Abstract
Bisphenol A (BPA) is a ubiquitous compound that is emerging as a possible toxicant during embryonic development. BPA has been shown to epigenetically affect the developing nervous system, but the molecular mechanisms are not clear. Here we demonstrate that BPA exposure in culture led to delay in the perinatal chloride shift caused by significant decrease in potassium chloride cotransporter 2 (Kcc2) mRNA expression in developing rat, mouse, and human cortical neurons. Neuronal chloride increased correspondingly. Treatment with epigenetic compounds decitabine and trichostatin A rescued the BPA effects as did knockdown of histone deacetylase 1 and combined knockdown histone deacetylase 1 and 2. Furthermore, BPA evoked increase in tangential interneuron migration and increased chloride in migrating neurons. Interestingly, BPA exerted its effect in a sexually dimorphic manner, with a more accentuated effect in females than males. By chromatin immunoprecipitation, we found a significant increase in binding of methyl-CpG binding protein 2 to the “cytosine-phosphate-guanine shores” of the Kcc2 promoter, and decrease in binding of acetylated histone H3K9 surrounding the transcriptional start site. Methyl-CpG binding protein 2-expressing neurons were more abundant resulting from BPA exposure. The sexually dimorphic effect of BPA on Kcc2 expression was also demonstrated in cortical neurons cultured from the offspring of BPA-fed mouse dams. In these neurons and in cortical slices, decitabine was found to rescue the effect of BPA on Kcc2 expression. Overall, our results indicate that BPA can disrupt Kcc2 gene expression through epigenetic mechanisms. Beyond increase in basic understanding, our findings have relevance for identifying unique neurodevelopmental toxicity mechanisms of BPA, which could possibly play a role in pathogenesis of human neurodevelopmental disorders.
In the central nervous system (CNS), neuronal chloride is a fundamental cellular parameter that dictates neurotransmission by GABA, the main inhibitory transmitter (1, 2). Equilibrium at low levels of intracellular chloride is maintained by potassium chloride cotransporter 2 (KCC2), which is exclusively responsible for chloride extrusion from mature neurons. Development of the CNS is characterized by initially high neuronal chloride in early neurons that correlates with their migrational phenotype, followed by a decrease during perinatal development. This highly dynamic change is supported by concomitant increase in KCC2 expression and referred to as the chloride shift (2–5). Low neuronal chloride underlies the function of GABA as the predominant inhibitory transmitter of the mature CNS, quintessential for normal functioning of all subsections of the CNS, including brain, spinal cord, and retina. The expression of KCC2 in developing neurons is sexually dimorphic, with significantly lower Kcc2 mRNA levels in males relative to females during the perinatal period (6). Moreover, the chloride shift is of fundamental relevance for migration of cortical inhibitory precursor neurons to their proper final locations in the brain’s cortex. Also, KCC2 has a nontransporter function as a perinatal synchronizer of cellular neuronal development, critically influencing length of neuronal spines and the density and function of neuronal synapses (7). Whereas some mechanistic detail is known in regards to modulation of the chloride shift, we do not know which environmental agents might interfere with it.
External chemical cues that expose the developing CNS during susceptible periods can corrupt CNS development. Bisphenol A [BPA or 2,2-bis-(4-hydroxy-phenyl)propane] is an extensively investigated environmental xenobiotic. BPA is widely used in the production of a wide range of consumer products including food- and drink-storage containers, medical equipment, dental sealants, and as paper coating in thermal printers. Annual production of BPA worldwide amounts to >3.6 × 106 tons. Not surprisingly, human exposure to BPA is widespread and global. Possibly indicative of increasing exposures, urinary BPA levels are higher in children than adults, despite earlier concerns about untoward health effects of BPA (8, 9). For example, increasing urinary levels of BPA correlate significantly with behavioral abnormalities in girls <4 y of age. Also, urinary levels of BPA correlate significantly with obesity levels in a large Chinese cohort, highlighting worldwide impact. Animal studies suggest that in utero and early postnatal exposure to BPA produce a broad range of adverse effects (10–12). Historically, endocrine-reproductive and immune function-related abnormalities were documented first, and impaired brain development and behavior have been linked to BPA exposure more recently (13–15).
Mechanisms of how BPA can affect CNS development, behavior and neuroendocrinium are elusive. Possibly BPA can reprogram gene regulation in the developing CNS by altering the epigenome because epigenetic effects of BPA have been described (16–18). Despite the relatively recent discovery of the relevance of epigenetic regulation for development and function of the nervous system, it is considered a fact that epigenetic gene regulation is critical for normal neural development and differentiation (19). Epigenetic modulation of gene expression is driven by covalent modifications of amino acid residues in histone proteins, changes in the methylation status of DNA, and by noncoding RNAs. These factors determine chromatin configuration and accessibility to transcription factors, which in turn recruit specific cofactors to regulatory DNA binding sites influencing transcription outcome. Exposure to ubiquitous environmental pollutants such as BPA can induce epigenetic changes with profound dysregulation of target genes (20, 21). Despite this compelling theoretical concept, little concrete experimental evidence is available in support of it.
