Abstract
Despite their nearly universal activation of mammalian target of rapamycin (mTOR) signaling, glioblastomas (GBMs) are strikingly resistant to mTOR-targeted therapy. We analyzed GBM cell lines, patient-derived tumor cell cultures, and clinical samples from patients in phase 1 clinical trials, and find that the promyelocytic leukemia (PML) gene mediates resistance to mTOR-targeted therapies. Direct mTOR inhibitors and EGF receptor (EGFR) inhibitors that block downstream mTOR signaling promote nuclear PML expression in GBMs, and genetic overexpression and knockdown approaches demonstrate that PML prevents mTOR and EGFR inhibitor-dependent cell death. Low doses of the PML inhibitor, arsenic trioxide, abrogate PML expression and reverse mTOR kinase inhibitor resistance in vivo, thus markedly inhibiting tumor growth and promoting tumor cell death in mice. These results identify a unique role for PML in mTOR and EGFR inhibitor resistance and provide a strong rationale for a combination therapeutic strategy to overcome it.
Keywords: mTORC1, glioma
Glioblastoma (GBM) is the most common malignant primary brain tumor of adults and one of the most lethal forms of cancer (1, 2). As a consequence of frequent EGF receptor (EGFR) amplification and/or activating mutation, other receptor tyrosine kinase amplifications and phosphatase and tensin homolog (PTEN) loss (3, 4), persistent hyperactivation of the phosphatidyl-inositol-3-kinase (PI3K) pathway is observed in nearly 90% of GBMs making the downstream effector, mammalian target of rapamycin (mTOR), a compelling drug target. mTOR links growth factor signaling through PI3K to energy and nutrient status, protein translation, autophagy, and tumor cell metabolism (5). Thus, mTOR is a critical integrator that regulates tumor growth, survival and, potentially, cancer drug resistance.
The allosteric mTOR inhibitor rapamycin has failed in the clinic as a treatment for GBM patients. We previously reported that in a clinical phase I trial for patients with recurrent PTEN-deficient GBM, rapamycin treatment led to Akt activation resulting in loss of negative feedback, consistent with the homeostatic regulatory role of mTOR complex I (mTORC1) as a negative regulator of PI3K/Akt signaling (6). Further, we demonstrated a critical role for mTOR complex II (mTORC2) as a critical mediator of rapamycin resistance through Akt and mTORC1-independent signaling pathways (7). These results have highlighted the potential role for mTOR kinase inhibitors, which block both mTOR signaling complexes, in the treatment of GBM and potentially other cancers.
The interconnectivity between mTOR signaling complexes suggests the possibility that multiple mechanisms of mTOR inhibitor resistance may exist, some of which may be clinically actionable. The promyelocytic leukemia (PML) gene may represent one such mechanism. PML is a pleiotropic tumor suppressor that plays multiple roles on cellular homeostasis such as apoptosis, proliferation, and senescence (8, 9). PML, as part of the retinoic acid receptor (RAR)/PML fusion protein identified in acute promyelocytic leukemia, represents one of the first molecular cancer targets amenable to targeted drug therapy (10, 11). Although the loss of PML protein expression is associated with tumor progression in many tumors (12), some tumors show paradoxically high levels of PML. For example, PML has been shown to be highly expressed in hematopoetic stem cells and in chemotherapy resistant, quiescent leukemia-initiating CML cells (13).
PML is also closely related to receptor tyrosine kinase (RTK)/PI3K/Akt/mTOR signaling pathway at multiple levels. PML has been reported to oppose the function of nuclear Akt (14) and was also identified as a repressor of mTOR through inhibition of Ras homolog enriched in brain (Rheb)–mTOR interaction during hypoxia (15). Further, PML is responsible for the repression of transcriptional activity from the EGFR promoter (16). Considering these factors, we hypothesized that PML might promote resistance to rapamycin, ATP-competitive mTOR kinase inhibitors, and EGFR tyrosine kinase inhibitors by controlling RTK/PI3K/Akt/mTOR signaling and cell cycle in GBM. Here, we examine the expression of PML in GBM cell lines and GBM-patient tissues; show that it is regulated by PI3K/Akt/mTOR signaling; demonstrate the impact of mTOR inhibition on PML expression; and then, using genetic and pharmacological approaches and correlations from clinical samples of patients treated with rapamycin or erlotinib, demonstrate a role for PML in preventing drug-induced apopotosis and promoting clinical resistance. Finally, we identify genetic and pharmacological approaches to overcome this drug resistance.
Results
PML Expression in GBM Patients.
