Abstract
Recent work on vertebrate hematopoiesis has uncovered the presence of deeply rooted similarities between fish and mammals at molecular and cellular levels. Although small animal models such as zebrafish are ideally suited for genetic and chemical screens, the study of cellular aspects of hematopoietic development in lower vertebrates is severely hampered by the complex nature of their histocompatibility-determining genes. Hence, even when hosts are sublethally irradiated before hematopoietic cell transplantation, stable and long-term reconstitution by allogeneic stem cells often fails. Here, we describe the unexpected observation that transplantation and maintenance of allogeneic hematopoietic stem cells in zebrafish homozygous for the c-mybt25127 allele, carrying a missense mutation (Ile181Asn) in the DNA binding domain can be achieved without prior conditioning. Using this model, we examined several critical parameters of zebrafish hematopoiesis in a near-physiological setting. Limiting dilution analysis suggests that the kidney marrow of adult zebrafish harbors about 10 transplantable hematopoietic stem cells; this tissue also contains thymus-settling precursors that colonize the thymic rudiment within days after transplantation and initiate robust T-cell development. We also demonstrate that c-myb mutants can be stably reconstituted with hematopoietic cells carrying specific genetic defects in lymphocyte development, exemplifying one of the many potential uses of this model in experimental hematology.
Keywords: ENU mutagenesis, chimera
Hematopoietic stem cells (HSCs) are self-renewing progenitor cells capable of multilineage differentiation. In mouse (1) and humans (2), HSCs can be isolated to near homogeneity based on species-specific expression of cell surface molecules. However, a universal pattern for phenotypic and/or molecular markers of HSC has yet to be defined (3); thus, long-term differentiation into all blood lineages after in vivo transplantation (4–6) remains the only reliable criterion for assessing HSC function (7), particularly in species other than mouse and humans (8, 9).
The outcome of hematopoietic cell transplantation depends on many variables. A major factor is the availability of specific stromal niches in hematopoietic tissue environments that are capable of supporting the long-term survival and differentiation of HSCs (10). In general, myeloablative conditioning, such as irradiation of the recipient, is a prerequisite for stable engraftment by donor HSCs. Preconditioning of the host serves two purposes: first, it weakens the immune system that would otherwise attack and destroy incoming cells of the donor, and second, it empties hematopoietic niches to allow the settling of donor cells in such supportive environments. Recently, a mouse model has been generated that stably accommodates histo-incompatible HSCs, which renders prior myeloablation of the host unnecessary; this was achieved by generating a mouse lacking T, B, and NK cells as a result of synergistic null mutations in Rag2 and Il2rg genes, in addition to impairing self-renewal of HSCs owing to the combined presence of two hypomorphic alleles of the c-kit locus (KitW/KitW-v) (11). Despite the complexity of its genetic background, the availability of such a “universal” acceptor for hematopoietic cell transplantation studies represents a major step forward and provides unprecedented opportunities for assessing the developmental potential of HSCs and other types of hematopoietic progenitor cells under near-physiological conditions in the mouse model. However, functionally comparable recipient strains have so far not been described for other species.
Another important factor determining the success of hematopoietic cell transplantation is the degree of immunological compatibility between host and donor tissues. This is determined by the structure of the polymorphic major histocompatibility (MHC) genes; identical MHC haplotypes are generally associated with favorable outcomes (12). In allogeneic situations, that is, when donor and host carry different MHC haplotypes, immunosuppression following transplantation is additionally used to reduce the activity of the immune system of the host and hence to increase the chances of successful long-term engraftment; moreover, the transplantation of highly purified HSC preparations lessens the incidence of graft versus host disease (GvHD) resulting from donor cells attacking host tissues.
The zebrafish has emerged as a versatile experimental model for studies on vertebrate hematopoiesis (13). This success is mostly due to its unique biological features such as high fecundity and transparency of embryos and larvae; moreover, different methods of genetic manipulation and the amenability to high-throughput genetic and chemical screens greatly increase its practical utility. However, the development of methods for hematopoietic cell transplantation in zebrafish is still in its infancy (8, 9, 14). The situation is further complicated by the lack of robust procedures for the purification of HSCs, the difficulty of generating isogenic lines (15, 16) that gives rise to a high frequency of histo-incompatibility between host and donor tissues (14), and the considerable toxicity of the required preconditioning regimens (14). In previous work, immune reactions against donor cells were circumvented by transplantation into immunologically immature hosts (before commencement of lymphoid development) (8); however, only very few cells can be transplanted into recipient embryos, which limits the practical utility of this approach. As an alternative, myeloablative irradiation of adult recipients before transplantation (9) combined with matching of haplotypes at several MHC loci (14) was shown to result in stable multilineage hematopoietic engraftment in a substantial fraction of transplant recipients. Preconditioning is associated with significant levels of toxicity (14); these side effects may also alter the environments supporting the maintenance and differentiation of HSCs, influencing the interpretation of outcomes in this setting.
