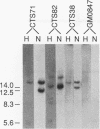
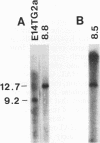
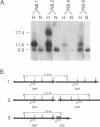
Abstract
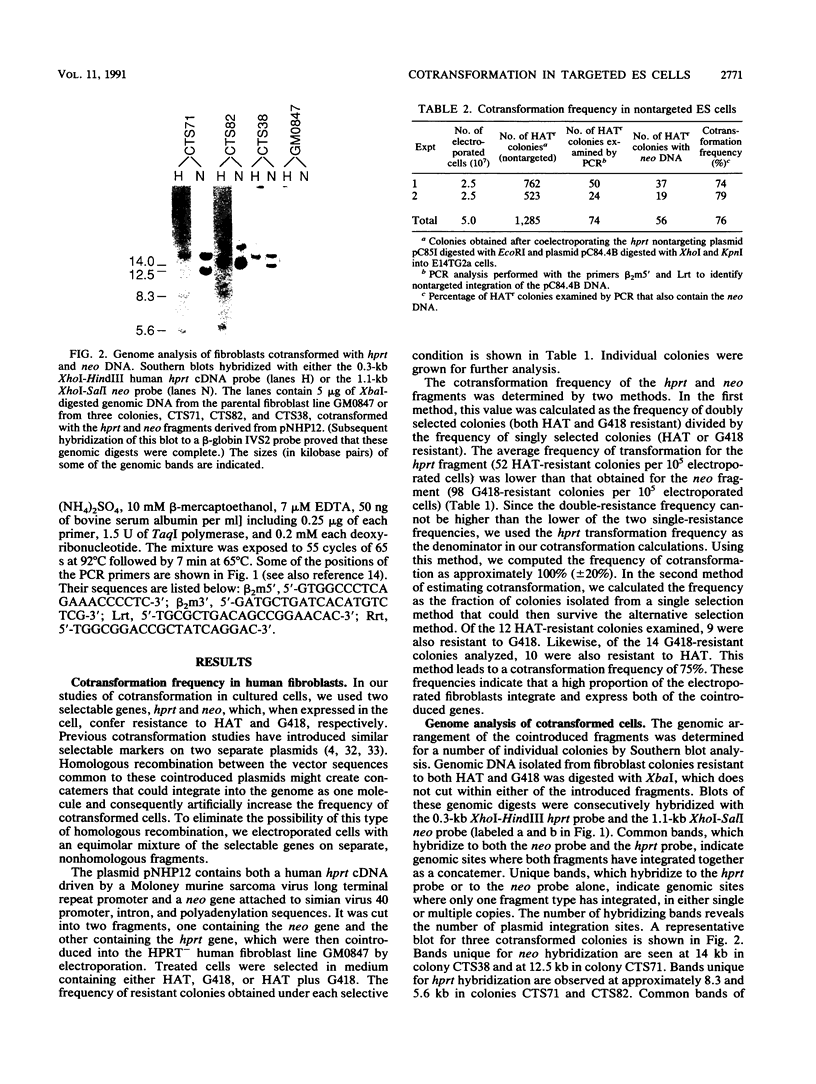
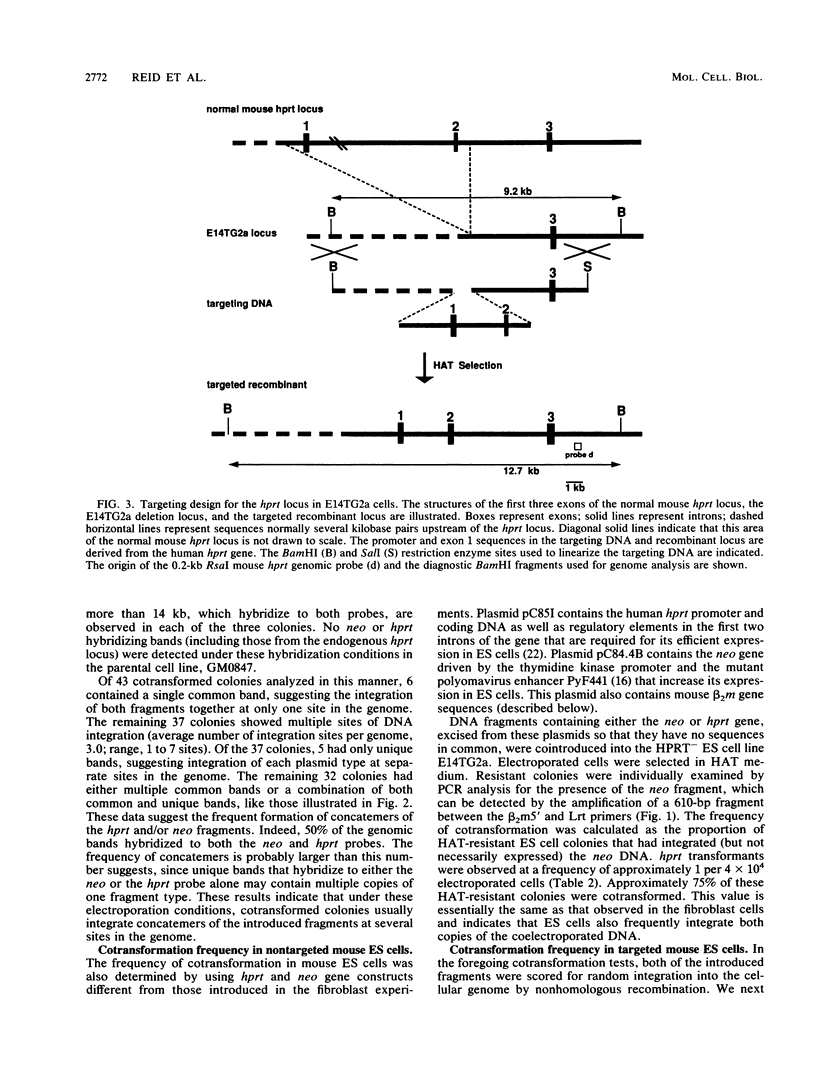
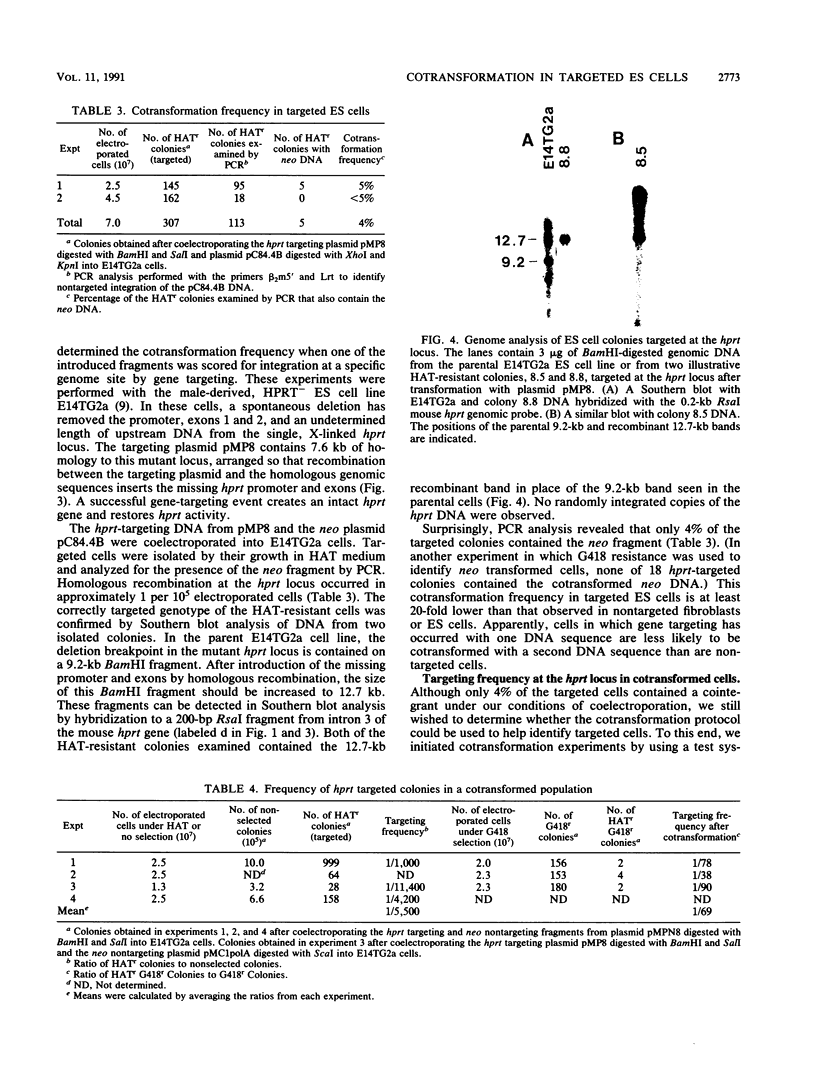
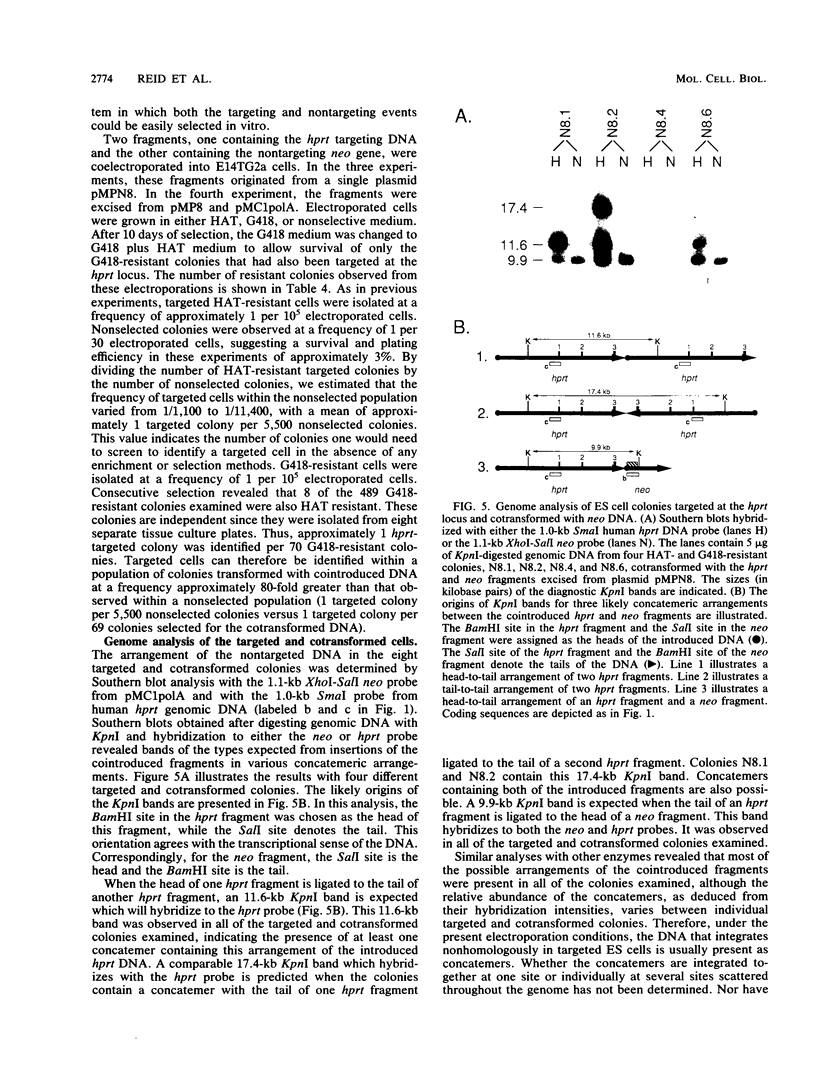
We have investigated cotransformation in mammalian cells and its potential for identifying cells that have been modified by gene targeting. Selectable genes on separate DNA fragments were simultaneously introduced into cells by coelectroporation. When the introduced fragments were scored for random integration, 75% of the transformed cells integrated both fragments within the genome of the same cell. When one of the cointroduced fragments was scored for integration at a specific locus by gene targeting, only 4% of the targeted cells cointegrated the second fragment. Apparently, cells that have been modified by gene targeting with one DNA fragment rarely incorporate a second DNA fragment. Despite this limitation, we were able to use the cotransformation protocol to identify targeted cells by screening populations of colonies that had been transformed with a cointroduced selectable gene. When hypoxanthine phosphoribosyltransferase (hprt) targeting DNA was coelectroporated with a selectable neomycin phosphotransferase (neo) gene into embryonic stem (ES) cells, hprt-targeted colonies were isolated from the population of neo transformants at a frequency of 1 per 70 G418-resistant colonies. In parallel experiments with the same targeting