methyl-CpG binding protein 2 (MECP2) is a gene-regulatory protein with particular relevance for normal neuronal functioning and is highly expressed in the brain. MECP2 has been shown to bind chromatin globally (22, 23). Gain- and loss-of-function mutations in humans cause the progressive neurodevelopmental disorder, Rett syndrome, which is the most common cause of mental retardation in girls. Results from Mecp2−/− and humanized MECP2(mut)-mice corroborate these findings. In terms of gene regulation, MECP2 is canonically known as part of repressor complexes, capable of binding methylated DNA and contributing to transcriptional repression of genes (24). MECP2 mediates transcriptional repression through interaction with histone deacetylases (25). Conversely, acetylation of histone proteins represents an active chromatin mark. Acetylation of histone proteins opens up chromatin, which is subsequently accessible to transcription factors so that transcription can be activated. Acetylation of lysine-9 of histone H3 (H3K9ac) has been reported to occupy regions surrounding the transcriptional start site (TSS) of genes (26).
Interestingly, the promoter region of the forebrain and major isoform of Kcc2, Kcc2b (referred to as Kcc2 from here), contains a typical 5′-cytosine-phosphate-guanine (CpG) island (CGI). More recently, the relevance of the shores of 5′-CpG islands, rather than the CGIs themselves, has been stressed in terms of dynamic regulation of gene activity by epigenetic mechanisms (27, 28).
To address the lack of experimental evidence for BPA relying on an epigenetic mechanism to change neuronal functioning, and also to advance understanding of how environmental agents modulate the perinatal chloride shift, we have examined the effects of BPA exposures on the chloride shift. Specifically, we have asked whether BPA exposure can affect Kcc2 gene expression during perinatal neural development in cortical neurons and whether BPA exerts its actions on Kcc2 and thus the chloride shift via epigenetic mechanisms. Overall, our results are affirmative, indicating that epigenetic mechanisms are usurped by BPA. Importantly, we were able to verify hallmarks of our findings in human primary neurons, thus validating our rodent-based mechanistic studies. Interestingly, we uncovered a sexual dimorphism in the detrimental impact of BPA on the developmental regulation of Kcc2 expression. These effects directly affect cortical development, the chloride shift, and a critical component of inhibitory neurotransmission in the CNS.
Results
BPA Retards Developmental Up-Regulation of Kcc2 mRNA in Perinatal Neurons and the Effect Can Be Rescued by Histone-Deacetylase Inhibitor.
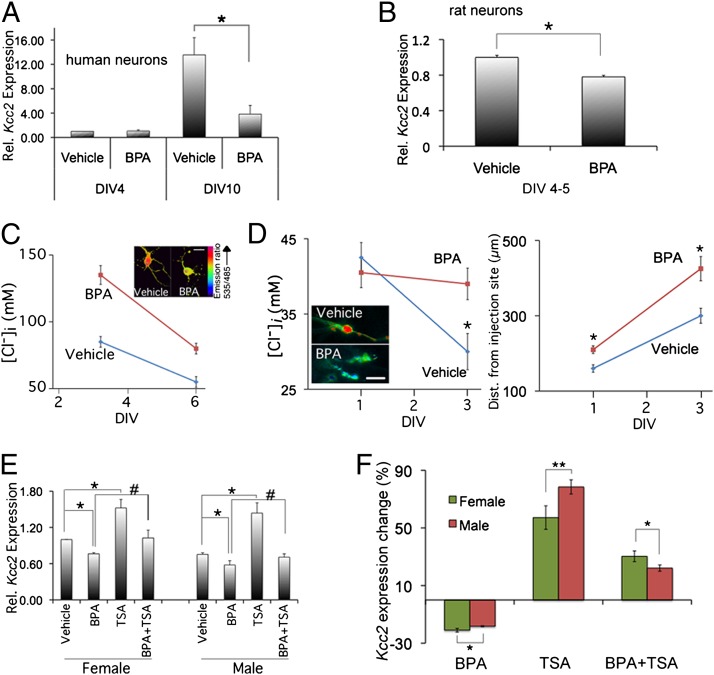
We intended to study the effects of BPA exposure on Kcc2 expression during the perinatal period. Using developing primary neurons, we established that 100 nM BPA significantly decreased KCC2/Kcc2 mRNA expression in cultured human and rat cortical neurons (Fig. 1 A and B) (29). Fig. S1 shows the dose–response effect of BPA on Kcc2 mRNA expression and viability in rat neurons. At a concentration of 100 nM BPA, cell viability was 95% (using trypan blue exclusion), but at 500-nM BPA cell viability was 70%. Hence we decided to treat neurons with 100 nM BPA throughout. Because an increase of KCC2 expression is a key reason for decrease of chloride in perinatal CNS neurons, we verified corresponding changes in reduction of intraneuronal chloride concentration, [Cl−]i. In fact, exposure of cultured rat neurons to 100 nM BPA led to increased [Cl−]i, as part of a delay, but not elimination of the chloride shift (Fig. 1C).
Fig. 1.
