Abstract
Neural slowing is commonly noted in older adults, with consequences for sensory, motor, and cognitive domains. One of the deleterious effects of neural slowing is impairment of temporal resolution; older adults, therefore, have reduced ability to process the rapid events that characterize speech, especially in noisy environments. Although hearing aids provide increased audibility, they cannot compensate for deficits in auditory temporal processing. Auditory training may provide a strategy to address these deficits. To that end, we evaluated the effects of auditory-based cognitive training on the temporal precision of subcortical processing of speech in noise. After training, older adults exhibited faster neural timing and experienced gains in memory, speed of processing, and speech-in-noise perception, whereas a matched control group showed no changes. Training was also associated with decreased variability of brainstem response peaks, suggesting a decrease in temporal jitter in response to a speech signal. These results demonstrate that auditory-based cognitive training can partially restore age-related deficits in temporal processing in the brain; this plasticity in turn promotes better cognitive and perceptual skills.
Keywords: aging, neuroplasticity
As we age, we may notice that we require more time to respond to sensory input. There are many potential biological causes for our slower responses, including: loss of myelin integrity (1), longer neural recovery times (2–4), decreased brain connectivity (5), and loss of neural synchrony (6). The consequences of neural slowing are pervasive, but one of the most distressing effects is difficulty hearing in background noise. Precise neural timing is an important factor for successful speech-in-noise perception (7), but older adults experience a breakdown in this precision (8, 9). This communication difficulty affects older adults’ quality of life as they begin to avoid places and social situations in which communication is compromised (10). Because amplification through hearing aids or assistive listening devices does not compensate for decreased temporal precision, a management strategy that specifically addresses deficits in timing is needed (11).
The effects of auditory training on neural responses in older populations have been evaluated in an animal model. After training on a frequency discrimination task, the auditory cortices of older rats showed greater neural synchrony in response to pulsed noise trains, likely mediated by increased levels of inhibitory neurotransmitters (12). In humans, short-term auditory training programs have yielded improvements in neural response timing and frequency representation in children (13) and young adults (14–16). Short-term training in visual discrimination has been linked to better working memory and cortical changes in older adults; training-related changes in the magnitude of visual-evoked cortical responses predict working memory improvements (17). Although short-term training-induced improvements in auditory memory and speed of processing have been demonstrated in older adults (18), the effects of short-term training on the aging auditory nervous system remain unknown. There is, however, evidence of long-term training advantages in older adults; musicianship, an example of long-term auditory training, can offset neural timing deficits in older adults (19) and is associated with better hearing in noise and auditory memory (20).
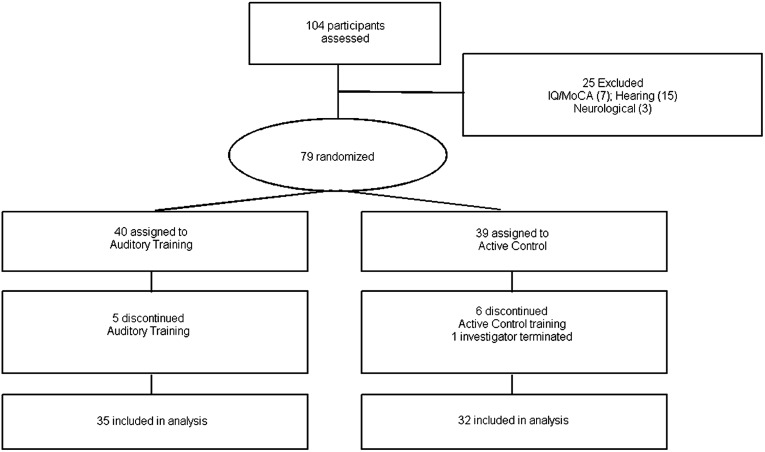
We hypothesized that training which directs focus to rapidly changing formant transitions in speech leads to faster neural timing and better speech-in-noise perception. To test this hypothesis, we randomly assigned older adults to one of two groups (Fig. 1).
Fig. 1.
Flow of participants randomly assigned to auditory training or active control groups.
The first group (auditory training; n = 35) completed an adaptive computer-based auditory training program that combines bottom-up perceptual discrimination exercises with top-down cognitive demands. The second group (active control; n = 32) participated in a general educational stimulation program that was matched for time and computer use to that of the auditory training group. We recorded auditory brainstem responses to the speech syllable [da] (Fig. 2) presented in quiet and noise and assessed speech-in-noise perception, short-term memory, and speed of processing before and after 8 wk of training. We expected that auditory training would induce earlier brainstem peak latencies at posttest compared with pretest, and that the effects of noise on response timing would be reduced. Given previously demonstrated cognitive and perceptual gains from both short-term (14, 18) and long-term (20) auditory training, we expected that auditory training would also improve speech-in-noise perception, short-term memory, and speed of processing.
Fig. 2.
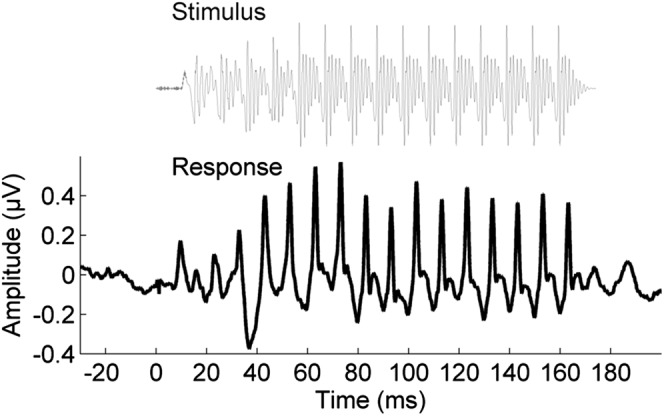
The stimulus waveform [da] (gray) and the grand average response waveform to the [da] presented in quiet (n = 67) at pretest (black).