construct, hprt-targeted cells were found at a frequency of 1 per 5,500 nonselected colonies. Thus, an 80-fold enrichment for targeted cells was observed within the population of colonies transformed with the cointroduced DNA compared with the population of nonselected colonies. This enrichment for targeted cells after cotransformation should be useful in the isolation of colonies that contain targeted but nonselectable gene alterations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Nairn R. S., Wilson J. H., Seidman M. M., Brotherman K. A., MacKinnon C., Scheerer J. B. Targeted homologous recombination at the endogenous adenine phosphoribosyltransferase locus in Chinese hamster cells. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4574–4578. doi: 10.1073/pnas.86.12.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. D. High-frequency homologous recombination between duplicate chromosomal immunoglobulin mu heavy-chain constant regions. Mol Cell Biol. 1989 Dec;9(12):5500–5507. doi: 10.1128/mcb.9.12.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. D., Shulman M. J. Homologous recombination between transferred and chromosomal immunoglobulin kappa genes. Mol Cell Biol. 1988 Oct;8(10):4041–4047. doi: 10.1128/mcb.8.10.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs S. S., Gregg R. G., Borenstein N., Smithies O. Efficient transformation and frequent single-site, single-copy insertion of DNA can be obtained in mouse erythroleukemia cells transformed by electroporation. Exp Hematol. 