Down-regulation of Kcc2 mRNA expression by BPA; rescue of the effect of BPA by HDAC inhibitor, TSA. (A) BPA (100 nM) down-regulates KCC2 mRNA expression in human primary cortical neurons.*P < 0.001, n = 4 independent experiments. (B) Likewise finding in rat neurons; the average reduction is ≥25%; P < 0.001; n = 10; DIV4-5. (C) In cultured rat cortical neurons, BPA exposure leads to increased [Cl−]i (clomeleon methodology) (Inset) (Scale bar, 15 µm.). Differences highly significantly different between BPA (100 nM) and vehicle, n ≥ 50 neurons per data point; *P < 0.01. (D) Slice culture from embryonic rat brain: BPA (100 nM) leads to attenuation of chloride shift in migrating cortical inhibitory precursor neurons. Note the overmigration of BPA-treated neurons. (Scale bar, 20 µm.) *P < 0.05 for [Cl−]i (n ≥ 25 neurons on DIV1, n ≥ 50 on DIV3) and *P < 0.001 for migration (n ≥ 70 on DIV1, n ≥ 99 on DIV3). (E) Down-regulatory effect of BPA (greater difference in females, indicating increased susceptibility), and rescue by coexposure BPA with TSA (100 nM). *P < 0.01, #P < 0.05; n = 3 independent experiments. (F) Quantification of sex-specific differences, suggesting an increased susceptibility of female neurons to BPA (P < 0.001, n = 10 independent experiments) and the rescuing effects of TSA (P < 0.05, n = 3).
Several reports have shown that prenatal exposure to low doses of BPA resulted in acceleration of neurogenesis, alteration of neuronal migration, and disruption of thalamocortical projections during cortical neural development (13, 30). Furthermore, chloride transporter function of KCC2 has previously been found critical for regulation of cortical interneuron precursor migration to their final destination in the layered cortex (31). This is a key developmental milestone for setting up layered cortical architecture and its proper functioning mediated by GABA-ergic interneuronal circuits. Against this background, we addressed whether BPA exposure of developing neurons would interfere with interneuron tangential migration. Our results indicate that exposure of cortical slices to BPA [at embryonic day (E) 14] significantly increased migration of interneurons by 25–50% [from days-in-vitro (DIV) 1 to DIV3]. In keeping with significantly decreased KCC2 expression and accelerated migration in BPA-exposed slices, neuronal [Cl−]i increased by DIV3 vs. vehicle treated (Fig. 1D and Fig. S2).
In extension of our previous finding that the pan-histone deacetylase (HDAC)-inhibitor, trichostatin-A (TSA), can accelerate the chloride shift by up-regulating Kcc2 expression (3, 4), we exposed rat primary neurons to BPA. We then addressed whether the resulting decrease of Kcc2 expression could be rescued by TSA. In view of the sexually dimorphic dynamics of the chloride shift (see Introduction), we conducted this experiment in male vs. female primary neurons. Interestingly, BPA exerted its effect in a sexually dimorphic manner. We found that the BPA-mediated decrease of Kcc2 expression is greater in female than in male neurons, 28% vs. 20% (Fig. 1 E and F). Moreover, TSA rescued effects of BPA in female neurons significantly stronger than in male neurons (Fig. 1F).
Knockdown of HDAC1 Rescues Down-Regulation of Kcc2 Expression by BPA.
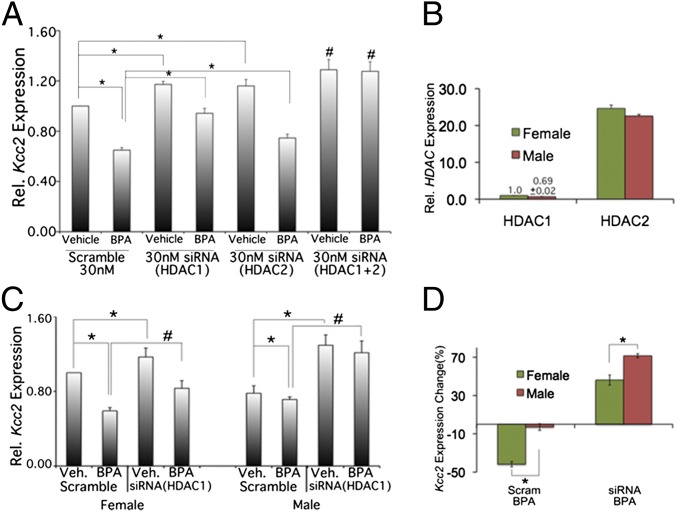
In view of the effect of a nonselective pan-HDAC-inhibitor, we addressed whether specific knockdown of class I HDACs, HDAC1 and HDAC2, would have comparable effects. Both are known to be expressed in the nucleus in developing CNS neurons. We first validated our siRNA assays (Fig. S3). A total of 40 nM siRNA caused >60% knockdown of Hdac1 and Hdac2 mRNA, respectively; cell viability was 90%. A total of 20 nM Hdac1 or Hdac2 siRNA increased Kcc2 mRNA levels significantly (20%). We decided to use 30 nM specific siRNA for effective knockdown without significant effect on viability. We observed increased Kcc2 mRNA expression by 20% (Fig. 2A). Double exposure had a mildly additive effect (30% increase). In terms of rescue of the effect of BPA, we observed a more moderate rescue with siRNA specific for Hdac2, and a more pronounced effect with siRNA specific for Hdac1 (Fig. S4). Combination led to enhancement of the rescue effect. The more moderate rescue of the effect of BPA by siRNA specific for HDAC2 may be explained by the relatively abundant (>20-fold) Hdac2 mRNA vs. Hdac1 (Fig. 2B). We next investigated sex specificity of Hdac1 knockdown, given the sex-specific effect of TSA and the effective rescue of the effect of BPA with Hdac1 siRNA. Hdac1 siRNA effects were indeed sex specific (Fig. 2C). Hdac1 knockdown significantly increased Kcc2 mRNA in non–BPA-exposed female neurons by 20% compared with 60% in male neurons. Rescue of the effect of BPA by Hdac1 siRNA was greater again in male compared with female neurons (Fig. 2 C and D). Although both methods–HDAC inhibition with TSA and Hdac1 knockdown with siRNA—share sex specificity, the sex predilection is inverse, namely, robust effects of TSA for female neurons and more appreciable effects of Hdac1 knockdown for male neurons. We tender again expression differences as a possible explanation: Hdac1 has 30% lower expression levels in male neurons vs. female. As an alternative, this could represent a genuinely sex-specific regulatory function of Hdac1, namely, that it is more critical for male neurons. TSA, inhibiting both class I and II HDACs, affects female neurons more appreciably because developmental Kcc2 up-regulation is more robust in female neurons.