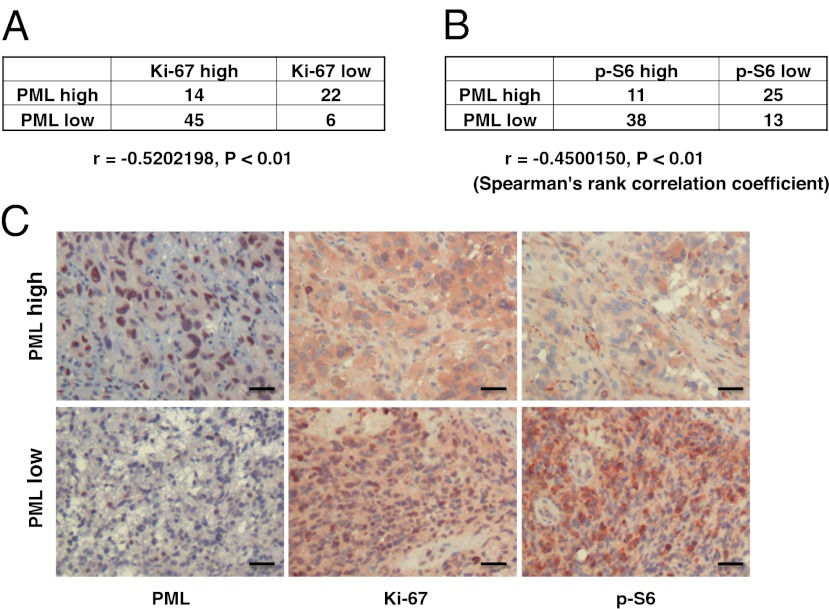
To examine the expression of PML in GBM patients, we performed immunohistochemical analysis of GBM by using a tissue microarray (TMA) consisting of multiple representative regions of tumor and adjacent normal tissue from 87 patients with primary GBMs (17, 18). Expression of PML, Ki-67, and phospho-S6 was analyzed and scored independently by two neuropathologists as high or low (scoring summarized in Fig. 1 A and B). PML was highly expressed in 41.4% of tumor samples (Fig. 1 A and B), and the expression was significantly inversely correlated with the cell proliferation marker Ki-67 (Fig. 1 A and C; r = −0.52, P < 0.01) and with mTORC1 signaling, as measured by S6 phosphorylation (Fig. 1 B and C; r = −0.45, P < 0.01).
Fig. 1.
PML is inversely correlated with proliferation rate and mTOR signaling in GBM clinical samples. (A and B) Tissue microarrays containing tumor samples from 87 GBM patients were stained by using PML, Ki-67, and p-S6 antibody, respectively. PML is highly expressed in 40% of GBM patients. Correlation analyses show PML significantly inversely correlate with Ki-67 (A) and p-S6 (B). (C) Immunohistochemical staining of (reddish brown) PML, Ki-67, and p-S6 from a representative GBM patient. (Magnification: 10×.) Nuclei were counterstained with hematoxylin (blue). (Scale bar: 100 μm.)
Rapamycin Induces Expression and Nuclear Aggregation of PML.
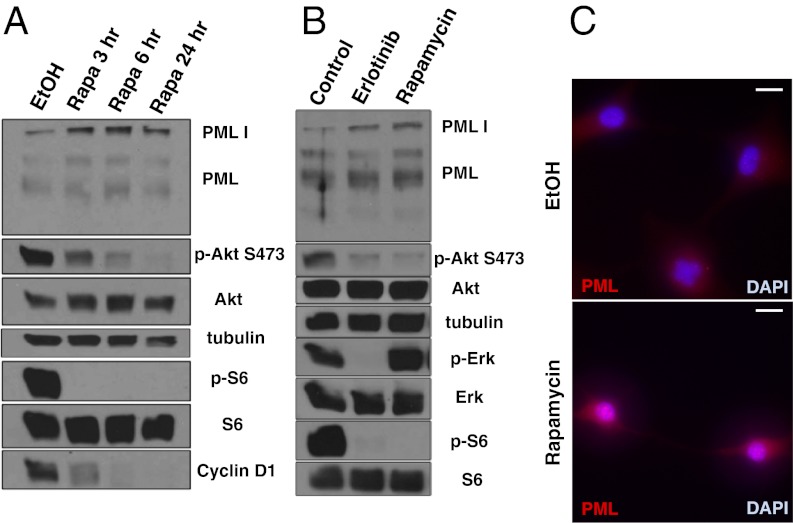
Next, we treated GBM cells and a GBM patient-derived cell culture with rapamycin to determine the effect of RTK/PI3K/mTOR inhibitor on PML (Fig. 2 A–C). Exposure to rapamycin treatment, or the ATP-competitive mTOR kinase inhibitor pp242, at doses sufficient to inhibit mTORC1 signaling, led to time-dependent increases in PML expression (Fig. 2 and Fig. S1). The EGFR tyrosine kinase inhibitor, erlotinib, which inhibited mTORC1 signaling downstream of EGFR, similarly elevated PML expression (Fig. 2B). Biochemical results were confirmed by fluorescent immunocytochemical analyses, demonstrating strongly granular patterns of PML staining in the nucleus, consistent with its reported distribution (Fig. 2C).
Fig. 2.
PI3K/Akt/mTOR inhibitors induce PML expression in GBM cells. (A) Western blot analysis of the effect of rapamycin treatment on PML expression in U87 cells. Cells are cultured in serum-free condition. (B) Effect of the EGFR inhibitor erlotinib and rapamycin on PML expression in GBM patient-derived cells. Cells are cultured under neurosphere conditions. (C) Immunofluorescence of PML (red) in U87 cells treated with rapamycin or control. Nuclei are stained with DAPI (blue). (Scale bar: 20 μm.)
Overexpression of PML Contributes to Decreasing PI3K/Akt/mTOR Signaling and a Slower Cell Cycle.