1986 Nov;14(10):988–994. [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Doetschman T., Gregg R. G., Maeda N., Hooper M. L., Melton D. W., Thompson S., Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987 Dec 10;330(6148):576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Doetschman T., Maeda N., Smithies O. Targeted mutation of the Hprt gene in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8583–8587. doi: 10.1073/pnas.85.22.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Jolly D. J., Okayama H., Berg P., Esty A. C., Filpula D., Bohlen P., Johnson G. G., Shively J. E., Hunkapillar T., Friedmann T. Isolation and characterization of a full-length expressible cDNA for human hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):477–481. doi: 10.1073/pnas.80.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 26;16(18):8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Hagemann L. J., Doetschman T., Hagaman J. R., Huang S., Williams P. J., First N. L., Maeda N., Smithies O. Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8927–8931. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Inactivating the beta 2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Linney E., Donerly S. DNA fragments from F9 PyEC mutants increase expression of heterologous genes in transfected F9 cells. Cell. 1983 Dec;35(3 Pt 2):693–699. doi: 10.1016/0092-8674(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Jolly D. J., Friedmann T., Verma I. M. A transmissible retrovirus expressing human hypoxanthine phosphoribosyltransferase (HPRT): gene transfer into cells obtained from humans deficient in HPRT. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4709–4713. doi: 10.1073/pnas.80.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman R., Domingo D., Trotter J., Trowbridge I. Selection and properties of a mouse L-cell transformant expressing human transferrin receptor. Nature. 1983 Aug 18;304(5927):643–645. doi: 10.1038/304643a0. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Pine D. S., Bourekas E. C., Potter S. S. Mys retrotransposons in Peromyscus leucopus and transgenic Mus musculus. Nucleic Acids Res. 1988 Apr 25;16(8):3359–3373. doi: 10.1093/nar/16.8.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
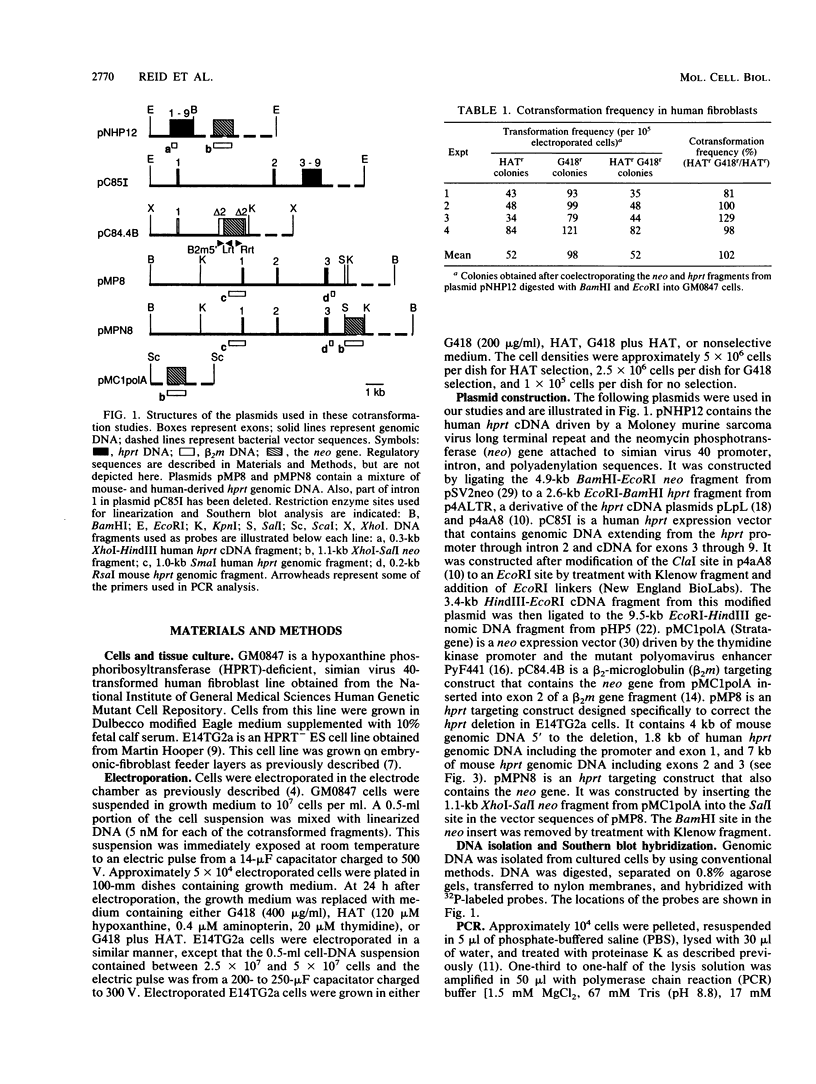
- Reid L. H., Gregg R. G., Smithies O., Koller B. H. Regulatory elements in the introns of the human HPRT gene are necessary for its expression in embryonic stem cells. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4299–4303. doi: 10.1073/pnas.87.11.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs C. D., Bates G. W. Stable transformation of tobacco by electroporation: evidence for plasmid concatenation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5602–5606. doi: 10.1073/pnas.83.15.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P. L., Goff S. P., Robertson E. J. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science. 1989 Nov 10;246(4931):799–803. doi: 10.1126/science.2554496. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P. L., Robertson E. J., Goff S. P. Targeted gene disruption of the endogenous c-abl locus by homologous recombination with DNA encoding a selectable fusion protein. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3210–3214. doi: 10.1073/pnas.87.8.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Nissen L., Collins C. Homologous recombination in hybridoma cells: dependence on time and fragment length. Mol Cell Biol. 1990 Sep;10(9):4466–4472. doi: 10.1128/mcb.10.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thompson S., Clarke A. R., Pow A. M., Hooper M. L., Melton D. W. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell. 1989 Jan 27;56(2):313–321. doi: 10.1016/0092-8674(89)90905-7. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Hayday A. C., Keating A. Electric field-mediated DNA transfer: transient and stable gene expression in human and mouse lymphoid cells. Mol Cell Biol. 1986 Feb;6(2):703–706. doi: 10.1128/mcb.6.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Keating A., Glynn S., McDonald K. Electric field-mediated gene transfer: characterization of DNA transfer and patterns of integration in lymphoid cells. Nucleic Acids Res. 1988 Jun 24;16(12):5515–5532. doi: 10.1093/nar/16.12.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wong E. A., Capecchi M. R. Homologous recombination between coinjected DNA sequences peaks in early to mid-S phase. Mol Cell Biol. 1987 Jun;7(6):2294–2295. doi: 10.1128/mcb.7.6.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989 Nov 23;342(6248):435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]