Fig. 2.
Knockdown of Hdac1 rescues the effect of BPA. (A) Hdac1 and Hdac2 critically contribute to the attenuating effects of BPA on Kcc2 expression in primary cortical neurons (mixed culture). Note rescue of the effects of BPA (Hdac1-specific siRNA > Hdac2-specific siRNA), and the most striking effect of a combination of Hdac1 and Hdac2 siRNA. *P < 0.05. #P < 0.01; n = 4). (B) Relative abundance of Hdac1 and Hdac2 mRNA in cortical neurons on DIV2. Note Hdac1 level set at “1” for female neurons; male neurons ∼60% Hdac1 of female neurons, Hdac1 ∼ <1/20 of Hdac2 expression, which may explain the negative result when targeting Hdac2 in primary neurons. (C) Sex-specific effects of Hdac1-specific siRNA. Note the more accentuated effects in male neurons. Hdac2-specific siRNA did not lead to a significant increase vs. BPA for either sex (not shown). *P < 0.05. #P < 0.01; n = 4 (D) Quantification of sex-specific differences from C. Note the increased effect of an Hdac1-specific siRNA on male neurons and increased rescue by Hdac1-specific siRNA of BPA effects in male neurons. This could possibly be due to sex-specific expression differences of Hdac1 (see B), which would render Hdac1 a more suitable target for an effective siRNA molecule.
Binding to Kcc2 Promoter Is Increased for MECP2 and Decreased for H3K9ac in Response to BPA Exposure.
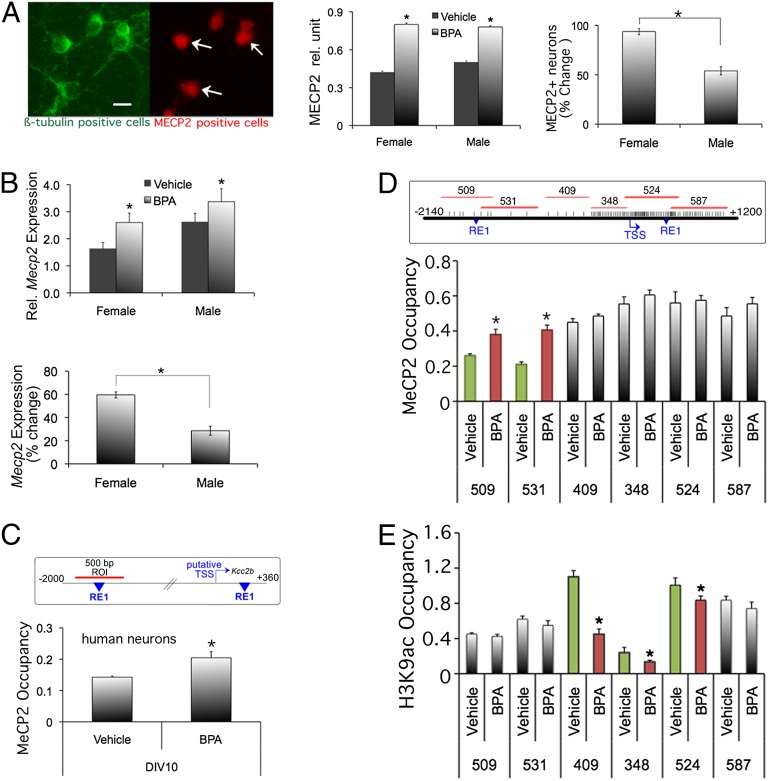
Previously, we have identified a second repressive element 1 (RE-1) DNA binding site 1.8 kb upstream of the Kcc2b TSS, in addition to a previously identified RE-1 in the first intron. We found that the dual RE-1 site of Kcc2 was critical for derepression of Kcc2 transcription during developmental up-regulation of KCC2 expression. We further showed that the two RE-1 sites bind MECP2 protein (4). Based on these findings, we addressed whether BPA exposure affects MECP2 abundance, in particular whether binding of MECP2 to Kcc2 DNA regulatory region increases. We obtained affirmative results. We found that BPA exposure led to increased numbers of MECP2-expressing neurons (Fig. 3A), and to increased abundance of Mecp2 mRNA (Fig. 3B) in primary cortical neurons. Moreover there was a sexual dimorphism in MECP2 mRNA and protein with significantly greater increase in female vs. male neurons (Fig. 3 A and B).
