Abstract
A transketolase reaction was catalyzed by cyanide ion under prebiotic conditions instead of its modern catalyst, thiamine pyrophosphate (TPP). Cyanide ion converted fructose plus glyceraldehyde to erythrose plus xylulose, the same products as are formed in modern biochemistry (but without the phosphate groups on the sugars). Cyanide was actually a better catalyst than was TPP in simple solution, where there is a negligible concentration of the C-2 anion of TPP, but of course not with an enzyme in modern biology. The cyanide ion was probably not toxic on prebiotic earth, but only when the oxygen atmosphere developed and iron porphyrin species were needed, which cyanide poisons. Thus, catalyses by TPP that are so important in modern biochemistry in the Calvin cycle for photosynthesis and the gluconic acid pathway for glucose oxidation, among other processes, were probably initially performed instead by cyanide ion until its toxicity with metalloproteins became a problem and primitive enzymes were present to work with TPP, or most likely its primitive precursors.
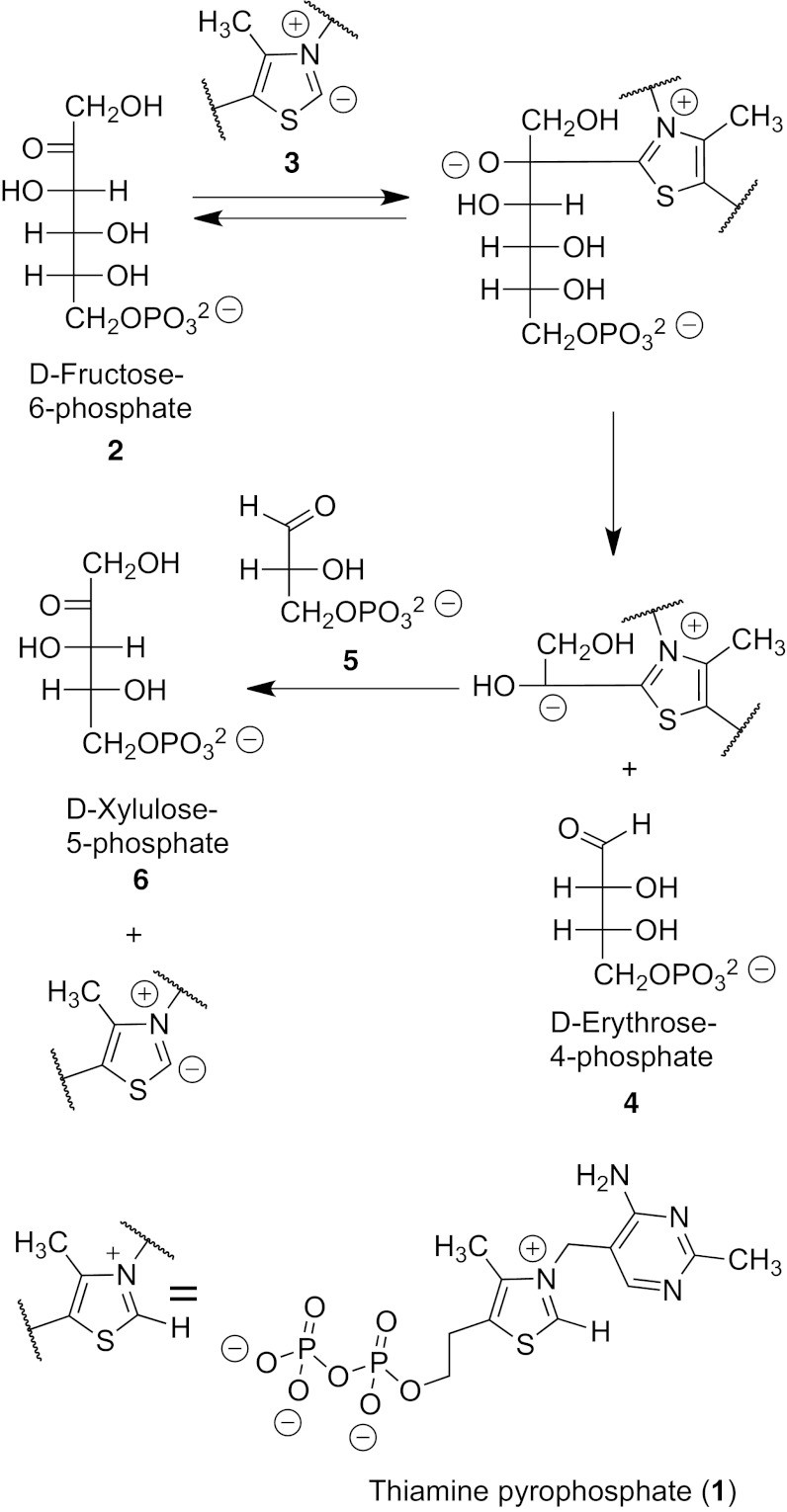
In modern biochemistry, transketolase reactions (1) play key roles in the Calvin cycle for photosynthesis and in the gluconic acid pathway for glucose conversion to carbon dioxide with ATP formation. The reactions are catalyzed by enzymes using thiamine pyrophosphate 1 (TPP) as the coenzyme. In a typical example (Fig. 1), the top two carbons of a ketosugar such as fructose-6-phosphate 2 (with an S stereocenter at carbon 3) are removed when the C-2 anion 3 of TPP adds to the carbonyl group of the ketosugar, allowing it to be removed as a TPP derivative of the hydroxyacetyl anion. This stays bound to the enzyme as the remaining