Results
Peak Timing Changes.
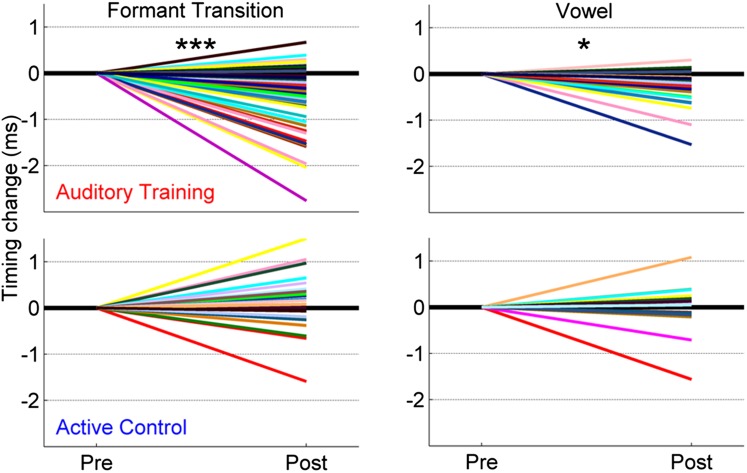
In the region of the response corresponding to the formant transition (cues that distinguish one speech sound from another; peaks at 34, 44, 54, and 64 ms), timing became earlier in the auditory training group in response to the syllable presented in quiet [F(1, 31) = 3.221, P = 0.025] and in noise [F(1, 31) = 7.816, P < 0.001]. These improvements were not seen in the active control group’s responses [quiet: F(1, 28) = 2.078, P = 0.110; noise: F(1, 28) = 1.231, P = 0.320]. A group-by-session interaction was found for the [da] presented in noise [F(1, 63) = 6.263, P < 0.001] but not in quiet [F(1, 63) = 0.756, P = 0.558], indicating that the auditory training group’s response timing improved relative to the active control group but only in the noise condition (Fig. 3). For the neural response to the vowel, there was a trending group-by-session interaction in noise [F(1, 57) = 1.193, P = 0.066]; specifically, only the auditory training group demonstrated earlier timing [F(1, 25) = 2.313, P = 0.043; active control: F(1, 22) = 1.095, P = 0.407]. In quiet, no changes occurred in either group [auditory training: F(1, 55) = 1.193, P = 0.342; active control: F(1, 52) = 0.786, P = 0.642] in the steady state. Fig. 4 displays changes in neural timing for individual participants.
Fig. 3.
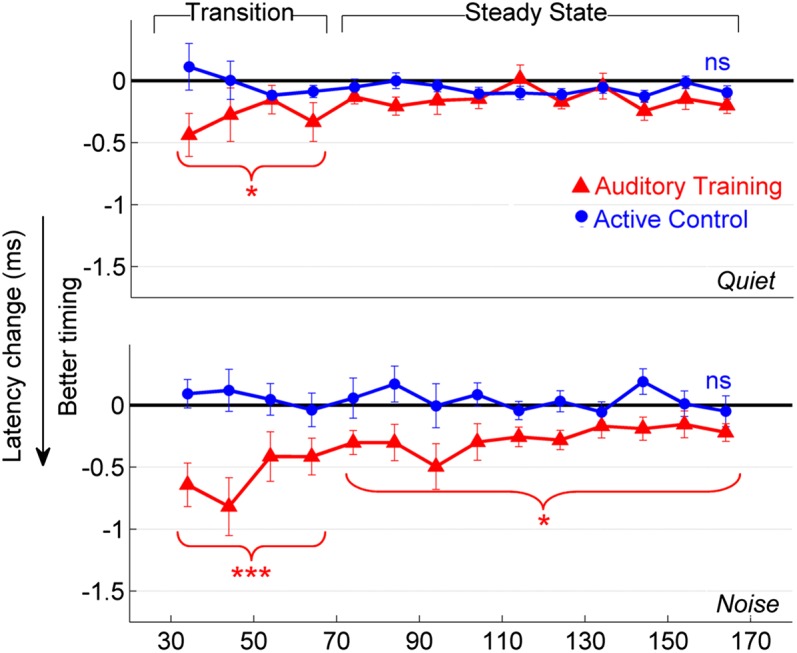
Changes in the neural response to [da] for peaks occurring every 10 ms (corresponding to the 100 Hz pitch of the stimulus) are displayed for the auditory training (red; n = 35) and active control (blue; n = 32) groups in quiet (Upper) and noise (Lower). The brackets indicate the transition and steady-state regions of the [da]. Improvements in timing were noted in the auditory training but not in the active control group. *P < 0.05, ***P < 0.001; Error bars: ±1 SE.
Fig. 4.
Individual average timing changes for the formant transition (Left) and steady-state vowel (Right) in the auditory training (Upper) and active control (Lower) groups for neural responses to speech in noise. *P < 0.05, ***P < 0.001.
We found that training reduced interpeak variability in noise [F(1, 34) = 7.478, P = 0.010; group × session interaction: F(1, 65) = 6.378, P = 0.014], but no training-induced changes were noted in the quiet condition or in the active control group (all P values > 0.10). As expected, the pretest interpeak variance in noise was greater than that in quiet (premean in noise: 1.05; premean in quiet: 0.89) (21, 22); therefore, we would expect to see greater changes in posttest variance in the noise condition. Importantly, change in variance relates to change in transition peak latency (r = 0.660, P < 0.001) in the trained group.
Changes in Effects of Noise on Response Timing.