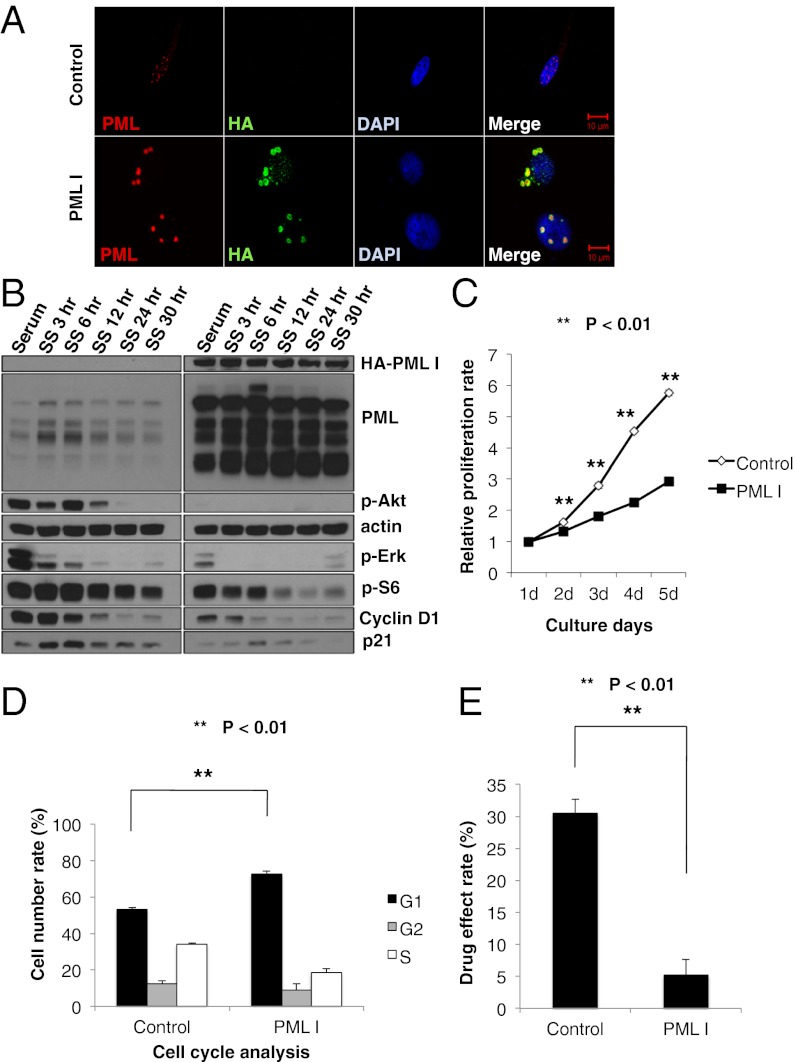
We hypothesized that PML might contribute to rapamycin, mTOR kinase inhibitor, and/or EGFR tyrosine kinase inhibitor resistance in GBM. Therefore, we performed retroviral transduction of PML I into U87 cells and examined the effect on PI3K/Akt/mTOR signaling and cell cycle progression. First, we performed double-immunofluorescent staining with PML and HA tag to confirm the overexpression of PML I (Fig. 3A). Compared with control, the retroviral-infected U87 cells expressed exogenous PML in both their nuclei and cytoplasm. Immunoblot analyses of these lysates demonstrated the increased expression of all PML isoforms, which would result from alternative splicing of the longest form, PML I (9). After confirmation of PML overexpression in these cell lines, we examined several PI3K/Akt/mTOR signaling proteins and cell cycle-related proteins by Western blotting. Akt and S6 phosphorylation were significantly decreased in U87 GBM cells that exogenously expressed PML I. Moreover, the cell cycle related proteins, cyclin D1 and cyclin-dependent kinase inhibitor 1 (p21), were also notably decreased, suggesting that PML contributes to decreasing PI3K/Akt/mTOR signaling and slowing down the cell cycle (Fig. 3B). We further performed cell proliferation assays by using these cell lines and confirmed that U87PML I cells were significantly less proliferative than control U87 cells (Fig. 3C; **P < 0.01).
Fig. 3.
PML overexpression decreases PI3K/Akt/mTOR signaling and slows down cell cycle. (A) Immunofluorescence in U87 control or hemagglutanin-tagged PML1 (HA-PMLI) infected cells. (Scale bar: 10 μm.) (B) Western blot analysis of PI3K/Akt/mTOR signaling pathway and cell cycle-related proteins performed on lysates from U87 control or HA-PMLI infected cells. Cells were placed in serum-free medium, cultured, and collected in each time course. (C) Proliferation of U87 control and HA-PMLI infected cells analyzed by WST assay. P value was determined by Student’s t test. (D) Effect of PMLI overexpression on cell cycle progression in U87 cells. Cell cycle distribution was performed by flow cytometric analysis. P value was determined by Student's t test. (E) Effect of treatment with rapamycin on growth of U87 control and HA-PMLI infected cells analyzed by WST assay. P value was determined by Student's t test.