Fig. 3.
BPA increases MeCP2 expression and MECP2 binding to Kcc2 promoter region, decreased binding of H3K9ac. (A, Left) Representative picture of MECP2 and neuronal β-tubulinIII staining. (Center) Increased MECP2 protein expression in primary rat cortical neurons on DIV5 after BPA exposure. (Right) Quantification of sexual dimorphism, note increased percentage of MECP2 increase in female neurons; *P < 0.01 n = 50 MECP2+ cells per group. (B) Increased Mecp2 mRNA expression in primary rat cortical neurons on DIV5 after BPA exposure. (Lower) Percentage of expression changes, notably females more than males. *P < 0.05, n = 3 independent experiments. (C, Upper) Map of human KCC2 promoter. Region-of-interest (ROI) is 500-bp fragment surrounding the upstream RE-1 site. (Lower) BPA treatment increased MECP2 protein binding to the KCC2 promoter CpG shore region of human primary cortical neurons (DIV5). *P < 0.05, n = 3 (D, Upper) Geography of the rat Kcc2 promoter with the transcriptional start site (TSS) and the double RE-1 repressor sites depicted in blue. Note the CpG island surrounding the TSS, as indicated by increased frequency of CpG sites (vertical bars). Red lines depict ROI, as amplified by specific PCR primers. (Lower) ChIP assay mapping of MECP2 binding to the Kcc2 proximal promoter of rat primary cortical neurons after exposure to BPA (vs. control) at DIV4. The greatest binding differences are accentuated by green and red bars, in this case the two CpG island “shores” fragments 509 and 531. Note statistically significant increase of MECP2 binding evoked by BPA. *P < 0.05, n = 3. (E) ChIP assay showing H3K9a occupancy on rat Kcc2 promoter and CpG shore region. The greatest binding differences in H3K9a occupancy after BPA treatment are accentuated by green and red bars, in this case the region surrounding the TSS; fragments 409, 348, and 524. *P < 0.05, n = 3.
We next used chromatin immunoprecipitation (ChIP) to determine MECP2 binding in cultured neurons exposed to BPA. Using mature human neurons (DIV10), we verified increased binding of MECP2 protein to the KCC2 promoter in response to BPA (Fig. 3C). To increase resolution of this finding, we took advantage of our rat primary cortical neuron methodology, to “map out” the Kcc2 promoter region with a series of adjacent PCR reactions (Fig. 3D, Upper and Fig. S5), covering a total of 3 kb flanking Kcc2b’s TSS. Our results show that BPA exposure resulted in increased binding of MECP2 protein to CpG shore regions 5′ of the TSS of Kcc2. We also noted that MECP2 binding to the CpG island region was consistently >0.5, irrespective of BPA exposure. This suggests that robust MECP2 binding can occur in nonmethylated regions such as CpG islands that surround the TSS of transcriptionally active genes, as is the case of Kcc2. We next asked whether the dynamic changes in MECP2 binding to the shores of the Kcc2 promoter upon BPA exposure were linked to DNA methylation. Our results indicate that there was no change in DNA methylation of CpG sites in the shore region surrounding the upstream RE-1 site, when comparing BPA exposed to control (Fig. S6).
BPA exposure resulting in increased binding of a repressor protein, MECP2, to the Kcc2 promoter prompted us to address whether histone proteins associated with an active chromatin mark were in turn down-regulated. We obtained affirmative results by applying ChIP specific for H3K9ac, and again using PCR assays as for MECP2 binding. BPA exposure resulted in significantly decreased H3K9ac binding to the rat Kcc2 promoter in three regions surrounding the TSS, one of them in a shore, showing the most dynamic regulation, and two in the CpG island that surrounds the TSS (Fig. 3E). Taken together, BPA exposure of primary cortical neurons resulted in increased binding of MECP2 and decreased binding of H3K9ac protein to the proximal promoter region of Kcc2. This provides a likely explanation for decreased Kcc2b expression and for the observed counterregulatory effects of inhibition of histone deacetylation.
Cortical Neurons from Offspring of BPA-Fed Mouse Dams Show Kcc2 Promoter Repression.