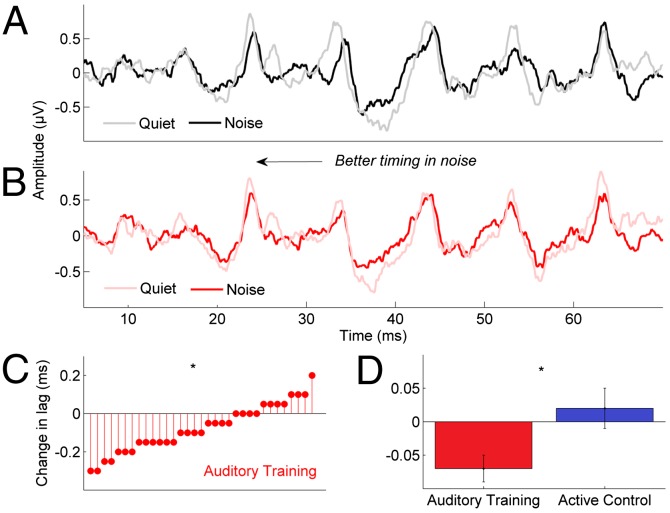
Cross-correlating responses in quiet and in noise indicated that the addition of background noise resulted in smaller shifts in neural response timing in the training group, objectively confirming the timing improvements noted from manual peak identification (Fig. 5). Smaller noise-induced timing shifts were observed from pretest to posttest in the auditory training [F(1, 34) = 4.928, P = 0.033] but not in the active control group [F(1,32) = 0.322, P = 0.574]. The group × session interaction was significant [F(1, 66) = 6.984, P = 0.010]. The average absolute quiet-to-noise shift was 0.07 ms, and the average change in timing, based on a change in quiet-to-noise lag, was 0.06 ms; therefore, we assume that this training-related change in lag is meaningful.
Fig. 5.
Quiet-to-noise timing shift differences. Responses in quiet and noise were cross-correlated to obtain an objective measure of timing (lag). (A) Before training, the expected noise-induced timing shift is observed, with the response in noise (black) lagging behind the response in quiet (gray) by 0.2–0.3 ms (shown in an individual participant). (B) After training, this individual’s response in noise (red) now overlays the response in quiet (pink) with minimal apparent lag. (C) Lag changes in each participant in the auditory training group, indicating a significant group change from pre- to posttest. (D) Mean group lag changes. *P < 0.01; error bars: ±1 SE.
Behavioral Gains.
The observed improvements in neural timing were complemented by perceptual and cognitive gains in the auditory training group but not the active control group, with significant group interactions for all measures [speech in noise: F(1, 66) = 8.739, P = 0.004; auditory short-memory: F(1, 66) = 10.567, P = 0.002; processing speed: F(1, 66) = 7.763, P = 0.007; Fig. 6; see Table 2 for pre- and posttraining scores]. Post hoc testing indicated improvements on all cognitive measures in the auditory training group after training [speech in noise: F(1, 34) = 25.343, P < 0.001; short-term memory: F(1, 34) = 29.590, P < 0.001; speed of processing: F(1, 34) = 5.842, P = 0.021]. The active control group did not show these improvements [speech in noise: F(1, 31) = 1.195, P = 0.283; auditory short-term memory: F(1, 31) = 0.101, P = 0.752; processing speed: F(1, 31) = 2.179, P = 0.150]. These cognitive improvements with training are consistent with previous reports (17, 18, 23).
Fig. 6.
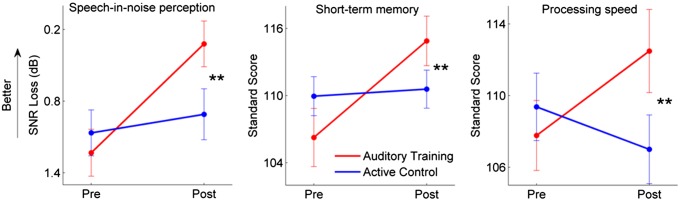
Improvements in speech-in-noise perception, short-term memory, and processing speed were only observed in the auditory training group. **P < 0.01 group × test interactions; error bars: ±1 SE.
Table 2.
Behavioral measures
| Auditory training |
Active control |
|||||
| Measure | Pretest | Posttest | P | Pretest | Posttest | P |
| Speech-in-noise perception | 1.13 (0.18) | 0.44 (0.23) | <0.001 | 1.06 (0.17) | 0.65 (0.21) | 0.283 |
| Auditory short-term memory | 105.46 (2.60) | 113.94 (2.13) | <0.001 | 110.00 (1.70) | 110.85 (2.130) | 0.752 |
| Processing speed | 108.77 (2.08) | 113.74 (2.31) | 0.021 | 109.74 (1.86) | 107.33 (1.85) | 0.150 |
Means (SDs) are displayed for pre- and posttest scores for speech-in-noise perception, auditory short-term memory, and processing speed for the auditory training and active control groups. P values are provided for pre- to posttest changes in each group.
Discussion
Our results demonstrate that short-term training induced neural plasticity in older adults in fundamental aspects of biological auditory processing. Specifically, we demonstrate a partial reversal of the age-related declines in neural temporal precision that have been demonstrated in older adults (19). Adaptive auditory training on consonant–vowel transitions results in earlier subcortical response timing to speech in noise. The greatest improvements were found in the response corresponding to the consonant–vowel transition, the perceptually vulnerable region of the syllable (24). We also found that interpeak variability decreased over the entire response in noise in the auditory training group. Concomitant behavioral and cognitive improvements were seen for perception of speech in noise, short-term memory, and processing speed.