Flow cytometric cell cycle analyses demonstrated an increased G1 fraction in U87PML I-expressing GBM cells (Fig. 3D; **P < 0.01). To determine whether this conferred rapamycin resistance, we treated U87PML I cells and control cells with rapamycin for 48 h and analyzed the drug effect by using WST-1 assays. PML I overexpression significantly reduced the growth inhibitory effect of rapamycin (Fig. 3E; **P < 0.01).
Interfering RNA-Mediated PML Knockdown Sensitizes GBM Cell Lines to mTOR and EGFR Kinase Inhibitor Treatment.
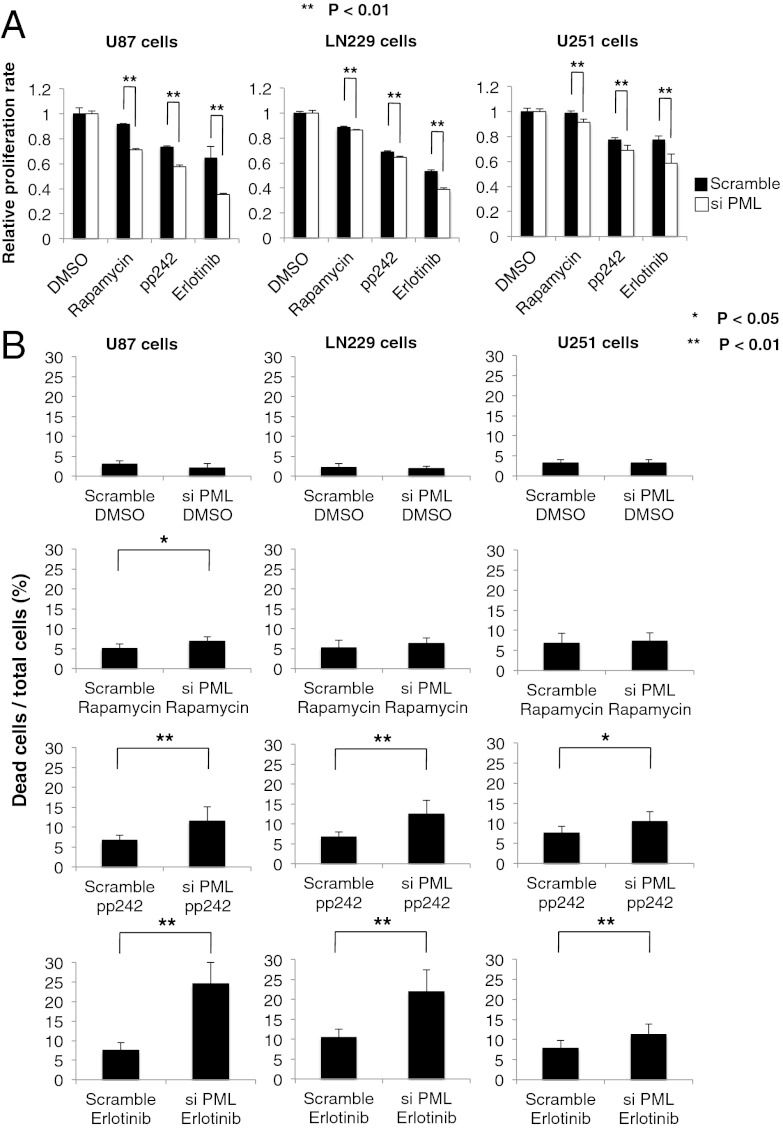
To confirm a specific role for PML in preventing mTOR and EGFR-kinase inhibitor-dependent cell death, we induced small interfering RNAs (siRNA)-mediated PML knockdown in multiple GBM cell lines and assessed its impact on response to rapamycin, pp242, and erlotinib. TUNEL analysis demonstrated that PML knockdown significantly sensitized all of the GBM cell lines to pp242 and erlotinib-mediated cell death (Fig. 4 A and B; *P < 0.05, **P < 0.01), which was confirmed by analysis of polyADP ribose polymerase (PARP) cleavage (Fig. S2). Of note, in contrast to pp242, rapamycin, which has less activity against mTORC2 than does pp242, induced minimal cell death even in the presence of PML knockdown, potentially suggesting a role for sustained mTORC2 signaling in mediating survival (7). Taken together, these data demonstrate that PML contributes to mTOR and EGFR kinase inhibitor resistance in GBM by suppressing tumor cell death, which can be reversed by pharmacological or genetic inhibition of PML.
Fig. 4.
PML knockdown sensitizes GBM cell lines to EGFR and mTOR targeted therapies. (A) Cell viability assays demonstrate a synergistic effect of PML knockdown and each indicated inhibitor. P values were determined by Student’s t test. (B) Effect of PML knockdown and each indicated inhibitor on multiple GBM cell lines analyzed by Trypan blue exclusion. P values were determined by Student’s t test.
As2O3 Abrogates pp242-Induced PML Up-Regulation and Sensitizes GBMs to mTOR Kinase Inhibitor-Mediated Cell Death.