In the present report, we introduce a zebrafish model for hematopoietic cell transplantation that overcomes the above limitations. We demonstrate that unmanipulated fish homozygous for the c-mybt25127 allele, carrying a missense mutation (I181N) in the highly conserved DNA binding domain (17), stably accept allogeneic hematopoietic cell transplants, thus obviating the need for myeloablation before transplantation. We have exploited this unexpected property of the c-myb mutant model to examine key parameters of HSC biology in zebrafish.
Results
Transplantation of Whole Kidney Marrow Leads to Sustained Hematopoietic Activity in c-myb Mutants.
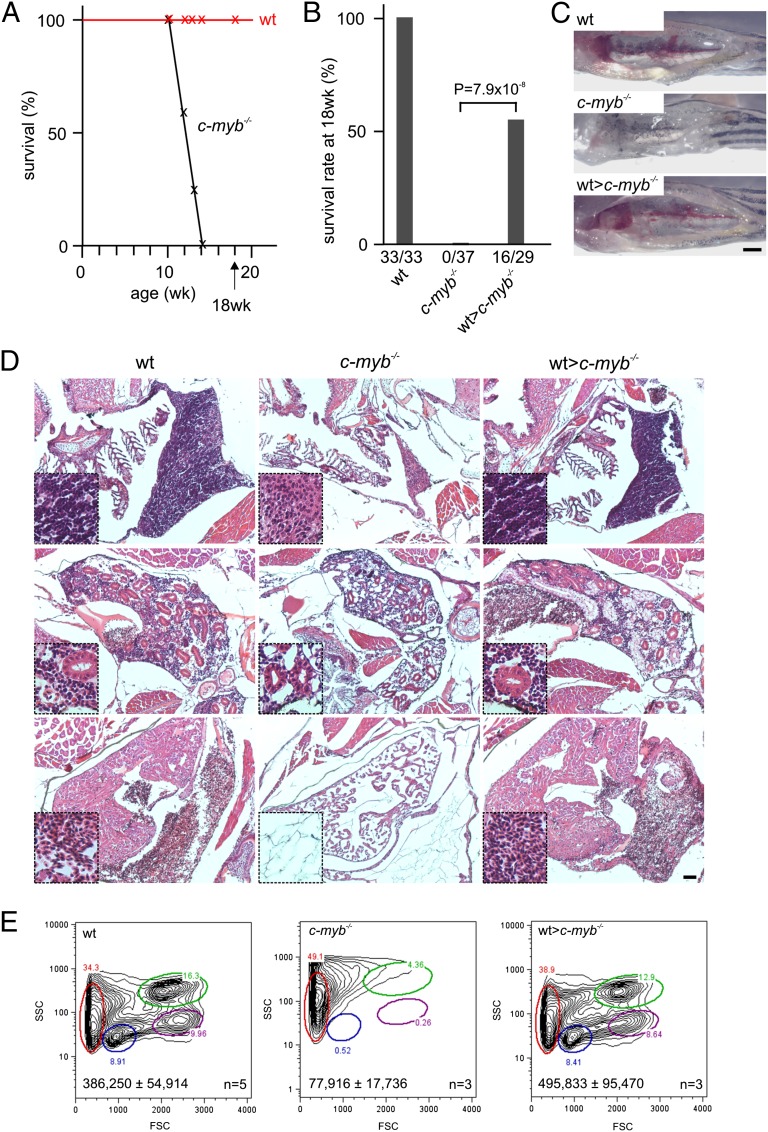
Fish homozygous for the c-mybt25127 allele encoding a mutant form of the c-myb transcription factor, c-mybI181N (hereafter referred to as c-myb mutants), lack definitive hematopoiesis (17). The mutant fish are easily distinguishable from their heterozygous or wild-type siblings by their smaller size, developmental retardation, anemia (accompanied by cardiac edema), and lack of sexual maturation. However, these mutants are capable of surviving for several weeks (Fig. 1A). Using retro-orbital injections (18), we transplanted unfractionated whole kidney marrow (WKM) cells (∼4 × 105 cells per recipient) from wild-type fish into 6–9-wk-old c-myb mutants; this time point was chosen because the fish have by then reached a size large enough to facilitate experimental manipulation yet are still healthy enough to cope with the transplantation procedure. The wild-type donor cells were transgenic for an ikaros:eGFP reporter gene, encoding an enhanced green fluorescent protein under the transcriptional control of the ikaros gene (19) to enable intravital tracking of the fluorescing transplanted cells. Although procedure-related mortality of about 50% was high, we established that a substantial fraction of transplanted mutant fish survived their untransplanted siblings by several months (Fig. 1B). Strikingly, the transplanted mutants grew rapidly, acquired a reddish complexion, lost their cardiac edema, and eventually exhibited the characteristic signs of sexual dimorphism. As a result, their phenotype was indistinguishable from that of their wild-type siblings about 5 wk after transplantation (Fig. S1A); accordingly, their lifespan was extended to well over 8 mo, the latest time point of our longitudinal observations. The macroscopic appearance of the head kidneys in transplanted c-myb mutants indicated the presence of hematopoiesis (Fig. 1C); likewise, productive thymus colonization was evident from the strong green fluorescence emanating from ikaros-expressing thymocytes (Fig. S1B). The characteristic features of ongoing hematopoietic activity were also observed in histological sections of thymus and kidney: the thymus was densely populated by lymphoid cells; the kidney of transplanted mutant fish exhibited the cellular pleomorphy of normal hematopoietic tissues (Fig. 1D). Furthermore, blood vessels were replete with mature erythrocytes and lymphoid and myeloid cells (Fig. 1D). In accordance with the histological appearance of kidney sections, flow cytometric analysis of WKM cell preparations indicated that the major cell populations, distinguishable by their light scatter characteristics (8), are present in normal proportions, thus supporting the notion of multilineage engraftment (Fig. 1E). The phenotypic normalization of hematopoietic tissue morphology is supported by the expression of genes indicative of restored hematopoietic activity (c-myb), including lymphoid (rag1) and myeloid (mpx) differentiation (Fig. S1 C and D). Collectively, these qualitative results indicated that the transplantation of WKM cells reestablished normal hematopoietic activity in c-myb mutants; this also holds true in quantitative terms, as the numbers of hematopoietic cells in the WKM cell preparations reach wild-type levels (Fig. 1E). No indication of the presence of GvHD, such as edema, ascites, and flaring of scales (14), was observed in any of the transplanted fish. To exclude the possibility that ikaros:eGFP transgenic wild-type hematopoietic cells stimulate hematopoietic activity of nontransgenic mutant recipient tissues, we examined the fraction of green cells in the WKM of donor, primary, and secondary (see below) transplant recipients; the percentage of green cells does not change in transplanted fish, indicating that nonfluorescent host cells do not contribute to hematopoietic recovery (Fig. S2A).
Fig. 1.
Reconstitution of the c-myb−/− mutant phenotype. (A) Survival of wild-type (n = 33) and c-myb−/− mutant (n = 37) fish; mutants do not survive longer than 14 wk of age. (B) Survival rates of wild-type, c-myb−/− mutants and mutant recipients transplanted with wild-type WKM (wt>c-myb−/−) transgenic for an ikaros:eGFP reporter assessed at 18 wk of age. All deaths occurring within the first 24 h after transplantation were considered to be procedural and thus not used for the calculation of survival rates. (C) Macroscopic appearance of head kidneys in wild-type, c-myb−/− mutants and transplanted mutant recipients; note that mutant kidney shows a complete lack of hematopoietic cells (17). (Scale bar, 1 mm.) (D) Hematoxylin/eosin staining of sections through the region of the thymus (Top), kidney (Middle), and heart (Bottom) of wild-type, c-myb−/− mutants and transplanted recipients at 9 wk of age (i.e., 3 wk after transplantation); insets are at fourfold higher magnifications. Note the lack of hematopoietic cells in the mutants and complete hematopoietic reconstitution in transplanted recipients. (Scale bar, 50 μm.) (E) Flow cytometric analyses of WKM cells of wild-type, c-myb−/− mutants and transplanted recipients as in D. FSC, forward light scatter; SSC, side light scatter. Circles denote different blood cell populations in adult wild-type fish: red, erythrocytes; blue, lymphocytes; magenta, precursors; green, myelomonocytes. Total cell numbers (mean± SD) in WKM preparations are indicated.
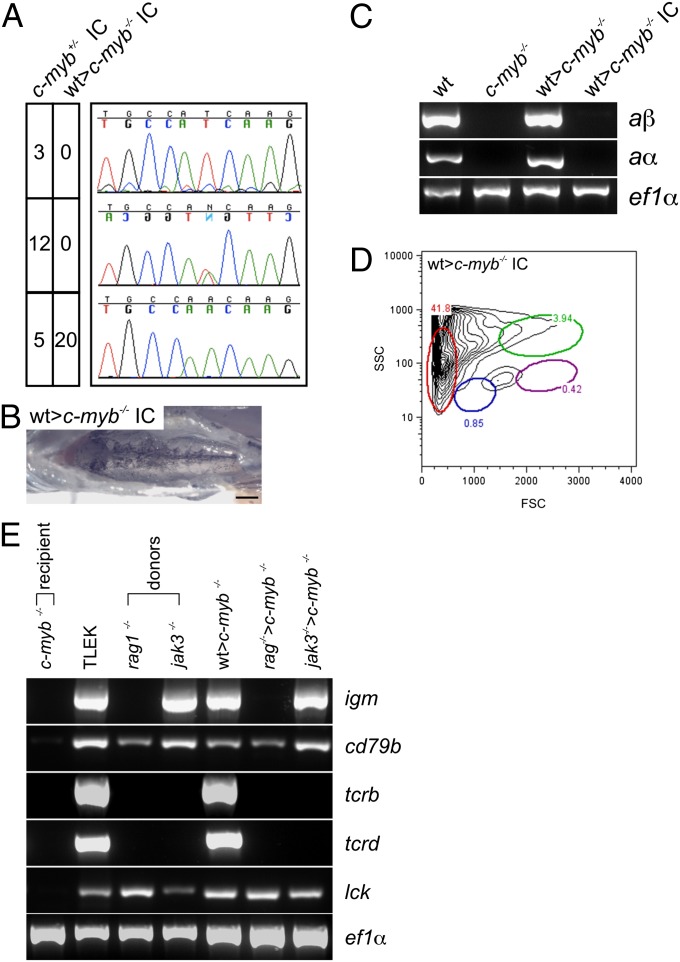
The above observations suggest that at least some of the nonhematopoietic phenotypes in c-mybt25127 homozygous fish, including early lethality, are not caused by the lack of wild-type c-myb activity but were an indirect consequence of failing definitive hematopoiesis. We examined this issue directly by assessing the fertility of successfully transplanted sexually dimorphic c-myb mutants (Fig. S1A). To this end, we mated hematopoietically rescued male and female c-myb mutant fish and examined the genotypes of their offspring. Whereas an incross (IC) of c-myb+/t25127 heterozygous fish yielded the expected wild-type, heterozygous, and homozygous mutant genotypes, all progeny of mutant matings were homozygous for the c-myb mutation (Fig. 2A) and exhibited the characteristic phenotypic abnormalities (Fig. 2 B–D). The transplantation of wild-type hematopoietic cells restores immune function to c-myb mutants; whereas all mutants die from overwhelming infection within 2–3 d of clipping their fins, the transplanted fish survive this insult (Fig. S2B).