What mechanisms might drive these neurophysiologic changes? Activity-driven increases in inhibitory neurotransmitters, such as gamma-aminobutyric acid (GABA), may be responsible for the observed changes. Our finding of decreased variability in interpeak latency suggests an inhibition-mediated decrease in temporal jitter. This in turn may drive the improvement in temporal precision for the fast-changing transition cues; due to the dynamic pitch content of the consonant–vowel transition, these peak latencies may be especially subject to jitter (25). Activation of inhibitory mechanisms is necessary for precise temporal processing (26). Decreases in the levels of inhibitory transmitters in the cochlear nucleus, inferior colliculus, and auditory cortex have been documented in aging animal models (26–28); these decreases are linked to a loss of temporal precision (29, 30). On the other hand, treatment designed to increase levels of inhibitory transmitters can reverse this loss. To illustrate, GABA receptor units increase following training to reverse damage to the frequency and temporal processing in the rat auditory cortex caused by noise exposure during development (31). Boosting inhibition through pharmalogic treatment can have similar effects; the use of a GABAergic agent improves temporal resolution in gerbils (32). Given that our older adults demonstrated improved neural timing, we propose that auditory-based training programs may increase inhibition, leading to enhanced temporal precision in older adults. The latency changes we found reflect, in part, increased neural precision or sharpened tuning, accomplished through activation of inhibitory sidebands (33).
Additional mechanisms might be responsible for the changes in our study, as both excitatory and inhibitory neurotransmitters may contribute to increased sharpness or neural tuning in the brainstem, as has been demonstrated in the auditory cortex (34). For example, release of acetycholine is necessary for certain types of learning, such as recognition memory (35), through mechanisms which include enhanced attention (36). Furthermore, stimulation of the nucleus basalis, the primary source of acetylcholine in the cerebral cortex, can increase cortical excitability and the transmission of action potentials (37); indeed, several studies have documented cortical reorganization and enhanced perception when auditory stimulation is paired with nucleus basalis stimulation (38–40). In fact, Froemke and colleagues showed that nucleus basalis stimulation paired with acoustic stimulation decreased variability of synaptic tuning in auditory cortex, thereby improving sensory perception and related behavior (41). Based on these studies, we conjecture that the training, with its built-in reward system, may stimulate the natural production of acetylcholine, leading to enhanced learning. Acetylcholine reduces neural firing variability (42), and thus may account for our finding of training-induced reductions in interpeak latencies.
These results complement previous work in which we have shown that life-long musical experience offsets age-related neural timing delays (19); here, we show that a partial reversal of these delays is possible with just 8 wk of training. The finding that training changes are specific to the transition is expected, given our previous finding of age-related latency delays in the transition region, but not the steady state, for responses to a speech syllable presented in quiet (25). Given this finding, we would not predict that auditory training decreases response latencies beyond what is expected in a young healthy nervous system. We assume that the extent of latency change is limited by biological constraints, such as neural conduction time, and that training cannot improve latencies beyond these limits. We did, however, find that the latency reductions were larger in noise than in quiet conditions. Because noise is known to delay peak latencies in the brainstem response (43), there may be a greater range for improvement in responses to stimuli presented in noise.
The most pronounced latency changes were in response to the formant transition in noise. There was also a latency reduction in the steady state in noise, albeit a smaller one than in the transition. Two mechanisms may be contributing to timing in the response: neural synchrony and neural recovery. The training effects were predominantly in the transition (∼0.5 ms), whereas in the steady state the change gradually decreased from ∼0.5 ms at the beginning to 0 ms at the end of the vowel. The training-induced latency changes seen in the early region of the steady state may arise from increased neural synchrony, potentially driven by a decrease in temporal jitter. If older adults’ long neural recovery time is unchanged, however, we would not expect latency differences at the offset of the stimulus.
Cognitive engagement was an important component of the current study. In the auditory training program, five of the six exercises combined adaptive demands on short-term memory and perception of consonant–vowel syllables. Because of its memory demands, the training would have engaged both memory and attention to perform the tasks accurately. Cognitive demands on memory increase reliance on perceptual cues via the prefrontal cortex (44). Therefore, the adaptive memory and perceptual demands of the training program may interact to strengthen the neural representation of speech in background noise conditions that are unfavorable for perception (45). The fact that the effects were greater in the consonant–vowel transition than the vowel portion of the [da] stimulus is consistent with evidence that perceptual learning leads to stimulus-specific changes in information processing; that is, perceptual training on transitions improves neural representation of transitions (46, 47). We also found improved speed of processing on a visual matching task in participants who had undergone training. Although the training focused on adaptively changing auditory stimuli, the program supplemented auditory stimuli with visual images, and participants likely used these images as an additional memory cue. Slower speed of processing mediates memory declines in older adults (48); therefore, we posit that increased speed of processing facilitated enhanced memory performance.
Given that the efferent pathway from the cortex to the inferior colliculus (IC) is critical for auditory learning (49), and that the IC is the putative generator of the subcortical frequency following response (50), it is not surprising that we found these neural changes in the auditory brainstem. In fact, after training, pitch pattern representation in the IC, as documented by functional magnetic resonance imaging, relates to representation of pitch patterns in the frequency following response and improved auditory word learning, suggesting a possible role for the IC in the mapping of sound to meaning (51).
In sum, our results demonstrate a partial reversal of age-related neural timing delays after 40 h of training over 8 wk; what remains unknown is the minimum amount of training necessary to induce these changes. Neural changes have been documented in young adults after just days or hours of training (15); in fact, neural changes can precede changes in perception with as little as one training session (52). Given evidence in animal models, however, older adults likely require a longer training regimen. For example, because adult owls require more incremental training than young owls for plasticity in auditory space mapping (53) older adult humans may require a more gradual increase in task difficulty level to improve perception. Therefore, future work should evaluate the time course of auditory training and the maintenance of treatment effects over time.