erythrose-4-phosphate fragment 4 dissociates. Then a new aldosugar such as glyceraldehyde-3-phosphate 5 is bound to the enzyme and the two-carbon piece on TPP is added to the aldehyde group, in this case forming xylulose-5-phosphate 6 (with a new 3-S stereocenter) after the TPP anion 3 is expelled. The S stereochemistry is normally assumed to reflect enzyme selectivity. In photosynthesis, xylulose then isomerizes to ribulose with a 3-R configuration.
Fig. 1.
A transketolase reaction catalyzed by the enzyme with TPP as the coenzyme.
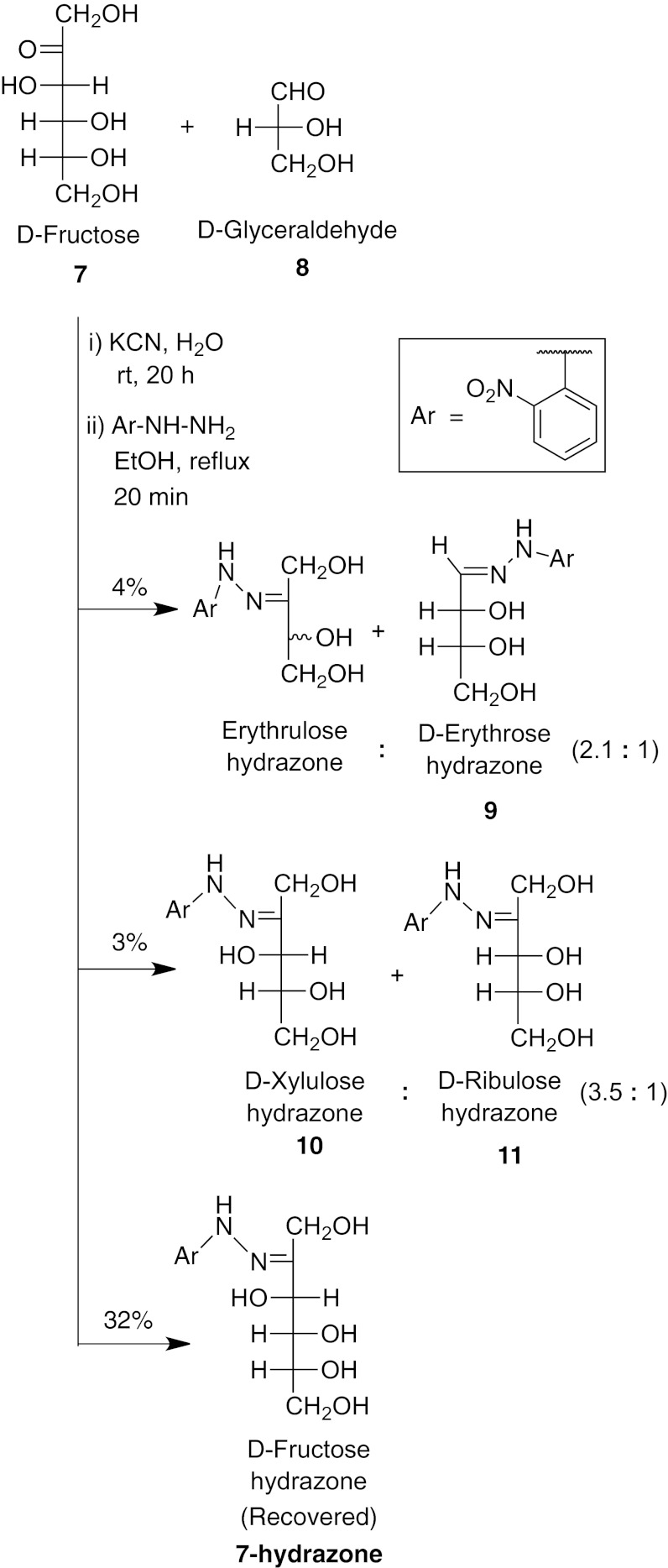
If such reactions were occurring in the prebiotic world, it is unlikely that a molecule as complex as TPP could have been the catalyst. Thus, we propose that the TPP anion 3 now used in biochemistry was instead a simple cyanide anion under prebiotic conditions. It would not be unacceptably toxic until iron compounds such as heme were developed to handle the oxygen atmosphere that developed after photosynthesis had begun. To test this idea, we have performed a transketolase reaction between D-fructose 7 and D-glyceraldehyde 8 with catalysis by cyanide ion (Fig. 2). We found that the major products were erythrose 9 and xylulose 10, parallel to the modern biochemical result (which involves sugar phosphates, not simple sugars). Strikingly, the new chiral center that is formed when a two-carbon piece is added to D-glyceraldehyde has mainly the S configuration of xylulose 10, not ribulose 11, as with the modern enzyme.
Fig. 2.
A nonenzymatic transketolase reaction catalyzed by cyanide ion under credible prebiotic conditions.
Procedures and Results
Transketolase Reaction of D-Fructose with D-Glyceraldehyde Catalyzed by Cyanide Ion.