Fig. 2.
Characterization of reconstituted c-myb−/− mutants. (A) Genotypes of offspring embryos resulting from in-crosses of c-myb+/− heterozygous fish and reconstituted female and male c-myb−/− mutant fish, respectively. The fecundity of reconstituted transplant recipients is indistinguishable from wild-type fish. The number of wild-type (Top panels), heterozygous (Middle panels), and homozygous mutant (Bottom panels) embryos are indicated as are representative sequence traces. (B) Macroscopic phenotype of the head kidney in the offspring of reconstituted c-myb−/− mutants. (Scale bar, 1 mm.) (C) Expression of adult hemoglobin alpha (aα) and beta (aβ) genes in WKM cells of wild-type (wt), c-myb−/− mutants; reconstituted mutants (wt>c-myb−/−); and offspring of reconstituted mutants (wt>c-myb−/− IC) as determined by RT-PCR at 11–12 wk of age; amplification with ef1α-specific primers serves as a control for cDNA integrity and amount. (D) Flow cytometry analysis of WKM cells from offspring of reconstituted mutant fish (see Fig. 1E legend for explanation of indicated cell populations); profile representative of three fish. (E) Expression of T- and B-cell–specific marker genes in TLEK fish (wild-type strain), c-myb−/− mutants, rag1- and jak3-deficient donor fish, and c-myb−/− recipients transplanted with wild-type (transgenic for the ikaros:eGFP reporter), rag1-, and jak3-deficient WKM cells. cd79b, gene encoding the igβ adaptor protein, constituting part of the B-cell receptor in B cells; igm, Ig µ heavy chain gene expressed in B cells; lck, gene encoding a receptor-associated tyrosine kinase expressed in T cells; tcrb, T-cell receptor β chain gene expressed in T cells; tcrd, T-cell receptor δ chain gene expressed in T cells.
Transfer of Genetically Impaired Hematopoietic Systems to c-myb Mutant Recipients.
The success of hematopoietic cell transplantations prompted us to examine the possibility of transferring genetically impaired hematopoietic tissues to allogeneic hosts. To this end, we chose two well-characterized genotypes for further study, rag1- (20) and jak3- (21) deficiencies. Phenotypically, these mutant genotypes rescued the characteristic anemia of c-myb mutants, as expected, but recapitulated the distinct lymphocyte lineage-specific defects; in particular, no somatic assembly of tcr and ig genes could be detected in rag1−/− fish and in jak3-deficient hematopoietic chimeras, T-cell development was affected to a greater extent than B-cell development (Fig. 2E).
Thymus-Settling Cells in Adult WKM.