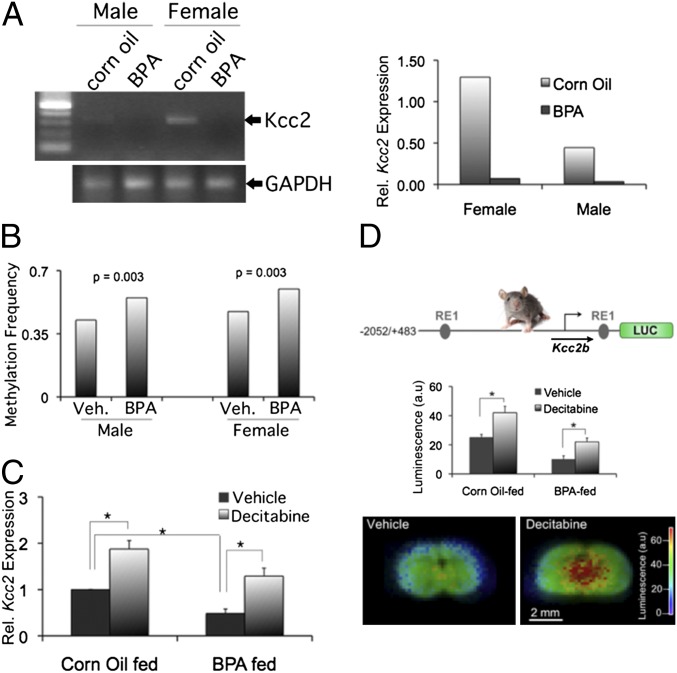
Next, we addressed whether results based on BPA exposure in primary neuronal tissue culture have a correlate in live animals. Previous reports have described changes in cortical brain development in mice exposed prenatally to BPA (13, 30). Therefore, we fed a BPA-containing diet (50 mg/kg chow) (32) to pregnant mouse dams and generated primary neuronal cultures from the cortices of P0 pups, separated by sex. Our results indicate that Kcc2 mRNA was significantly down-regulated in response to in utero BPA exposure. We recapitulated a critical component of the sexually dimorphic chloride shift in our cultures, namely a more robust Kcc2 expression in female than in male neurons. Strikingly, in utero BPA exposure resulted in greatly diminished expression for both sexes, so that the difference in BPA vs. control was more robust for female neurons (Fig. 4A). We next used bisulfite sequencing to determine if in utero BPA exposure affected CpG methylation of the Kcc2 promoter. We confirmed this to be the case for the shore region (Fig. 4B and Fig. S7), with equal net effect for both sexes. To test functional significance of CpG methylation, we applied the DNA methyltransferase inhibitor, decitabine. This resulted in increased Kcc2 expression in control-exposed neurons, indicating that DNA methylation has a role in regulatory control of perinatal Kcc2 expression (Fig. 4C). Repressive effects of BPA on Kcc2 expression were partially rescued by decitabine, suggesting a critical role for DNA methylation. Based on this evidence, we tested direct effects of in utero BPA exposure on the Kcc2 promoter. We also wanted to better understand whether the effects of BPA were recapitulated in the organotypic context of the brain. To address these questions, we took advantage of a mouse line carrying a knock-in at the Rosa26 locus (33), in which 2.5 kb of the Kcc2 regulatory region drives luciferase (LUC) gene activity. We cultured brain slices after in utero BPA exposure. We treated slices with decitabine or control vehicle (Fig. 4D). Results indicate that (i) in utero BPA exposure led to down-regulation of Kcc2-LUC reporter, and (ii) decitabine could partially rescue these effects. These findings indicate that DNA methylation exerts a repressive effect on 2.5 kb of the Kcc2 promoter surrounding the TSS, and that this is relevant in live animals and in the organotypic context of the brain. Moreover, BPA enhances repression, which depends on DNA methylation.
Fig. 4.
Cortical neurons from offspring of BPA-fed mouse dams show Kcc2 promoter repression. (A) Sexually dimorphic delay in chloride shift of primary cortical neurons at DIV4 derived from P0 mouse pups, born to BPA or corn oil-exposed dams. (Right) Quantitation of mRNA levels: Note increased difference for female neurons. (B) Significant methylation increase relative to vehicle in the CpG dinucleotides with equal net effect for both sexes. At least 25 clones were sequenced per CpG dinucleotide. P values determined by Fisher’s exact test. (C) Significant decrease of Kcc2 expression in mixed primary cortical neurons. Decitabine (2 µM) rescues the effect of BPA; DIV4, n = 3 independent experiments, *P < 0.01 (D, Top) Design of 2.5-kb mouse Kcc2–LUC construct (33). (Middle) Significant decrease of Kcc2 promoter-Luc activity in slice cortical culture in response to BPA. Decitabine (2 µM) rescues the effect of BPA, DIV3; n = 4 independent experiments, *P < 0.05. (Bottom) Representative luminescence heatmap of DIV3 organotypic cortical slice culture prepared from offspring of BPA-fed dams. Note increased luminescence in response to decitabine treatment.
Discussion
Taken together, we have discovered that the neurodevelopmental increase in expression of the Kcc2 gene in cortical neurons, a pivotal change in the mode of operation of the central nervous system, is delayed and suppressed by exposure to BPA. Our findings were established in rodent models and verified in human systems, an important validation step given health relevance of our findings and ongoing public concern about ubiquitous BPA exposure. BPA decreases Kcc2 mRNA expression, subsequently affecting KCC2 protein expression, and neuronal chloride extrusion. We demonstrate delayed chloride extrusion, plus its impact on migration of cortical inhibitory precursor neurons. Reduction of KCC2 will likely affect the nontransporter function of the protein (7), which affects neuronal process formation, synaptic maturation, and dendritic spine length. Besides the effects of BPA on Kcc2, additional effects on Nkcc1 neuronal chloride transporter gene regulation cannot be excluded and will be elucidated in future studies.