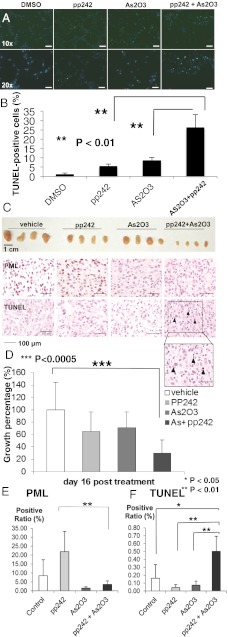
Arsenic trioxide (As2O3) has long been used as a therapeutic agent for promyelocytic leukemia (19–21). Besides its cell toxicity, As2O3 has been shown to target PML for degradation through a sumoylation-dependent process leading to PML polyubiquitination and proteosomal degradation (11, 13, 22–24). Therefore, we investigated the effect of As2O3 on reduction of PML in U87 cells. Single As2O3 treatments reduced PML expression at both low (0.15 μM) and high concentrations (2 μM) and decreased proliferation in serum-containing growth condition (Fig. S3 A and B). Notably, a high concentration (2 μM) induced an increase in p53 levels and decreased levels of cyclin D1 expression (Fig. S3A). The ability of low dose As2O3 to inhibit proliferation in the absence of p53 induction is consistent with previous papers (13, 25) suggesting that lower concentration of As2O3 mediates its effect by reducing PML levels and not by inducing DNA damage. Neither pp242, nor As2O3 alone, promoted extensive tumor cell death. In contrast, As2O3 (0.15 μM) significantly and synergistically promoted pp242- dependent apoptotic cell death, as measured by cleaved caspase and TUNEL staining, independent of any effect on p53 phosphorylation (*P < 0.01; Fig. 5 A and B and Fig. S3C).
Fig. 5.
As2O3 reduces PML and sensitizes GBM cells to mTOR-targeted therapies. (A) Representative images demonstrating TUNEL staining (green) to assess apoptotic effect of pp242 and As2O3 (2 μM) on U87 cells in vitro. Nuclei are stained blue. (B) Quantification of TUNEL staining. P values were determined by Student’s t test. (C) Representative photographs of U87 GBM xenografts treated daily with vehicle, pp242 (60 mg/kg per day by oral gavage), As2O3 (2.5 mg/kg intraperitoneally), or combination (n = 8 mice per condition). Images of representative PML and TUNEL stains. (D) Quantification demonstrating greater than threefold reduction in tumor size for mice treated with combined pp242 and As2O3 (P < 0.005). (E and F) Quantification of PML and TUNEL xenograft tumor staining from each treatment conditions.
Therefore, we analyzed the effect of combining the mTOR kinase inhibitor pp242 with As2O3 on PML expression, cell death, and tumor size in U87 GBM xenografts (Fig. 5 C–F). Sixteen days of treatment with pp242 and As2O3, significantly reduced the growth of GBMs by nearly threefold (P < 0.0005) and induced TUNEL-positive cell death, an effect that was not detected with either pp242 or As2O3 monotherapy (Fig. 5 C, D, and F). Importantly, As2O3 also abrogated the pp242-mediated up-regulation of PML expression (Fig. 5E). Ki-67 staining was also diminished, although the decrease failed to reach statistical significance (Fig. S4). Taken together, these results demonstrate that As2O3 dramatically synergizes with mTOR kinase inhibition to promote GBM cell death and block tumor growth in vivo.
Immunohistochemical Analyses of PML Expression in GBM Patients Treated with Rapamycin or Erlotinib.
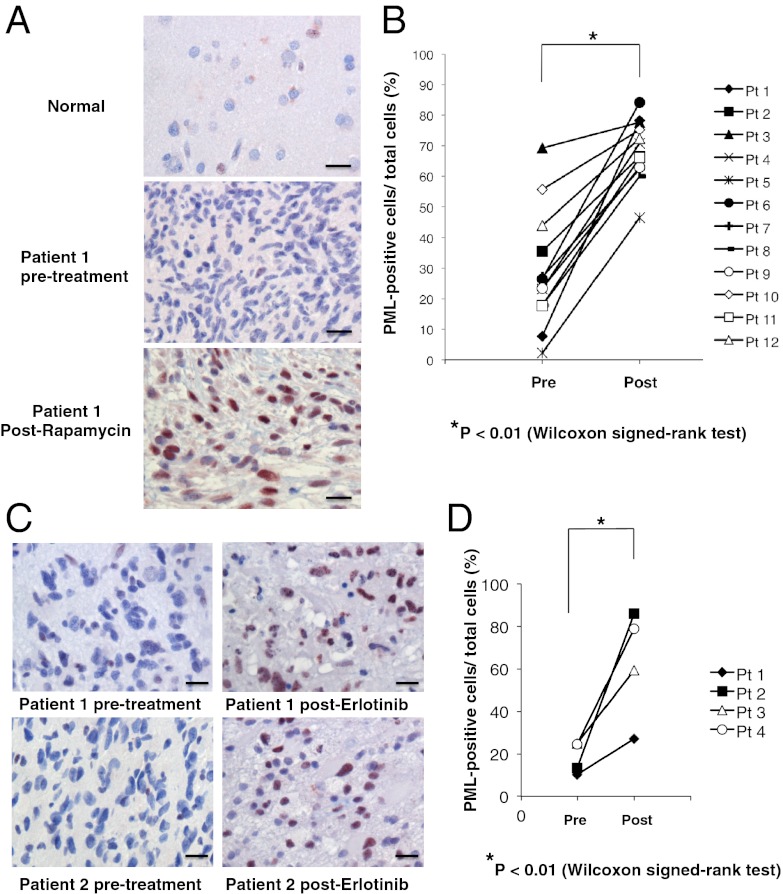
Finally, to establish clinical relevance and to determine whether PML up-regulation is associated with mTOR and EGFR inhibitor resistance in GBM patients, we performed immunohistochemical analyses of tumor samples obtained from two “biopsy-treat-biopsy” paradigm phase I clinical trials, for which tumor tissue was obtained 7–10 d after treatment with rapamycin or lapatinib (details presented in refs. 6 and 26). As shown in Fig. 6, rapamycin (Fig. 6 A and B) and erlotinib (Fig. 6 C and D) treatment were both associated with significantly enhanced nuclear PML expression (*P < 0.01).