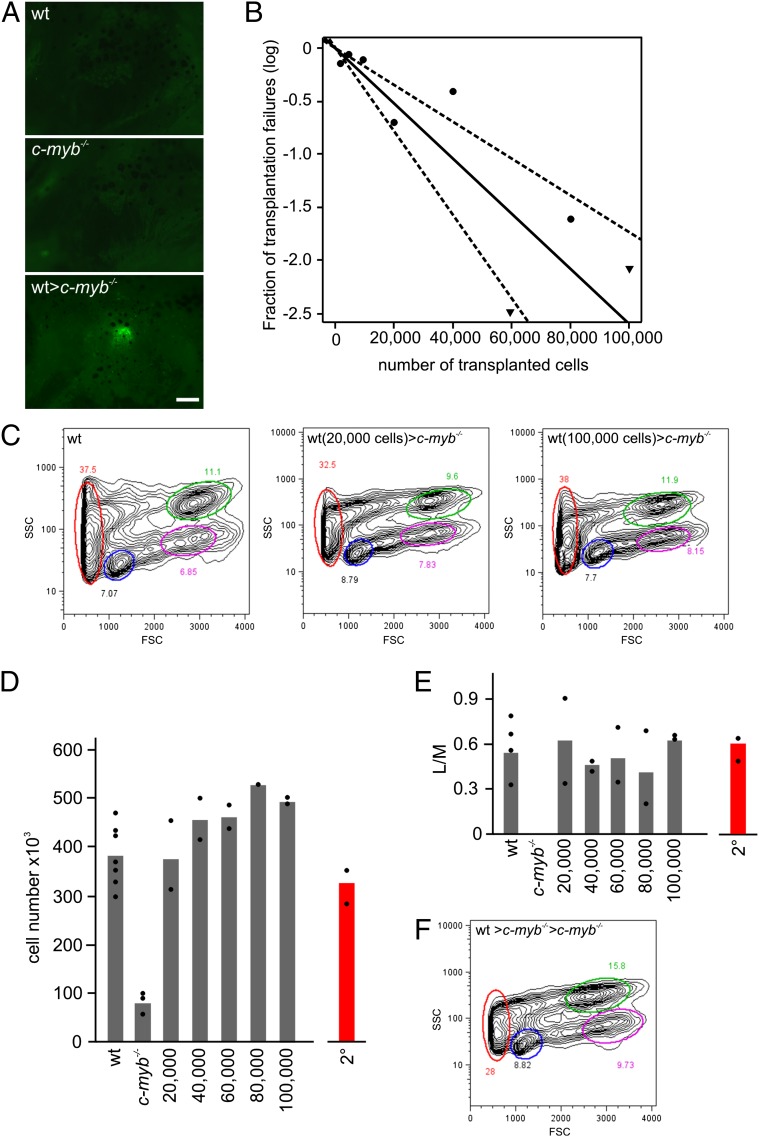
Under physiological conditions, T-cell development in the thymus is initiated by importation of T-cell precursors (22). Alymphoid c-myb mutants represent a unique opportunity to examine the presence of thymus-settling cells in zebrafish WKM. To this end, we transplanted WKM from adult wild-type fish transgenic for an ikaros:eGFP reporter gene (19) to enable noninvasive monitoring of the process of thymus colonization by fluorescence microscopy. Thymus colonization, as detected by the presence of eGFP-positive cells in the thymus rudiment, takes place in less than 7 d after transplantation (Fig. 3A). These results also demonstrate that the thymic epithelium remains receptive for progenitors for an extended period; in previous experiments using ikaros-deficient fish (22), the observed dormancy period of thymic niches was in the order of 2 wk, considerably shorter than the 6–7 wk observed here.
Fig. 3.
Long-term multilineage reconstitution of c-myb−/− mutant fish. (A) Thymus colonization in c-myb−/− recipients transplanted with wild-type ikaros:eGFP-transgenic WKM cells. Photographs (side views) were taken 7 d after transplantation. (Scale bar, 50 μm.) (B) Limiting dilution analysis of hematopoietic reconstitution of c-myb−/− mutant fish. (C) Flow cytometric analysis of WKM of wild-type fish (Left) and stably reconstituted c-myb−/− mutants after transplantation of 20,000 (Middle) and 100,000 (Right) wild-type WKM cells (see Fig. 1E legend for explanation of indicated cell populations). (D) Cellularity of WKM of wild-type (wt) fish, c-myb−/− mutants, and mutants stably reconstituted after transfer of the indicated numbers of WKM wild-type cells (9 wk after transplantation). The mean values (bars) and results of individual fish are shown. The number of cells found in WKM in secondary transplant recipients (2°) is depicted in red. (E) Multilineage reconstitution as measured by the ratio of cells in lymphoid (L) and myeloid (M) gates in flow cytometric analyses for fish depicted in D. (F) Flow cytometric analysis of WKM of stably reconstituted c-myb−/− mutants after transplantation of WKM of primary recipients (see Fig. 1E legend for explanation of indicated cell populations).
Frequency of Repopulating Hematopoietic Precursor Cells.
Using limiting dilution (23), we determined the number of transplantable HSCs in WKM preparations. Success rates of engraftment and stability of hematopoietic reconstitution were assessed 9 wk after transplantation. The results indicate that ∼1 in 38,140 (confidence interval, 1/57,742; 1/25,550) cells of wild-type WKM (Fig. 3B) possess long-term multilineage hematopoietic reconstitution properties (Fig. 3C), compatible with previous estimates (14). This indicates that ∼10 transplantable HSCs are present in the WKM of a single adult zebrafish.
Next, we examined whether a dose–response relationship existed between the input number of wild-type WKM cells and the resulting steady-state hematopoietic activity in rescued c-myb mutants, as measured by the number of cells in WKM preparations. Irrespective of the numbers of cells in the original inoculum, cellular yields from WKM preparations were always the same (Fig. 3D), with normal proportions of hematopoietic cell lineages (Fig. 3E). These results indicate that c-myb mutants provide appropriate niches conducive to the survival, self-renewal, and differentiation of exogenous HSCs that are contained in WKM cell preparations and suggest that transferred HSCs proliferate to occupy all available niches in the c-myb mutant recipient. Stable engraftment of hematopoietic cells was also observed in secondary c-myb mutant recipients, indicating that stem cells capable of contributing to all hematopoietic lineages had colonized the primary transplant recipients (Fig. 3 D–F).