Future development of treatment protocols should consider mechanisms to directly address factors that inhibit plasticity in older adults, such as changes in the balance of excitatory and inhibitory neurotransmitters (54). Kilgard and colleagues have paired vagus nerve stimulation (VNS) with sensory stimulation to induce plasticity, taking advantage of the known effects of VNS for releasing neurotransmitters (55). This noninvasive stimulation has been effective for reversing the maladaptive plasticity associated with tinnitus in rats. Perhaps this type of noninvasive stimulation or another technique that would pair training with natural activation of the brain’s reward centers [such as through the use of music (56) or action video games (57)] can be used to increase the effectiveness of therapies designed to enhance perception and cognition in older adults.
These results have broad implications for the management of perceptual, cognitive, and neural declines in older adults, as well as for the understanding of plasticity in an aging population. In particular, participants experienced improvement in speech-in-noise perception, indicating that the expected decreases in perceptual abilities that accompany aging are amenable to training. Auditory training, therefore, should be considered for the management of communication difficulties in older adults and may serve to mitigate some of the psychosocial sequelae that can exacerbate aging effects, such as depression and social isolation (10).
Materials and Methods
Participants.
One hundred and four participants were recruited from the greater Chicago area to engage in a study on hearing in noise in older adults; 67 (38 female) are included in the analysis (see Fig. 1). All participants were between the ages of 55 and 70 y (mean, 63.0; SD, 3.7 y). By choosing this age range, we hoped to establish an effect of training that is uncomplicated by the potential confounds of older age, such as severe hearing loss, increased medication use, and greater cognitive declines that are more prevalent in older individuals.
Audiometric thresholds were measured at both test sessions bilaterally at octave intervals from 0.125 to 8 kHz, including interoctave intervals at 3 and 6 kHz, plus 10 kHz and 12.5 kHz. In all participants, pure-tone averages (average threshold from 0.5 to 4 kHz) were ≤45 dB hearing level (HL) bilaterally. No asymmetries (>15 dB HL difference at two or more frequencies between ears) were noted. All participants had normal click-evoked auditory brainstem responses (defined as a Wave V latency < 6.8 ms bilaterally; 100-μs click presented at 80 dB SPL at 31.25 Hz) and no interaural Wave V latency difference ≥ 0.2 ms.
No participant had a history of neurologic conditions, and all participants were screened for dementia using a cutoff score of 22/30 on the Montreal Cognitive Assessment (MOCA) (58). Participants had normal IQ (≥85 on the Wechsler Abbreviated Scale of Intelligence; ref. 59). All procedures were reviewed and approved by the Northwestern University Institutional Review Board. Participants provided informed consent and were compensated for their time.
Auditory Training.
Participants were seen for behavioral and electrophysiological testing and then randomly assigned to one of two conditions: auditory training and active control. (Toward the end of the study enrollment, group assignment was targeted to ensure that groups were matched on sex, hearing, age, and IQ.) These two conditions involved 8 wk of in-home auditory activities. The auditory training group completed Brain Fitness cognitive training (Posit Science). Brain Fitness is an auditory-based cognitive training program comprising a series of six adaptive exercises designed to improve the speed and accuracy of auditory information processing; this is accomplished by exaggerating and then adaptively compressing the rapidly changing and perceptually vulnerable (21) formant transition period of speech syllable envelopes. Many different consonant–vowel transitions were used, especially stops and fricatives paired with different vowels in various linguistic contexts, including syllables, words, sentences, and stories. The training employs adaptively contracting transitions paired with increased memory demands in these contexts. The six exercises include discriminating pairs of frequency-modulated sweeps, discriminating between similar syllables, repeating back sequences of syllables and words, matching pairs of syllables and words, remembering multipart commands, and remembering details of stories. Several of these tasks are multimodal, integrating auditory and visual attention and working memory (see ref. 18 for additional details on the training program). We expected that greater memory demands would focus attention on the speech components needed to discriminate between similar-sounding words and syllables, and in so doing would modulate neural activity via top-down mechanisms to make these differences more salient (44, 60). We have previously shown that precise temporal encoding of the formant transition is degraded in older adults (19, 25) and is critical for speech-in-noise perception (21) and so hypothesized that adaptive training on this cue combined with increased memory demands would provide benefits for hearing in noise. Completion of training was verified through automated online logs. Previous studies have documented improvements in cognitive processing, efficiency, and memory after Brain Fitness training and we expected to replicate these effects (18). The active control group watched a series of educational DVDs about art, science, history, etc., and answered multiple choice questions that required careful listening and focused attention to the DVD content; only participants who scored above chance on the questions were included. Both participants were contacted on a regular basis throughout the training to ensure adherence to the program. The active control training was devised to be as similar to the auditory training as possible in terms of time and modality. However, the active control training had none of the adaptability or remediation offered by the auditory training software, nor did it focus on perception of any specific speech cue.
Both groups completed training at home on personal computers with supplied headphones (Koss UR/29, Koss). Training lasted for 1 h/day, 5 d/week, for 8 wk. After training, participants returned to the laboratory to repeat the behavioral and electrophysiological battery.
Groups were matched on sex both between and within group (χ2 tests, all P > 0.1) and for age, hearing thresholds (each frequency 0.125–12.5 kHz bilaterally), click latency, IQ, and test–retest interval (t tests, all P > 0.1). Group profiles are summarized in Table 1.
Table 1.