Potassium cyanide (6.5 mg, 0.10 mmol) and D-fructose 7 (45 mg, 0.25 mmol) were dissolved in water (200 μL) and stirred for 16 h at room temperature. The pH was 10.2. During this time, we observed the formation of fructose cyanohydrin [a similar reaction was reported at pH 9.5 over 60 h (2)]. An aqueous solution of D-glyceraldehyde 8 (200 μL of 2.5 M, 0.50 mmol) was added, and it was stirred at room temperature for another 4 h. To the reaction mixture, ethanol (2.5 mL) was added, followed by 2-nitrophenylhydrazine mixed with 30% water (248 mg, 1.13 mmol), and the mixture was heated to reflux for 20 min. After cooling the mixture to room temperature, the solvents were removed under vacuum, the residue was dissolved in water (20 mL), and the solution was filtered. The filtrate was concentrated under vacuum and the residue was purified by preparative TLC on a silica plate with fluorescent indicator, using a solvent mixture of CHCl3/MeOH (4:1), to obtain 2-nitrophenylhydrazones of tetroses (Rf 0.7), pentoses (Rf 0.5), and hexoses (Rf 0.2). They were isolated and identified by comparison with authentic samples.
The results are shown in Fig. 2. The small amount of ribulose 11 seen could be a secondary product from equilibration of xylulose, because holding pure xylulose at 52 °C for 24 h at pH 12 with KOH converted the xylulose to a 2.5:1 ratio of xylulose to ribulose. Starting with D-glyceraldehyde, the D-xylulose was observed with only 10% ee, showing that some racemization of the D-glyceraldehyde had also occurred under our conditions. At the same pH 10.2 in the absence of cyanide, we have not observed any formation of four- or five-carbon sugars.
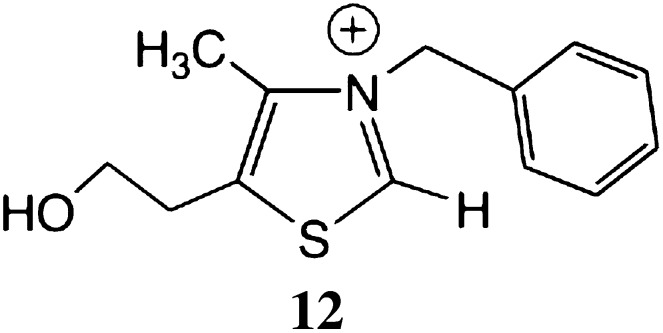
Transketolase Reaction of D-Fructose with D-Glyceraldehyde Catalyzed by Thiazolium Ions.