Allogeneic Hematopoietic Cell Transplantation.
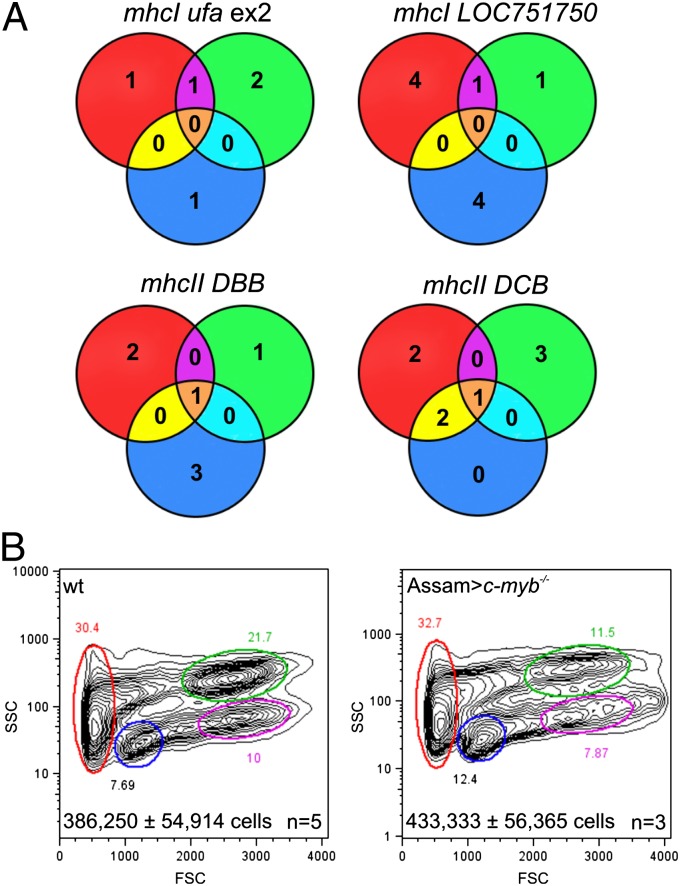
Two major factors impede the use of hematopoietic cell transplantation in fish biology. First, isogenic lines as a means of establishing histocompatibility between host and donor are exceedingly difficult to maintain owing to severe in-breeding depression (15, 16). Second, unlike mammals in which the MHC genes are encoded at a single chromosomal locus, fish possess several MHCI and MHCII loci that are situated on different chromosomes (24). Because of the fragmented nature of the MHC locus, MHC genotypes resulting from genetic crosses in fish species are difficult to predict. Although initial attempts at resolving the relative biological contribution of different MHC loci to the outcome of hematopoietic cell transplantation have been reported (14), transplantations across histocompatibility barriers remain a major challenge. Intriguingly, the experiments described above collectively suggested the possibility of successful allogeneic transplantations using c-myb−/− recipients, although the host–donor pairs were initially not examined for congruency at the MHC loci. To examine this issue more specifically, we surveyed the diversity of some mhcI and mhcII genes; these experiments revealed the presence of considerable allelic polymorphism (Fig. 4A; Figs. S2 and S3). For instance, when WKM cells from the Assam strain, a recently established wild-type zebrafish line (25), were transferred into c-myb mutants, successful hematopoietic reconstitution could be easily achieved, despite substantial allelic differences between MHC alleles of different strains (Fig. 4B; Figs. S2 and S3). Collectively, these analyses support the notion that unfractionated allogeneic WKM cells containing hematopoietic progenitor cells can be successfully transferred into unmanipulated c-myb mutants to achieve stable hematopoietic reconstitution.
Fig. 4.
Allogenic hematopoietic cell transplantation. (A) Analysis of allelic diversity of mhcI and mhcII genes in c-myb−/− mutants (red circles), Tg(ikaros:eGFP) wild-type fish (green circles), and Assam wild-type fish (blue circles). The Venn diagrams indicate the extent of overlap as determined by nucleotide sequences of the relevant parts of the indicated mhc genes (see Table S1 for primer sequences and Fig. S3 for protein sequence comparisons). (B) Flow cytometric analysis of WKM of wild-type and c-myb−/− mutants reconstituted with WKM cells of the Assam strain, 9 wk after transplantation (see Fig. 1E legend for explanation of indicated cell populations); representative profiles are shown. Total cell numbers (mean± SD) in WKM preparations are indicated.