Group profiles
| Criterion | Auditory training | Active control |
| Age, years | 62.5 (3.2) | 63.6 (4.1) |
| Females:males | 21:14 | 18:14 |
| Test–retest delay, weeks | 9.8 (1.3) | 9.9 (2.3) |
| Pure-tone average (hearing 0.5–4 kHz; dB HL) | 17.9 (9.8) | 18.2 (6.7) |
| High-frequency hearing (6–8 kHz; dB HL) | 29.2 (16.5) | 30.0 (15.2) |
| Click Wave V latency, ms | 6.0 (0.3) | 6.0 (0.4) |
| IQ, standard score | 118 (11) | 120 (13) |
| MoCA score | 27.57 (1.96) | 27.31 (1.50) |
Groups are matched on all demographic criteria. Means (SDs) are displayed for age, sex distributions, pre- and posttraining testing intervals, hearing, click latency, and IQ.
Behavioral Measures.
Speech-in-noise perception.
Participants’ speech-in-noise perception was evaluated with the Quick Speech-in-Noise Test (QuickSIN, Etymotic Research). The QuickSIN is a nonadaptive clinical measure of speech-in-noise perception; sets of six sentences are presented in a background of four-talker babble (three female, one male) binaurally at 70-dB HL through insert earphones (ER-2, Etymotic Research). The first sentence in each set is presented at a +25 dB signal-to-noise ratio (SNR), and the SNR decreases by 5 dB for each of the five subsequent sentences down to a 0 dB SNR. The total number of 5 target words repeated correctly in each set of six sentences (maximum 30) is subtracted from 25.5 to obtain the SNR loss (dB), defined as the difference between an individual’s speech-in-noise threshold and the average speech-in-noise threshold (61). The SNR scores are averaged across four lists to obtain a composite SNR score. Different sentence sets were presented before and after training.
Auditory short-term memory.
The Woodcock–Johnson III Tests of Cognitive Abilities (62) Memory for Words subtest was used to evaluate short-term memory. Participants repeat aurally presented sequences of up to seven words in the same order as they were presented. Age-normed scores were used for analysis.
Processing speed.
The Woodcock–Johnson III Tests of Cognitive Abilities Visual Matching subtest was used to evaluate speed of cognitive processing. Participants are presented with 60 printed sets of six numbers, and in 3 min have to identify (and circle) two identical numbers within each set. The numbers range from single- to triple-digit numbers. Age-normed scores were used for analysis.
Group means and standard deviations for pre- and posttraining behavioral measures are displayed in Table 2. There were no significant differences in auditory training and active control group performance on these measures at pretest [QuickSIN: t(65) = 0.501, P = 0.618; short-term memory: t(65) = 1.422, P = 0.160; visual matching: t(65) = 0.678, P = 0.500].
Electrophysiology.
Stimulus and recording.
A six-formant, 170-ms speech syllable [da] was synthesized in Klatt (63) at a 20-kHz sampling rate (refer to ref. 25 for details). The [da] was presented in two conditions: in quiet and in two-talker babble background noise (presented at a +10 dB SNR). In cases of hearing loss (thresholds > 20 dB HL at any frequency from 0.25 to 6 kHz for each ear), the frequencies in the [da] were selectively amplified with the NAL-R algorithm (64) using custom routines in MATLAB (Mathworks) to create binaural stimuli customized for each ear. Twenty participants in the auditory training group and 23 participants in the active control received altered stimuli. As individual hearing thresholds did not change over the course of training, no participant required differently amplified stimuli for the pre- and posttesting session.
Recording.
Stimuli were presented binaurally through electrically shielded insert earphones (ER-3; Etymotic Research) at 80 dB SPL, with an 83-ms interstimulus interval in alternating polarities using Neuroscan Stim2 (Compumedics). Subcortical responses were recorded differentially at 20 kHz with Ag-AgCl electrodes in a vertical electrode montage (Cz active, forehead ground, earlobe references) with all impedances < 5 kΩ using Neuroscan Acquire 4.3.
Data reduction.
Brainstem responses were offline bandpass filtered from 70 to 2000 Hz in Neuroscan Edit (12 dB/octave roll-off, zero phase-shift), and then epoched with a −40- to 213-ms time window, relative to stimulus onset at 0 ms. Responses to the two polarities were added to reduce interference from stimulus artifact and cochlear microphonic on the response (65). Responses were amplitude-baselined relative to the prestimulus period (−40 to 0 ms). Final averages consisted of 6,000 sweeps (3,000 in each polarity) in each condition. A grand average response is presented in Fig. 1.
Data Analysis.
Peak picking.
Two trained peak-pickers, blind to participant group (auditory training/active control) and test session (pre/post) manually identified the major negative-going peaks of interest in the brainstem response. An additional third peak-picker confirmed selections. The peaks of interest in the formant transition were referenced to their expected latencies at 34, 44, 54, and 64 ms. In the steady state the peaks of interest were referenced to 74, 84, 94, …, 164 ms. Interpeak variance was determined by calculating the SD of the interpeak differences in the FFR (34–44, 44–54, …154–164) pre- and posttraining.
Quiet-to-noise correlations.
To obtain an objective measure of training-related improved timing in noise, the similarity of the responses in the two conditions (quiet and noise) was assessed with a cross-correlation technique (66). Correlation coefficients (Pearson’s r) are computed by shifting the response waveform in noise relative to that in quiet (±2 ms) until the maximum correlation coefficient is achieved. Fisher z′-scores were used for statistical analyses (67). We measured this shift (lag) between the two responses before and after training in the region corresponding to the onset and transition as an objective corollary of our peak-picking measures of neural timing improvements, in this case relative to a response in quiet.
Statistical analyses.
