson A. Demonstration of urinary N-acetyltyramine in patients with neuroblastoma. Clin Chim Acta. 1967;16(1):180–183. doi: 10.1016/0009-8981(67)90289-6. [DOI] [PubMed] [Google Scholar]
- 30.Vogel G. Tropical Diseases. Mystery disease haunts region. Science. 2012;336(6078):144–146. doi: 10.1126/science.336.6078.144. [DOI] [PubMed] [Google Scholar]
- 31.Tudos AJ, Besselink GJ, Schasfoort RBM. Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry. Lab Chip. 2001;1(2):83–95. doi: 10.1039/b106958f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.