When a simple thiazolium salt 12 (Scheme 1), or thiamine, or TPP was used as the catalyst for the reaction of D-fructose with D-glyceraldehyde in phosphate buffer of pH 8, the pH we had used for previous thiazolium-catalyzed reactions (3, 4), the reaction was very slow and less efficient than that of cyanide ion-catalyzed reaction (Scheme 1).
Scheme 1.
A simple synthetic catalyst.
For example, the thiazolium salt catalyzed reaction was not complete even after 7 d and yielded only 2% of four-carbon sugars (4:1 erythrolose/erythrose) and 0.1% of five-carbon sugars. The tiny yield makes the identification of the dominant product not yet certain.
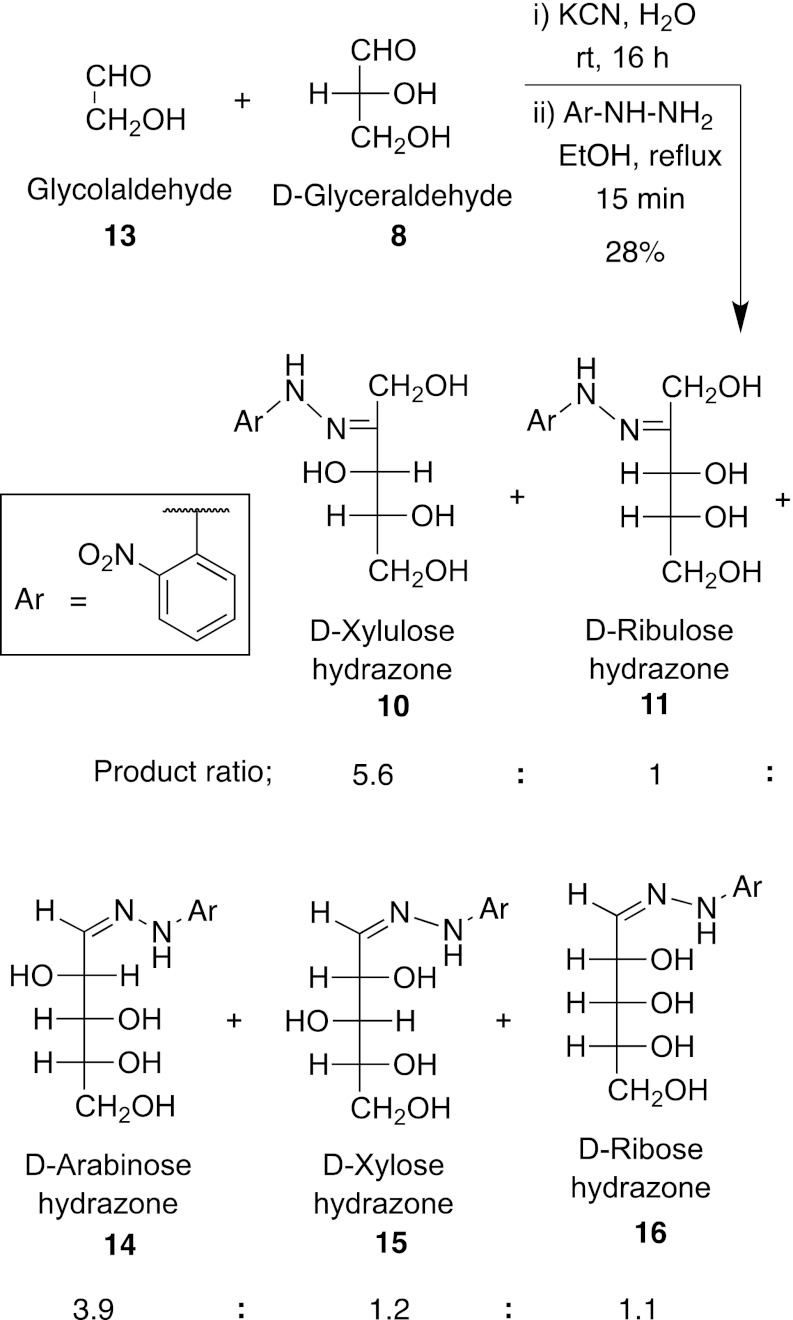
Ketolase Reaction of Glycolaldehyde 12 with D-Glyceraldehyde Catalyzed by Cyanide Ion (Fig. 3).
Fig. 3.
A nonenzymatic ketolase reaction catalyzed by cyanide ion under credible prebiotic conditions.