Fig. 6.
Rapamycin and erlotinib treatment induces PML expression in GBM patient tumor tissues. (A) Immunohistochemical staining (reddish brown) of PML before and after treatment with rapamycin. Nuclei were counterstained with hematoxylin (blue). (B) Quantification of immunohistochemical staining from >1,000 cells from at least three representative areas of each tumor before and after rapamycin treatment. P value was determined by Wilcoxon signed-rank test. (C) Immunohistochemical staining (reddish brown) of PML before and after treatment with erlotinib. Nuclei were counterstained with hematoxylin (blue). (D) Quantification of immunohistochemical staining from >1,000 cells from at least three representative areas of each tumor before and after erlotinib treatment. P value was determined by Wilcoxon signed-rank test. (Scale bars: 50 μm.) (Magnification: 20×.)
Discussion
PML is a pleiotropic tumor suppressor protein that is lost in many cancer types (12, 27). PML negatively regulates Akt-mTOR signaling (14, 28) and suppresses PTEN loss- induced prostate tumorigenesis (14) and mTOR-dependent renal carcinoma progression (28). We provide evidence from preclinical models and in patients that PML suppresses Akt/mTOR signaling and proliferation (Fig. 1). However, PML is also commonly overexpressed in cancer, including in GBM (12, 29), and has been shown to promote a range of activities that may enhance the growth and progression of cancer, including oncogene-induced senescence (29), hematopoetic stem cell maintenance, and breast cancer tumor cell survival through a peroxisome proliferator-activated receptor (PPAR)-γ/fatty acid oxidation-dependent pathway (30, 31). Further, PML has been shown to mediate resistance of leukemias to chemotherapy by supporting maintenance of a “quiescent” tumor cell population (13). Its impact on cancer drug resistance, including drugs that target mTOR or its upstream effectors, in solid tumors including GBM is less clear. Through integration of preclinical studies with analysis of tumor tissue from patients in phase I clinical trials, we demonstrate an important role for PML in mediating mTOR and EGFR inhibitor resistance in GBM. These results present evidence that mTOR inhibition promotes PML up-regulation in patients and that this up-regulation of PML mediates drug resistance.
It is tempting to speculate that PML promotes this resistance by inducing a “quiescent state” through inhibition of Akt/mTOR signaling. However, we cannot formally exclude the possibility that PML may drive resistance through its metabolic prosurvival effects. In fact, this possibility is consistent with our previous observation that EGFR mutant GBMs have enhanced reliance on fatty acid synthesis for survival (17), creating enhanced dependence on fatty acid oxidation for survival (32). Futures studies will be needed to determine the mechanisms by which PML promotes drug resistance in GBM.
mTOR has emerged as a critical target in GBM because it is persistently hyperactivated downstream of the most common GBM alterations including EGFR amplification, EGFR variant III (EGFRvIII) mutation, platelet-derived growth factor receptor (PDGFRα) and hepatocyte growth factor receptor (c-MET) amplification, and PTEN loss (3). We have demonstrated that mTOR inhibition is required for the efficacy of EGFR-targeted therapies (33), suggesting a mechanistic basis by which EGFR tyrosine kinase inhibitors (TKIs) may also potently up-regulate PML expression to promote drug resistance. For both EGFR TKIs and mTOR kinase inhibitors, the potential for converting a cytostatic response, which often yields minimal benefit, to a cytotoxic response by pharmacologically abrogating PML, could potentially represent a significant clinical advance. Our demonstration of a synergism between the two classes of compound in cell death induction underscores this possibility.
Pharmacologically targeting PML represents one of the most exciting success stories for the principle of molecularly guided therapies (10, 11). As2O3 targets PML for degradation through a SUMOylation-dependent process (34), potently promoting long-term remission in patients and mice with acute promyelocytic leukemia bearing the PML/RAR fusion (24). Its role in solid cancers has yet to be established. However, As2O3 given with standard chemotherapy can be tolerated by GBM patients, as demonstrated in recent clinical trials (35). The results presented here suggest a clinically actionable strategy to combine resistance by combining As2O3 with mTOR kinase and EGFR TKIs for the treatment of GBM patients.
Materials and Methods
Cell Lines.
U87, LN229, and U251 GBM cell lines were cultured as previously described (18, 26). Brain tumor samples were collected after surgical resection under University of California, Los Angeles (UCLA) institutional review board-approved protocols between 1999 and 2011 from patients who gave informed consent, and graded by the neuropathologist in accordance with World Health Organization-established guidelines. Neurosphere cultures were prepared as described (36). Full details are provided in SI Materials and Methods.
