Abstract
Chemokines and chemokine receptors, has been shown to be involved in metastatic process of prostate cancer (PCa). In this study, we have show that primary PCa tissues and cell lines (LNCaP and PC3) express CXCR5, a specific chemokine receptor for the CXCL13. Expression of CXCR5 was significantly higher (P < 0.001) in PCa cases than compared to normal match (NM) tissues. CXCR5 intensity correlated (R2 = 0.97) with Gleason score. While prostate tumor tissues with Gleason scores ≥ 7, displayed predominantly nuclear CXCR5 expression patterns, PCa specimens with Gleason scores ≤ 6 showed predominantly membrane and cytoplasmic expression patterns that were comparable to benign prostatic hyperplasia (BPH). Similar to tissue expression, PCa cell lines expressed significantly more CXCR5 than normal prostatic epithelial cells (PrECs) and CXCR5 expression was distributed among intracellular and extracellular compartments. Functional in vitro assays showed higher migratory and invasive potentials toward CXCL13, an effect that was CXCR5-mediated. In both PCa cell lines, CXCL13 treatment increased the expression of collagenase-1 or matrix metalloproteinase-1 (MMP-1), collagenase-3 (MMP-13), stromelysin-1 (MMP-3), stromalysin-2 (MMP-10), and stromelysin-3 (MMP-11). These data demonstrate the clinical and biological relevance of the CXCL13-CXCR5 pathway and its role in PCa cell invasion and migration.
Keywords: chemokine, prostate cancer, tissue expression
Introduction
PCa is one of the leading causes of cancer-related deaths among men in US and the major cause of death from PCa is metastasis.1, 2 Many factors have been implicated in this process, but the precise mechanisms for the directional migration and invasion of tumor cells into specific organs is unknown.3, 4 Dissemination of cancer cells from the primary tumor site is one of the key steps of metastasis.5 MMPs are generally present in greater quantities in and around malignant tumor tissues than in the vicinity of normal, benign, or pre-malignant tissues. The highest expression patterns of MMPs occur in the area of active invasion around the tumor-stroma interface.6 Indeed, the presence of these factors has been associated with malignancies and tumor aggressiveness.7–11 While cell invasion assays demonstrated that MMP levels influence the ability of cells to penetrate native and reconstituted basement membranes, the modulators of MMP expression are not entirely known.12
Chemokines and their corresponding receptors appear to play an important role in the directional migration of hematopoietic cells to specific anatomical sites.13 Recent studies have shown similar to their role leukocyte trafficking, chemokines and chemokine receptors are involved in cancer metastasis. Indeed, it is widely held that the CXCL12-CXCR4 pathway plays a major role in PCa metastasis. 14–16 Recently, CXCR317, CXCR718, CX3CR119, CCR520, CCR721, and CCR922 have been shown to be involved in the adhesion, migration, and/or invasion of PCa cells. It is therefore likely that multiple chemokine receptors are involved in directing the multi-step process of PCa progression, including MMP expression.
The chemokine receptor CXCR5 is primary expressed by the B cells and certain T cells and controls their migration into and within lymph nodes. However, recent studies have shown that CXCR5 is expressed by the colon carcinoma and mediates metastasis to the liver.23 Others have shown relatively higher CXCR5 mRNA expression by some PCa cell lines.17, 24 In this study, we have show that CXCR5 mRNA and protein are significantly elevated in PCa cell lines. CXCL13, the ligand for CXCR5, mediates differential MMP mRNA expression and active protein secretion by PCa cell lines in a CXCR5-dependent fashion, demonstrating the biological significance of this chemokine-chemokine receptor axis. In addition to these functional studies, we show CXCR5 are highly expressed in PCa tissues than compared to NM tissues.
Materials & Methods
Cell lines and cell culture
PC3 and LNCaP cell lines were obtained from the American Type Culture Collection (ATCC). PrECs were obtained from Clonetics-Biowhittaker and cultured in prostate epithelial basal medium. PC3 cells were initially cultured in Ham’s F12K medium with 2mM L-glutamine and adjusted to contain 1.5 g/l sodium bicarbonate (ATCC) with 10% fetal bovine serum (FBS). After five passages in Ham’s F12K media, PC3 cells were switched to RPMI-1640 at 37°C and 5% CO2 with 10% fetal bovine serum FBS. LNCaP cells were cultured in RPMI-1640 with 10% FBS at 37°C with 5% CO2.