We have also examined possible ketolase reactions in which the two-carbon piece is derived from glycolaldehyde, rather than removed from fructose. Potassium cyanide (2.4 mg, 0.04 mmol) was dissolved in water (200 μL) and stirred for 5 min, and then an aqueous solution of glycolaldehyde (200 μL of 2.5 M, 0.50 mmol) was added and it was stirred at room temperature. After 5 min, an aqueous solution of D-glyceraldehyde (100 μL of 2.5 M, 0.25 mmol) was added, and the reaction mixture was stirred for 16 h at room temperature. To the reaction mixture, ethanol (2.5 mL) was added followed by 2-nitrophenylhydrazine mixed with 30% water (248 mg, 1.13 mmol), and the reaction mixture was heated to reflux for 15 min. After cooling to room temperature, solvents were removed under vacuum, and the residue was dissolved in water (20 mL) and filtered. The filtrate was concentrated under vacuum and the residue was purified by preparative TLC as above using a solvent mixture of CHCl3:MeOH (4:1) to obtain pentose hydrazones (Rf 0.5).
Again, we see the formation of xylulose as the major product (Table 1) along with ribulose and other five-carbon aldosugars such as arabinose, xylose, and ribose. However, in an alternate pathway, addition of glycolaldehyde to glyceraldehyde by an aldol reaction, not a ketol reaction, could form xylose that could then perhaps isomerize to xylulose. To examine this, we treated D-xylose with a catalytic amount of cyanide ion in water. After it was stirred for 16 h at room temperature, we observed the formation of only 3% D-xylulose in the reaction mixture, with 97% remaining xylose that we do not see in the cyanide-catalyzed ketolase reaction. Thus, xylulose is not chiefly being produced by an aldol and then isomerase sequence.
Table 1.
Ketolase reaction catalyzed by thiazolium salt at pH 8
| Entry | 5-Carbon sugars* | Product ratio† |
| 1‡ | Xylulose | 4.2 |
| 2 | Ribulose | 1 |
| 3 | Arabinose | 1.2 |
| 4 | Xylose | 0.5 |
| 5 | Ribose | 0.4 |
| 6 | Lyxose | 0.4 |
*Isolated as 2-nitrophenyl hydrazone derivative.
†Determined by 1H-NMR spectra.
‡Enantiopurity of D-xylulose was determined by HPLC analysis as 1.1% ee.
As another possibility, the glyceraldehyde could isomerize to dihydroxyacetone that could then add to glycolaldehyde in an aldol reaction forming xylulose. In this case, starting with D-glyceraldehyde the xylulose would be racemic, whereas in the ketolase reaction or the aldol addition of glycolaldehyde to D-glyceraldehyde the product xylulose would not be racemic. We find the formation of D-xylulose but with only 3.7% ee. At the same pH (10) with buffer in the absence of cyanide ion, we find no reaction. Therefore, this aldol process can account for some of the xylulose product, but not all, if we assume that cyanide ion can act as a general base catalyst of the aldol reaction as well as a nucleophilic catalyst of the ketolase reaction.
Ketolase Reaction of Glycolaldehyde 12 with D-Glyceraldehyde Catalyzed by Thiazolium Ions.
When thiazolium salt 12, thiamine hydrochloride, or TPP was used as the catalyst for the reaction of glycolaldehyde with D-glyceraldehyde in phosphate buffer of pH 8, the reaction was again very slow and less efficient than that of the cyanide ion catalyzed reaction. Even after 7 d the starting compounds were not completely consumed. Five-carbon sugars (17% yield) were identified as their 2-nitrophenylhydrazones. In the case of the thiazolium salt catalysts, the preferred formation of D-xylulose was again observed with 1.1% ee so some racemization had occurred.
Discussion
Many prebiotic reactions of sugars are relatively simple, involving aldol additions catalyzed by various bases, such as the synthesis of D-glyceraldehyde from formaldehyde and glycolaldehyde catalyzed by amino acids (5, 6). However, the transketolase reaction is different. It requires the special catalysis that thiamine pyrophosphate furnishes presently, but this complex molecule must have occurred on primitive earth well after photosynthesis made it necessary. The transketolase reaction is a central part of the Calvin path for photosynthesis, as well as the gluconic acid pathway for glucose oxidation, but a simpler catalyst must have been used in prebiotic times. In the Calvin cycle, xylulose-5-phosphate is then isomerized to ribulose-5-phosphate, which is then phosphorylated to the 1,5-ribulose diphosphate that is the recipient of carbon dioxide in the photosynthesis process.