Discussion
Here, we present a versatile model for studies on the hematopoietic system of zebrafish. We demonstrate that c-myb mutant fish readily accept allogeneic hematopoietic cell transplants without prior conditioning, thus providing the best possible physiological setting available to date to study the function of HSCs in a lower vertebrate. Whereas mice deficient for c-myb die of anemia in midgestation (26), the unique physiology of fish allows c-myb mutants to survive into adulthood in the absence of definitive hematopoiesis (17); this species-specific difference highlights the importance of exploring a wide range of animal models to identify those most suitable for addressing a specific biological problem. Because of its practicability, we expect that the c-myb mutant model described here will lend itself to several other applications that would have been essentially impractical under previous experimental schemes owing to the complexity and unclear functional relevance of the many loci collectively defining the MHC genotype. In addition to competitive repopulation assays using cells of different genotypes, c-myb mutants might also prove useful in experiments involving transplantations of tumors such as leukemic cells and solid tumors; we also envisage the use of this model in interspecific hematopoietic reconstitutions.
Apart from the practical advantage of working with a single gene deficiency provided by our model system, our results reveal key features of zebrafish HSC biology. The adult kidney marrow contains HSCs, at a concentration of ∼1/40,000 cells; considering that leukocytes comprise ∼50% of cells in the WKM preparations used for transplantation, this frequency is surprisingly similar to the frequency of HSCs in mouse bone marrow that is estimated to be in the order of 1/10,000 nucleated cells (27). It also suggests that the adult zebrafish kidney contains a total of about 10 transplantable HSCs, a surprisingly low number, but nonetheless compatible with estimates of the number of active HSCs sustaining hematopoiesis in the mouse (4). Collectively, our experiments support the notion that the properties of zebrafish HSCs are similar to those of mammals, further validating the use of the zebrafish hematopoietic system for in-depth genetic and chemical screening (28, 29).
Our results are also pertinent to the question of HSC niche formation and maintenance. We have demonstrated that c-myb mutants lack definitive hematopoiesis (17), either as a result of impaired function or complete absence of HSCs. If the former is true, the successful establishment of hematopoietic chimerism described here suggests that mutant HSCs are at a substantial competitive disadvantage when confronted with wild-type HSCs; if the latter holds, the mutant kidney must either contain preformed yet empty niches (generated and maintained in HSC-independent autonomous fashion) or be capable of elaborating such niches in response to incoming HSCs. Our finding that transplantations of WKM cells always result in wild-type levels of hematopoietic cells argues against the presence of normal numbers of HSCs in the kidney marrow of mutant fish, unless their competitive fitness for niche occupancy is very low. On the other hand, the impaired fitness of mutant HSCs would appear incompatible with their ability to survive the extended periods of time before transplantation takes place in the present experimental scheme. Therefore, we favor the explanation that c-myb mutants entirely lack definitive HSCs. It follows that the niches for HSCs either function cell autonomously or that the microenvironment is poised to generate them when required.
The successful hematopoietic transplantations across histocompatibility barriers observed here highlight the role of the recipients’ impaired immunocompetence. The use of early embryos as recipients (8) avoids the problem of rejection of transplanted cells; however, there is a limitation in terms of the number of cells that can be transplanted. To overcome this problem, adult fish must be used as recipients, but they in turn require irradiation to destroy the immune system before transplantation (9, 14). The c-myb mutants used here as recipients lack cells of the main hematopoietic lineages that are derived from HSCs, namely erythrocytes, myeloid cells, and lymphocytes. Although long-lived myelomonocytic cells that are generated during embryonic stages are still present in the adult fish, these cells do not seem to elicit unwanted graft versus host reactions by donor effector cells. Further study is required to ascertain whether this is due to the low number of embryonic myelomonocytic cells, to their developmentally imprinted inability to function as antigen-presenting cells (APCs), or to their intrinsic c-myb deficiency. In any case, the absence of GvHD in transplanted c-myb mutants indicates that the newly emerging T-cell compartment derived from donor HSCs—selected by host thymic epithelial cells and donor APCs—becomes tolerant to host tissues.
Methods
Zebrafish Stocks.
Zebrafish (Danio rerio) wild-type strains (TLEK and Assam—a kind gift of Dr. S. Zala, Konrad Lorenz Institute of Ethology, Vienna, Austria) and the transgenic (Tg) ikaros:eGFP (19) and the mutant c-mybt25127 (17) lines are kept at the Max Planck Institute of Immunobiology and Epigenetics. All procedures were conducted in accordance with institutional guidelines under a license from the local government and approved by the Institutional Animal Care and Use Committee at the Max Planck Institute of Immunobiology and Epigenetics.
Transplantation Procedure.
WKM cells from adult fish were harvested as described (8) and finally resuspended at 2,000–40,000 cells/μL in 0.9× PBS supplemented with 5% (vol/vol) FCS; cells were injected retro-orbitally (18) in a volume of 10 μL.
Flow Cytometry.
Flow cytometric analysis of light-scatter characteristics of WKM cells was carried out as described (8); staining with hydroxystilbamidine (Enzo Life Sciences; final concentration 1 μg/mL) was used to exclude dead cells.
Whole-Mount RNA in Situ Hybridization.
Whole-mount RNA in situ hybridization was performed with digoxigenin-labeled RNA riboprobes as described (17).
Gene-Expression Analysis.
Primers used for RT-PCR (17, 30, 31) are listed in Table S1.
MHC Genotyping.