Furthermore, we found that neurons treated with TSA (pan-HDAC inhibitor) and decitabine (DNA methyltransferase inhibitor) increased Kcc2 expression. This clearly indicates a critical role for epigenetic regulation in the control of the chloride shift. Notably, decitabine and TSA partially rescued the BPA effects. Importantly for the objective of this study, we show that BPA has epigenetic effects that affect regulation of a key event in perinatal neural development. The epigenetic mechanism of BPA exposure is corroborated by the effects of siRNA specific for Hdac1 and the effect of combined knockdown of Hdac1 and -2. Furthermore, our findings reemphasize the relevance of the less abundant HDAC1.
The effects of BPA showed a sexual dimorphism, which is based on the sexually dimorphic perinatal up-regulation of Kcc2, slower in male neurons. BPA is more potent to retard Kcc2 up-regulation in females. Likewise the pan-HDAC inhibitor, TSA, showed an increased potency in female neurons. In contrast, siRNA specific for Hdac1 affected male neurons more potently in rescuing the effects of BPA. These results notwithstanding, it remains an unanswered question which specific HDAC is key in female neurons mediating the effect of BPA on Kcc2 expression. The effect of BPA to increase MECP2 expression was more prominent in female neurons as well. This also indicates, in a more general sense, that BPA-mediated dysregulation of MECP2 contributes to sexually dimorphic maldevelopment of the brain. BPA-evoked regulation of MECP2 is a relevant finding because Rett syndrome-related human hereditary disorders, affecting exclusively females, are not only caused by MECP2 loss of function, but also by the more rare gain-of-function mutations. Thus, our present findings, in which we are recording related results in rat, mouse, and human neurons, raise the question of whether BPA exposure could predispose to neurodevelopmental disorders including autism-spectrum–related diseases.
In terms of a mechanism of damage, the effects of BPA exposure on migration of cortical inhibitory precursor neurons have to be emphasized. These effects will result in a disordered cortical layer architecture leading to impaired inhibitory cortical neural networks. Alterations in cortical cytoarchitecture have been described previously (13, 34). At the cellular level of the neuron, delayed expression of Kcc2 by exposure to BPA will have impact through the relative lack of the nontransporter function of KCC2 on neuronal differentiation. This might be an important additional mechanism together with the desynchronized migration and corrupted organization of cortical layers. The effects of BPA on Kcc2 gene expression might therefore have relevance for behavioral abnormalities observed in response to in utero BPA exposure (16, 17, 35). BPA-evoked behavioral abnormalities can even be traced to the F2 generation, suggesting epigenetic mechanisms such as changes in DNA methylation. In humans, gestational exposure to BPA was significantly correlated with behavioral and mood abnormalities in daughters more than in sons of such pregnancies (36). Our findings raise the question whether this is due to the effect of BPA exposure on MECP2 expression and DNA binding and subsequent retardation of the neural chloride shift in these daughters.
We are confident that our findings will improve understanding of how environmental exposure to BPA can affect gene regulation of a critical gene for neuronal function and neural development. Identification of the Kcc2 promoter as a target for the effects of BPA and subsequent changes of gene expression and function of KCC2 allow us to ask follow-up questions in a rational manner, such as whether these effects involve estrogen receptor signaling, and if so, which specific estrogen receptor. In a more general sense, what signaling mechanisms are upstream of the epigenetic effects of BPA? Because decreased Kcc2 expression has been implied in an array of neuropsychiatric diseases, such as chronic pain, epilepsy, and traumatic injury, the question also arises of whether earlier BPA exposures predispose for later development of these conditions. These will be worthy topics for future studies. Finally, we remain aware that BPA may affect gene- and cellular regulation in neural cells beyond the Kcc2 promoter in cortical neurons in the perinatal period.
Methods
More extensive descriptions can be found in SI Methods.
Primary Cultures of Embryonic Cortical Neurons and BPA Exposure.
Primary cortical neurons from rodents were prepared as described previously (4). Rodent neurons were typically studied on DIV4-5. Primary human cortical neurons were established from 16- to 21-wk-old human fetal brain tissue specimens as described previously (37, 38). For sex-specific studies, genomic DNA was typed using Y and X chromosome-specific primers.
For BPA, stock solution was dissolved in ethanol at 10 mM and then diluted with PBS. Trypan blue exclusion test was used to determine cell viability after BPA exposure.
BPA-Feeding Protocol.
Feeding protocol was carried out according to ref. 32. At birth [postnatal day (P) 0; newborn] pups were euthanized and brain tissue was collected for further processing.
ChIP Protocol.
The ChIP assay was carried out as described previously (4).
Immunocytochemistry.
Immunochemistry labeling of cells was carried out as previously described (4).
Cortical Slice Culture and Migration Assay.
Cortical slices from rat embryos (E14) were electroporated and then cultured as described (31, 39). A glass pipette filled with a DNA construct, pCAG-Clomeleon was inserted into the median ganglionic eminence (MGE) region of the ventricular and subventricular zones. After electroporation, culture inserts were transferred into 35-mm culture dishes. Clomeleon emission ratios were obtained over time from individual neurons and converted into intracellular chloride concentration ([Cl−]i) (4, 40). After initial imaging on DIV1, the medium was changed, BPA (100 nM) or vehicle control was added, and slices were reimaged at DIV3. To quantify migration, fluorescent cells were traced on low magnification images. Distance from the injection sites was determined for each cell by using in IgorPro macro (Wavemetrics).