Repeated measures analysis of variance (RmANOVA) was used to compare neural and behavioral measures before and after training, with test session (pre/post) modeled as a within-subject factor and group (auditory training/active control) as a between subject factor. Multivariate RmANOVA was used to compare overall timing differences in the transition (34, 44, 54, 64 ms) and steady state (74, 84, 94, 104, …, 164 ms) of the brainstem responses. Normality of all variables was ensured with the Shapiro–Wilk test (all P > 0.1) and homogeneity of variance with Levene’s test. Mauchly’s test of sphericity was used in RmANOVAs, and the Greenhouse–Geisser correction was applied when the sphericity assumption was violated. Bonferroni corrections were applied as appropriate for multiple comparisons; P values reflect two-tailed tests.
Acknowledgments
We thank the participants in our study and Jane Hornickel, Adam Tierney, Sarah Drehobl, and Trent Nicol for their comments on the manuscript. This work was supported by National Institutes of Health Grants T32 DC009399-01A10 and R01 DC10016 and the Knowles Hearing Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.H.R. is a guest editor invited by the Editorial Board.
References
- 1.Lu PH, et al. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J Clin Exp Neuropsychol. 2011;33(10):1059–1068. doi: 10.1080/13803395.2011.595397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience. 2011;192(0):619–630. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 3.Recanzone GH, Engle JR, Juarez-Salinas DL. Spatial and temporal processing of single auditory cortical neurons and populations of neurons in the macaque monkey. Hear Res. 2011;271(1-2):115–122. doi: 10.1016/j.heares.2010.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998;18(7):2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forstmann BU, et al. The speed-accuracy tradeoff in the elderly brain: A structural model-based approach. J Neurosci. 2011;31(47):17242–17249. doi: 10.1523/JNEUROSCI.0309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider B, Pichora-Fuller MK. Age-related changes in temporal processing: Implications for speech perception. Semin Hear. 2001;22(03):227–240. [Google Scholar]
- 7.Kraus N, et al. Consequences of neural asynchrony: A case of AN. J Assoc Res Otolaryngol. 2000;01:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozorg Grayeli A, et al. Diagnostic value of auditory brainstem responses in cerebellopontine angle tumours. Acta Otolaryngol. 2008;128(10):1096–1100. doi: 10.1080/00016480701881803. [DOI] [PubMed] [Google Scholar]
- 9.Tremblay KL, Piskosz M, Souza P. Aging alters the neural representation of speech cues. Neuroreport. 2002;13(15):1865–1870. doi: 10.1097/00001756-200210280-00007. [DOI] [PubMed] [Google Scholar]
- 10.Heine C, Browning CJ. Communication and psychosocial consequences of sensory loss in older adults: overview and rehabilitation directions. Disabil Rehabil. 2002;24(15):763–773. doi: 10.1080/09638280210129162. [DOI] [PubMed] [Google Scholar]
- 11.Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003;114(7):1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 12.de Villers-Sidani E, et al. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci USA. 2010;107(31):13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo NM, Hornickel J, Nicol T, Zecker S, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behav Brain Funct. 2010;6(1):60. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song JH, Skoe E, Banai K, Kraus N. Training to improve hearing speech in noise: biological mechanisms. Cereb Cortex. 2012;22(5):1180–1190. doi: 10.1093/cercor/bhr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song JH, Skoe E, Wong PCM, Kraus N. Plasticity in the adult human auditory brainstem following short-term linguistic training. J Cogn Neurosci. 2008;20(10):1892–1902. doi: 10.1162/jocn.2008.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carcagno S, Plack CJ. Subcortical plasticity following perceptual learning in a pitch discrimination task. J Assoc Res Otolaryngol. 2011;12(1):89–100. doi: 10.1007/s10162-010-0236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry AS, et al. The influence of perceptual training on working memory in older adults. PLoS ONE. 2010;5(7):e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith GE, et al. A cognitive training program based on principles of brain plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J Am Geriatr Soc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience offsets age-related delays in neural timing. Neurobiol Aging. 2012;33(7) doi: 10.1016/j.neurobiolaging.2011.12.015. 1483.e1–e4. [DOI] [PubMed] [Google Scholar]
- 20.Parbery-Clark A, Strait DL, Anderson S, Hittner E, Kraus N. Musical experience and the aging auditory system: Implications for cognitive abilities and hearing speech in noise. PLoS ONE. 2011;6(5):e18082. doi: 10.1371/journal.pone.0018082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson S, Skoe E, Chandrasekaran B, Kraus N. Neural timing is linked to speech perception in noise. J Neurosci. 2010;30(14):4922–4926. doi: 10.1523/JNEUROSCI.0107-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parbery-Clark A, Skoe E, Kraus N. Musical experience limits the degradative effects of background noise on the neural processing of sound. J Neurosci. 2009;29(45):14100–14107. doi: 10.1523/JNEUROSCI.3256-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson Sabes J, Sweetow RW. Variables predicting outcomes on listening and communication enhancement (LACE) training. Int J Audiol. 2007;46(7):374–383. doi: 10.1080/14992020701297565. [DOI] [PubMed] [Google Scholar]
- 24.Miller GA, Nicely PE. An analysis of perceptual confusions among some English consonants. J Acoust Soc Am. 1955;27(2):338–352. [Google Scholar]
- 25.Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci. 2012;32(41):14156–14164. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211(Pt 11):1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes LF, Turner JG, Parrish JL, Caspary DM. Processing of broadband stimuli across A1 layers in young and aged rats. Hear Res. 2010;264(1-2):79–85. doi: 10.1016/j.heares.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: Role of inhibitory inputs. J Neurosci. 2005;25(47):10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton JP, Frisina RD, Ison JR, O'Neill WE. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J Comp Physiol A. 1997;181(2):161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- 30.Ison JR, Allen P. A diminished rate of “physiological decay” at noise offset contributes to age-related changes in temporal acuity in the CBA mouse model of presbycusis. J Acoust Soc Am. 2003;114(1):522–528. doi: 10.1121/1.1577553. [DOI] [PubMed] [Google Scholar]