Antibodies and Reagents.
We used antibodies directed against the following: phosopho-Akt Ser473, Akt, phospho-S6 Ser235/236, S6, phospho-Erk, Erk, CyclinD1, cleaved PARP (Cell Signaling); β-actin, p21 (Sigma); phospho-EGFR Tyr1086 (Invitrogen); EGFR (Millipore); PML (for Western blotting, Abcam; for immunohistochemisry, Santa Cruz). Reagents used are rapamycin, As2O3, polybrene (Sigma), erlotinib (ChemieTex), pp242 (Chemdea). Full details of immunoblot analysis are provided in SI Materials and Methods. Stock solutions of inhibitor for rapamycin were made by dissolving in ethanol, erlotinib, and pp242 were made by dissolving in DMSO (Sigma) and stored at −20 °C. Inhibitors were added to each well at final concentrations of 10 nM, 10 μM, and 2 μM, respectively. An equal concentration of ethanol or DMSO served as control. As2O3 was diluted by PBS and 10 M NaOH, then pH was adjusted at 8.0 by 12 M HCl.
Plasmid, Retroviral Infection, and siRNA Transfection.
Plasmid 22(pLNCX) encoding hemagglutinin (HA) tag-expression construct was obtained from the I.K. laboratory (37). Full details are available in SI Materials and Methods. Transfection of siRNA into GBM cell lines was carried out by using Lipofectamine RNAiMAX (Invitrogen) in full serum, with medium change after 24 h. On-TARGET plus SMARTpool siRNAs (Dharmacon) specifically targeting PML (catalog no. L-006547-000005) and nontargeting control pools of siRNAs (catalog no. D-0018-10-10-05) were used at 10 nM, and cells were harvested 48 h after transfection.
Cell Proliferation and Death Assays.
Relative proliferation to control cells with vehicle treatment was checked with a WST-1 Cell Proliferation Assay Kit (Millipore). Cell death was assessed by Trypan blue exclusion (Invitrogen). Full details are given in SI Materials and Methods.
Cell Cycle Analyses.
Cells were fixed in 70% ethanol diluted in PBS, and the samples were stored at −20 °C. The fixed cells were resuspended in PBS containing 20 μg/mL propidium iodide (Sigma) and 10 μg/mL RNase A (Sigma), and incubated for 10 min at 37 °C. Flow cytometric analysis was performed by using FACSCalibur flow cytometer (Becton Dickinson).
TUNEL Staining and Immunofluorescence Analysis.
For TUNEL staining, cells were placed in eight-well chamber slides, incubated with TUNEL Reaction Mixture (Roche) at 37 °C for1 h in the dark, and visualized with a fluorescene microscope (Olympus BX-61). Ten separate, randomly chosen fields on each chamber were imaged, and the numbers of TUNEL-positive cells and whole nuclei were counted. For immunofluorescence analysis with indicated antibodies, cells were fixed with 4% paraformaldehyde in PBS for 10 min, washed twice in PBS, incubated with primary antibodies in PBS containing 3% BSA at 4 °C overnight, and detected with appropriate fluorescence-conjugated secondary antibodies. Full details are presented in SI Materials and Methods.
In Vivo Studies.
We suspend 1.25 × 106 U87 GBM cells in 100 μL of Matrigel, PBS 1:2 solution, and injected them subcutaneously into the right flank of each 4- to 5-wk-old athymic nude mice. Tumors were measured with an electronic caliper, and volumes were calculated by using width (a), length (b), and depth (c) measurements (V = a × b × c). Ten days after injection, mice were treated daily with vehicle, 60 mg/kg pp242 by gavage, 2.5 mg/kg intraperitoneally injected As2O3 or their combination, respectively. Mice were euthanized when tumor volume of treated mice reached statistical significance compared with control groups. Mice were euthanized in accordance with the University of California at San Diego Institutional Guidelines for Animal Welfare and Experimental Conduct.
Immunohistochemical Assays, Tissue Microarrays, and Image Analysis-Based Scoring.
Immunohistochemical staining and analysis of two GBM TMAs was performed, as described (6, 26). Among 140 cases, 87 GBM patient tissue cores were available for analysis based on sufficient high quality tissue. Staining intensity was scored independently by two pathologists who were unaware of the findings of the molecular analyses. See SI Materials and Methods for full details.
Statistical Analysis.
Results are shown as mean ± SEM. χ2 for independence test was used to assess correlations between various molecular markers on TMAs. For nonparametric clinical trial data, Wilcoxon rank test was used. Other comparisons in cell proliferation assays, cell death assays, and TUNEL staining were performed with Student’s t test, as by analysis of variance, appropriate. P < 0.05 was considered as statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. George Thomas for helpful discussions and comments on this paper. A.I. and J.K. were supported in part by a grant from the Japan Society for the Promotion of Science. A.I. was also supported by a grant from the Uehara Memorial Foundation. B.G. is supported by a Marie Curie Fellowship from the European Commission- PIOF-GA-2010-271819. C.Z. is supported by an American-Italian Cancer Foundation postdoctoral research fellowship. This work was supported by National Institutes of Health (NIH) Grants NS73831 and CA119347 (to P.S.M.), by the Ziering Famiy Foundation in memory of Sigi Zeiring (P.S.M. and T.F.C.), the Ben and Catherine Ivy Foundation (P.S.M. and T.F.C.), and NIH Grant P01-CA95616 (to W.K.C.). W.K.C. is a Fellow of the National Foundation for Cancer Research.