Seven individuals, each resulting from in-crosses of c-myb+/−, Tg(ikaros:gfp), and Assam strains, respectively, were genotyped for different mhcI and mhcII genes by PCR using primers listed in Table S1. The PCR products from two mhcI and mhcII genes each were cloned into pGEM-T Easy vector (Promega) and sequenced (Fig. S4).
Supplementary Material
Acknowledgments
We thank D. Diekhoff for help and the Max Planck Society and the Deutsche Forschungsgemeinschaft for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219847110/-/DCSupplemental.
References
- 1.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Notta F, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 3.Larochelle A, et al. Human and rhesus macaque hematopoietic stem cells cannot be purified based only on SLAM family markers. Blood. 2011;117(5):1550–1554. doi: 10.1182/blood-2009-03-212803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 5.Dao MA, Yu XJ, Nolta JA. Clonal diversity of primitive human hematopoietic progenitors following retroviral marking and long-term engraftment in immune-deficient mice. Exp Hematol. 1997;25(13):1357–1366. [PubMed] [Google Scholar]
- 6.Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001;2(1):75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 7.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1(3):263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Traver D, et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4(12):1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 9.Traver D, et al. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood. 2004;104(5):1298–1305. doi: 10.1182/blood-2004-01-0100. [DOI] [PubMed] [Google Scholar]
- 10.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 11.Waskow C, et al. Hematopoietic stem cell transplantation without irradiation. Nat Methods. 2009;6(4):267–269. doi: 10.1038/nmeth.1309. [DOI] [PubMed] [Google Scholar]
- 12.Woolfrey A, Anasetti C. Allogeneic hematopoietic stem-cell engraftment and graft failure. Pediatr Transplant. 1999;3(Suppl 1):35–40. doi: 10.1034/j.1399-3046.1999.00068.x. [DOI] [PubMed] [Google Scholar]
- 13.Huang HT, Zon LI. Regulation of stem cells in the zebra fish hematopoietic system. Cold Spring Harb Symp Quant Biol. 2008;73:111–118. doi: 10.1101/sqb.2008.73.029. [DOI] [PubMed] [Google Scholar]
- 14.de Jong JLO, et al. Characterization of immune-matched hematopoietic transplantation in zebrafish. Blood. 2011;117(16):4234–4242. doi: 10.1182/blood-2010-09-307488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monson CA, Sadler KC. Inbreeding depression and outbreeding depression are evident in wild-type zebrafish lines. Zebrafish. 2010;7(2):189–197. doi: 10.1089/zeb.2009.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinya M, Sakai N. Generation of highly homogeneous strains of zebrafish through full sib-pair mating. G3 (Bethesda) 2011;1(5):377–386. doi: 10.1534/g3.111.000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soza-Ried C, Hess I, Netuschil N, Schorpp M, Boehm T. Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved. Proc Natl Acad Sci USA. 2010;107(40):17304–17308. doi: 10.1073/pnas.1004640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pugach EK, Li P, White R, Zon L. Retro-orbital injection in adult zebrafish. J Vis Exp. 2009;(34):1645. doi: 10.3791/1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajoghli B, et al. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138(1):186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Wienholds E, Schulte-Merker S, Walderich B, Plasterk RH. Target-selected inactivation of the zebrafish rag1 gene. Science. 2002;297(5578):99–102. doi: 10.1126/science.1071762. [DOI] [PubMed] [Google Scholar]
- 21.Iwanami N, et al. Genetic evidence for an evolutionarily conserved role of IL-7 signaling in T cell development of zebrafish. J Immunol. 2011;186(12):7060–7066. doi: 10.4049/jimmunol.1003907. [DOI] [PubMed] [Google Scholar]
- 22.Hess I, Boehm T. Intravital imaging of thymopoiesis reveals dynamic lympho-epithelial interactions. Immunity. 2012;36(2):298–309. doi: 10.1016/j.immuni.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Smyth GK. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1-2):70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Sültmann H, Mayer WE, Figueroa F, O’HUigin C, Klein J. Organization of Mhc class II B genes in the zebrafish (Brachydanio rerio) Genomics. 1994;23(1):1–14. doi: 10.1006/geno.1994.1452. [DOI] [PubMed] [Google Scholar]
- 25.Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish. 2007;4(1):21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 26.Mucenski ML, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65(4):677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 27.Mayle A, Luo M, Jeong M, Goodell MA. Flow cytometry analysis of murine hematopoietic stem cells. Cytometry A. 2013;83(1):27–37. doi: 10.1002/cyto.a.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns CE, et al. A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood. 2009;113(23):5776–5782. doi: 10.1182/blood-2008-12-193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jing L, Durand EM, Ezzio C, Pagliuca SM, Zon LI. In situ hybridization assay-based small molecule screening in zebrafish. Curr Protoc Chem Biol. 2012;4(2):143–160. doi: 10.1002/9780470559277.ch110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownlie A, et al. Characterization of embryonic globin genes of the zebrafish. Dev Biol. 2003;255(1):48–61. doi: 10.1016/s0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 31.Chan F-Y, et al. Characterization of adult α- and β-globin genes in the zebrafish. Blood. 1997;89(2):688–700. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.