In our work detailing how thiamine catalysis occurs generally (3), we had pointed out its relationship to such reactions as the benzoin condensation, normally catalyzed by cyanide ion. Thus, we now propose that cyanide ion performed reactions such as the transketolase process on prebiotic earth, and probably other reactions for which present-day biology uses TPP. When an oxygen atmosphere developed, and species such as iron porphyrins became important, the toxic cyanide was replaced, ultimately with the complex molecule TPP.
We find that cyanide ion is a better catalyst for the transketolase reaction than is TPP, or simpler thiamine, or even simpler 10. However, in the modern world, TPP has clear advantages. Most important, it is not toxic because its anion is not present in significant concentration at pH 8 or 10 (the pKa is ∼18), except for its local concentration in the enzyme pocket next to the substrate. This is the main reason that cyanide ion is a better catalyst for our reactions. With the yield actually seen with TPP and an estimate of pKa 18, we conclude that the TPP C-2 anion 3 is actually more effective as a catalyst than is cyanide ion; it is simply present in much lower concentration. Also, TPP is a large molecule that can bind strongly to enzymes, along with the substrates, giving it both rate and selectivity advantages. With the appropriate enzyme, it is much faster and more selective compared with simple cyanide ion. Thus, TPP replaced cyanide ion at some point in earth’s history, once enzymes were developed, but simple cyanide ion made processes such as the transketolase reaction possible in earlier times.
The mechanism of the reaction of fructose with nonenzymatic catalysts must involve the addition of cyanide ion or the C-2 anion 3 of a thiazolium salt to the carbonyl group of fructose, which then fragments to a carbonyl adduct of glycolaldehyde and a molecule of erythrose. In the absence of another aldose such as glyceraldehyde, this fragmentation must be reversible, whereas with an excess of glyceraldehyde, which we use, the two-carbon piece adds to it in a competition with erythrose to form xylulose. Thus, we examined another way to form the same product. We started with glycolaldehyde, equilibrated it with the catalyst, and then added D-glyceraldehyde. We call this process the ketolase reaction, in a sense the second half of the transketolase reaction. Again, we ran the reaction for 16 h with cyanide ion at pH 10.2 but with the thiazolium salts at pH 8.0 for 7 d. The results were similar to those in the full transamination processes, but not identical because the relative concentrations were not the same.
It is striking that there is no cleavage of fructose by cyanide ion in the transketolase reaction until the glyceraldehyde is added, and no erythose or glycolaldehyde could be detected. Of course, glyceraldehyde cannot cause the fructose cleavage. The cleavage of fructose by cyanide ion must be reversible, and with an unfavorable equilibrium. After cleavage, the cyanohydrin anion of glycolaldehyde simply adds back to the erythrose, and the overall transketolase reaction is seen only when glyceraldehyde can intercept this cyanohydrin anion in competition with the erythrose. Our ketolase reaction starts with the anion of erythrose cyanohydrin, and glyceraldehyde is its only receptor molecule. This explains why the yield of product is quite respectable in the ketolase reaction, in contrast with the very modest yield in the transketolase case.
Acknowledgments
Support of this work by a grant from the National Aeronautics and Space Administration is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
References
- 1.Schenk G, Duggleby RG, Nixon PF. Properties and functions of the thiamin diphosphate dependent enzyme transketolase. Int J Biochem Cell Biol. 1998;30(12):1297–1318. doi: 10.1016/s1357-2725(98)00095-8. [DOI] [PubMed] [Google Scholar]
- 2.Perlin AS, Purves CB. Kiliani's reduction of glucose and fructose cyanohydrins to the corresponding heptanoic acids and lactones. Can J Chem. 1953;31:227–235. [Google Scholar]
- 3.Breslow R. On the mechanism of thiamine action. IV. Evidence from studies on model system. J Am Chem Soc. 1958;80:3719–3726. [Google Scholar]
- 4.Zhao H, Foss FW, Jr, Breslow R. Artificial enzymes with thiazolium and imidazolium coenzyme mimics. J Am Chem Soc. 2008;130(38):12590–12591. doi: 10.1021/ja804577q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslow R, Cheng ZL. On the origin of terrestrial homochirality for nucleosides and amino acids. Proc Natl Acad Sci USA. 2009;106(23):9144–9146. doi: 10.1073/pnas.0904350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslow R. A likely possible origin of homochirality in amino acids and sugars on prebiotic earth. Tetrahedron Lett. 2011;52:2028–2032. [Google Scholar]