Footnotes
The authors declare no conflict of interest.
†This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217602110/-/DCSupplemental.
References
- 1.Furnari FB, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yecies JL, Manning BD. Transcriptional control of cellular metabolism by mTOR signaling. Cancer Res. 2011;71(8):2815–2820. doi: 10.1158/0008-5472.CAN-10-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloughesy TF, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5(1):e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K, et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardi R, Pandolfi PP. Role of PML and the PML-nuclear body in the control of programmed cell death. Oncogene. 2003;22(56):9048–9057. doi: 10.1038/sj.onc.1207106. [DOI] [PubMed] [Google Scholar]
- 9.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8(12):1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 10.Andre C, et al. The PML and PML/RARalpha domains: From autoimmunity to molecular oncology and from retinoic acid to arsenic. Exp Cell Res. 1996;229(2):253–260. doi: 10.1006/excr.1996.0368. [DOI] [PubMed] [Google Scholar]
- 11.Chen GQ, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88(3):1052–1061. [PubMed] [Google Scholar]
- 12.Gurrieri C, et al. Loss of the tumor suppressor PML in human cancers of multiple histologic origins. J Natl Cancer Inst. 2004;96(4):269–279. doi: 10.1093/jnci/djh043. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453(7198):1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trotman LC, et al. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441(7092):523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardi R, et al. PML regulates p53 stability by sequestering Mdm2 to the nucleolus. Nat Cell Biol. 2004;6(7):665–672. doi: 10.1038/ncb1147. [DOI] [PubMed] [Google Scholar]
- 16.Vallian S, et al. Transcriptional repression by the promyelocytic leukemia protein, PML. Exp Cell Res. 1997;237(2):371–382. doi: 10.1006/excr.1997.3801. [DOI] [PubMed] [Google Scholar]
- 17.Guo D, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal. 2009;2(101):ra82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu KV, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69(17):6889–6898. doi: 10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aronson SM. Arsenic and old myths. R I Med. 1994;77(7):233–234. [PubMed] [Google Scholar]
- 20.Mathews V, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: Durable remissions with minimal toxicity. Blood. 2006;107(7):2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 21.Soignet SL, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339(19):1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 22.Lallemand-Breitenbach V, et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J Exp Med. 2001;193(12):1361–1371. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Thé H, Chen Z. Acute promyelocytic leukaemia: Novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10(11):775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- 24.Lallemand-Breitenbach V, Zhu J, Chen Z, de Thé H. Curing APL through PML/RARA degradation by As2O3. Trends Mol Med. 2012;18(1):36–42. doi: 10.1016/j.molmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S, Tsuchida T, Kawakami K, Shi C, Kawamoto K. Effect of As2O3 on cell cycle progression and cyclins D1 and B1 expression in two glioblastoma cell lines differing in p53 status. Int J Oncol. 2002;21(1):49–55. [PubMed] [Google Scholar]
- 26.Mellinghoff IK, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 27.Gambacorta M, et al. Heterogeneous nuclear expression of the promyelocytic leukemia (PML) protein in normal and neoplastic human tissues. Am J Pathol. 1996;149(6):2023–2035. [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardi R, et al. Pml represses tumour progression through inhibition of mTOR. EMBO Mol Med. 2011;3(5):249–257. doi: 10.1002/emmm.201100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scaglioni PP, et al. Translation-dependent mechanisms lead to PML upregulation and mediate oncogenic K-RAS-induced cellular senescence. EMBO Mol Med. 2012;4(7):594–602. doi: 10.1002/emmm.201200233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito K, et al. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18(9):1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carracedo A, et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest. 2012;122(9):3088–3100. doi: 10.1172/JCI62129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cvrljevic AN, et al. Activation of Src induces mitochondrial localisation of de2-7EGFR (EGFRvIII) in glioma cells: Implications for glucose metabolism. J Cell Sci. 2011;124(Pt 17):2938–2950. doi: 10.1242/jcs.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang MY, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66(16):7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XW, et al. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 2010;328(5975):240–243. doi: 10.1126/science.1183424. [DOI] [PubMed] [Google Scholar]
- 35.Grimm SA, et al. Phase I study of arsenic trioxide and temozolomide in combination with radiation therapy in patients with malignant gliomas. J Neurooncol. 2012;110(2):237–243. doi: 10.1007/s11060-012-0957-6. [DOI] [PubMed] [Google Scholar]
- 36.Geschwind DH, et al. A genetic analysis of neural progenitor differentiation. Neuron. 2001;29(2):325–339. doi: 10.1016/s0896-6273(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen LA, et al. Physical and functional link of the leukemia-associated factors AML1 and PML. Blood. 2005;105(1):292–300. doi: 10.1182/blood-2004-03-1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.