Cortical slices from P0 pups exposed in utero to BPA and carrying the Kcc2-LUC reporter were cultured (33), and LUC activity was determined over time.
Mouse Gene Targeting of ROSA26 Locus for Knock-in of a Kcc2-LUC Construct.
These animals have been described in a recent publication (33).
Statistical Analysis.
Data are shown as mean and SEM of at least three independent experiments. Each independent experiment was conducted in triplicate. P < 0.05 was considered significant, determined by t test or ANOVA with post hoc Tukey analysis.
Supplementary Material
Acknowledgments
This study was supported by funding from Duke University, the Klingenstein Fund, the National Institutes of Health [R21NS066307 (to W.B.L) and HD38466 and AG16573 (to J.B.)], and intramural funds from the National Institute of Environmental Health Sciences (to L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300959110/-/DCSupplemental.
References
- 1.Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28(6):278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 3.Rivera C, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 4.Yeo M, Berglund K, Augustine G, Liedtke W. Novel repression of Kcc2 transcription by REST-RE-1 controls developmental switch in neuronal chloride. J Neurosci. 2009;29(46):14652–14662. doi: 10.1523/JNEUROSCI.2934-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17(1):81–86. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Perrot-Sinal TS, Sinal CJ, Reader JC, Speert DB, McCarthy MM. Sex differences in the chloride cotransporters, NKCC1 and KCC2, in the developing hypothalamus. J Neuroendocrinol. 2007;19(4):302–308. doi: 10.1111/j.1365-2826.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 7.Li H, et al. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56(6):1019–1033. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: What it tells us about our toxic burden and how it assist environmental medicine physicians. Altern Med Rev. 2010;15(2):101–109. [PubMed] [Google Scholar]
- 10.Cabaton NJ, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect. 2011;119(4):547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golub MS, et al. Bisphenol A: Developmental toxicity from early prenatal exposure. Birth Defects Res B Dev Reprod Toxicol. 2010;89(6):441–466. doi: 10.1002/bdrb.20275. [DOI] [PubMed] [Google Scholar]
- 12.Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 2012;354(1-2):74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh K, Yaoi T, Fushiki S. Bisphenol A, an endocrine-disrupting chemical, and brain development. Neuropathology. 2012;32(4):447–457. doi: 10.1111/j.1440-1789.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- 14.Masuo Y, Ishido M. Neurotoxicity of endocrine disruptors: Possible involvement in brain development and neurodegeneration. J Toxicol Environ Health B Crit Rev. 2011;14(5-7):346–369. doi: 10.1080/10937404.2011.578557. [DOI] [PubMed] [Google Scholar]
- 15.Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167(3):741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25(6):1084–1093. doi: 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59(3):296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaoi T, et al. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376(3):563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Feil R, Fraga MF. Epigenetics and the environment: Emerging patterns and implications. Nat Rev Genet. 2011;13(2):97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- 20.Hou L, Zhang X, Wang D, Baccarelli A. Environmental chemical exposures and human epigenetics. Int J Epidemiol. 2012;41(1):79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skene PJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 2010;37(4):457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S, et al. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron. 2011;72(1):72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annu Rev Cell Dev Biol. 2011;27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- 25.Noh KM, et al. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci USA. 2012;109(16):E962–E971. doi: 10.1073/pnas.1121568109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40(7):897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irizarry RA, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41(2):178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones PA. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 29.Vandenberg LN, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118(8):1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komada M, et al. Maternal bisphenol A oral dosing relates to the acceleration of neurogenesis in the developing neocortex of mouse fetuses. Toxicology. 2012;295(1-3):31–38. doi: 10.1016/j.tox.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62(1):53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liedtke W, et al. 2012. Highly conductive carbon nanotube matrix accelerates developmental chloride extrusion in central nervous system neurons by increased expression of chloride transporter KCC2. Small, 10.1002/smll.201201994.
- 34.Nakamura K, Itoh K, Sugimoto T, Fushiki S. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci Lett. 2007;420(2):100–105. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K, et al. Prenatal and lactational exposure to low-doses of bisphenol A alters adult mice behavior. Brain Dev. 2012;34(1):57–63. doi: 10.1016/j.braindev.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Braun JM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelsman A, et al. GVS-111 prevents oxidative damage and apoptosis in normal and Down’s syndrome human cortical neurons. Int J Dev Neurosci. 2003;21(3):117–124. doi: 10.1016/s0736-5748(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 38.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26(22):6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polleux F, Ghosh A. The slice overlay assay: A versatile tool to study the influence of extracellular signals on neuronal development. Sci STKE. 2002;2002(136):pl9. doi: 10.1126/stke.2002.136.pl9. [DOI] [PubMed] [Google Scholar]
- 40.Pond BB, et al. The chloride transporter Na(+)-K(+)-Cl- cotransporter isoform-1 contributes to intracellular chloride increases after in vitro ischemia. J Neurosci. 2006;26(5):1396–1406. doi: 10.1523/JNEUROSCI.1421-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.