- 31.Guo F, et al. Auditory discrimination training rescues developmentally degraded directional selectivity and restores mature expression of GABA(A) and AMPA receptor subunits in rat auditory cortex. Behav Brain Res. 2012;229(2):301–307. doi: 10.1016/j.bbr.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 32.Gleich O, Hamann I, Klump GM, Kittel M, Strutz J. Boosting GABA improves impaired auditory temporal resolution in the gerbil. Neuroreport. 2003;14(14):1877–1880. doi: 10.1097/00001756-200310060-00024. [DOI] [PubMed] [Google Scholar]
- 33.Williams AJ, Fuzessery ZM. Differential roles of GABAergic and glycinergic input on FM selectivity in the inferior colliculus of the pallid bat. J Neurophysiol. 2011;106(5):2523–2535. doi: 10.1152/jn.00569.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426(6965):442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 35.Warburton EC, et al. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38(6):987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- 36.Herrero JL, et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454(7208):1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14(2):132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- 38.Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998;1(8):727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puckett AC, Pandya PK, Moucha R, Dai W, Kilgard MP. Plasticity in the rat posterior auditory field following nucleus basalis stimulation. J Neurophysiol. 2007;98(1):253–265. doi: 10.1152/jn.01309.2006. [DOI] [PubMed] [Google Scholar]
- 40.Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Froemke RC, et al. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2012;16(1):79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinke W, et al. Cholinergic modulation of response properties and orientation tuning of neurons in primary visual cortex of anaesthetized Marmoset monkeys. Eur J Neurosci. 2006;24(1):314–328. doi: 10.1111/j.1460-9568.2006.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burkard R, Hecox KE. The effect of broadband noise on the human brain-stem auditory evoked response. III. Anatomic locus. J Acoust Soc Am. 1987;81(4):1050–1063. doi: 10.1121/1.394677. [DOI] [PubMed] [Google Scholar]
- 44.Kuo B-C, Yeh Y-Y, Chen AJW, D’Esposito M. Functional connectivity during top-down modulation of visual short-term memory representations. Neuropsychologia. 2011;49(6):1589–1596. doi: 10.1016/j.neuropsychologia.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahum M, Nelken I, Ahissar M. Stimulus uncertainty and perceptual learning: Similar principles govern auditory and visual learning. Vision Res. 2010;50(4):391–401. doi: 10.1016/j.visres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Banai K, Lavner Y. Perceptual learning of time-compressed speech: More than rapid adaptation. PLoS ONE. 2012;7(10):e47099. doi: 10.1371/journal.pone.0047099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortiz JA, Wright BA. Differential rates of consolidation of conceptual and stimulus learning following training on an auditory skill. Exp Brain Res. 2010;201(3):441–451. doi: 10.1007/s00221-009-2053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clay OJ, et al. Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. J Aging Health. 2009;21(4):547–566. doi: 10.1177/0898264309333326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci. 2010;13(2):253–260. doi: 10.1038/nn.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology. 2010;47(2):236–246. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandrasekaran B, Kraus N, Wong PCM. Human inferior colliculus activity relates to individual differences in spoken language learning. J Neurophysiol. 2012;107(5):1325–1336. doi: 10.1152/jn.00923.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremblay K, Kraus N, McGee T. The time course of auditory perceptual learning: neurophysiological changes during speech-sound training. Neuroreport. 1998;9(16):3557–3560. doi: 10.1097/00001756-199811160-00003. [DOI] [PubMed] [Google Scholar]
- 53.Linkenhoker BA, Knudsen EI. Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature. 2002;419(6904):293–296. doi: 10.1038/nature01002. [DOI] [PubMed] [Google Scholar]
- 54.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: From molecular to behavioral interventions. J Neurosci. 2010;30(45):14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engineer ND, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nat Rev Neurosci. 2010;11(8):599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- 57.Bavelier D, Green CS, Pouget A, Schrater P. Brain plasticity through the life span: learning to learn and action video games. Annu Rev Neurosci. 2012;35(1):391–416. doi: 10.1146/annurev-neuro-060909-152832. [DOI] [PubMed] [Google Scholar]
- 58.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 59.Zhu J, Garcia E. The Wechsler Abbreviated Scale of Intelligence (WASI) New York: Psychological Corporation; 1999. [Google Scholar]
- 60.Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17(3):507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- 61.Killion MC, Niquette PA, Gudmundsen GI, Revit LJ, Banerjee S. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J Acoust Soc Am. 2004;116(4 Pt 1):2395–2405. doi: 10.1121/1.1784440. [DOI] [PubMed] [Google Scholar]
- 62.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 63.Klatt D. Software for a cascade/parallel formant synthesizer. J Acoust Soc Am. 1980;67:971–995. [Google Scholar]
- 64.Byrne D, Dillon H. The National Acoustic Laboratories’ (NAL) new procedure for selecting the gain and frequency response of a hearing aid. Ear Hear. 1986;7(4):257–265. doi: 10.1097/00003446-198608000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Campbell T, Kerlin JR, Bishop CW, Miller LM. Methods to eliminate stimulus transduction artifact from insert earphones during electroencephalography. Ear Hear. 2012;33(1):144–150. doi: 10.1097/AUD.0b013e3182280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skoe E, Kraus N. Auditory brain stem response to complex sounds: a tutorial. Ear Hear. 2010;31(3):302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1975. [Google Scholar]