RNA isolation and gene expression analysis
Human mRNA sequences for CXCR5, MMP-1, MMP-3, MMP-2, MMP-9, MMP-10, MMP-11, MMP-13, and 18S rRNA were obtained from National Institutes of Health National Center for Biotechnology Information (NIH-NCBI) gene bank database accession numbers NM000579, NM002421, NM002422, NM004530, NM004994, NM002425, NM005940, NM002427 and X00686.1, respectively. These sequences were then used to design primers for real-time polymerase chain reaction (RT-PCR) analysis, which generated amplicons 101, 83, 155, 95, 79, 94, 107, 176, and 149 base pairs in size for CXCR5 MMP-1, MMP-3, MMP-2, MMP-9, MMP-10, MMP-11, MMP-13, and 18S rRNA, respectively. Primers were designed using the primer 3 software program from the Whitehead Institute at the Massachusetts Institute of Technology (MIT). Thermodynamic analysis of the primers was conducted using the following computer programs: Primer Premier™ (Integrated DNA Technologies) and MIT Primer III. The resulting primer sets were compared against the entire human genome to confirm specificity and ensure that the primers flanked the mRNA splicing regions.
Following the manufacturer’s protocols, Tri-reagent™ (Molecular Research Center) was used to isolate total RNA from untreated PCa cells and PrECs or similar cells treated with 100 ng/ml of CXCL13. Potential genomic DNA contamination was removed from these samples by treating them for 15 minutes at 37 C with RNase-free DNase (Invitrogen). RNA was precipitated and resuspended in RNA Secure™ (Ambion). cDNA was generated by reverse transcribing 1.5 μg of total RNA using Taqman™ reverse transcription reagent (Applied Biosystems), according to the manufacturer’s protocols, and amplified with specific cDNA primers, using SYBR® Green PCR master mix reagents (Applied Biosystems). The levels of copies (> 10) of mRNA relative to 18S rRNA copies of these targets were evaluated by RT-PCR analysis using the BioRad Icycler and software. Gene expression analysis experiments were repeated three times.
Flow cytometry analysis of CXCR5 expression
Fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CXCR5 antibody was purchased from R&D Systems; FITC-conjugated mouse IgG2a monoclonal immunoglobulin, as isotype control, was purchased from BD Pharmingen. PCa cells and PrECs were washed 3 times in phosphate buffered saline (PBS) supplemented with 0.5% bovine serum albumin (BSA) and treated with 1.0 μg of Fc Block™ (Pharmingen) per 106 cells for 15 minutes at room temperature (RT). For CXCR5 surface staining, Fc-blocked cells were washed two times with FACS buffer (1% BSA in PBS) and stained with 1.0 μg of FITC-conjugated mouse anti-human CXCR5 or FITC-conjugated mouse IgG2a isotype control antibodies per 106 cells at 4°C for 40 minutes. Alternatively, to stain for both surface and intracellular expression of CXCR5, cells were fixed and permeabilized by incubation with BD Cytofix/Cytoperm solution for 20 min (BD-PharMingen). Next, cells were washed two times with BD perm/wash solution for 10 min at 4 C. Fixed and permeabilized cells were then stained using FITC-conjugated mouse anti-human CXCR5 antibody as described for surface staining. Cells were washed twice with FACS buffer and 0.25 μg of 7-Amino-Actinomycin D (7AAD; BD Pharmingen) per 106 cells for nuclear staining. Subsequently, cells were washed with FACS buffer and resuspended and stored in 500 μl of 2% paraformaldehyde solution. Approximately, 105 cells were analyzed by flow cytometry using a FACScan flow cytometer and CellQuest™ software (BD-PharMingen). Similarly, 106 of stained cells were analyzed by multispectral imaging flow cytometry using an ImageStream system and IDEAS 3.0 software (Amnis).
Migration and invasion assays
CXCL13 was obtained from PeproTech. Unlabeled, mouse anti-human CXCR5 antibody was purchased from R&D Systems. Migration and invasion studies were performed using BD BioCoat™ migration or Matrigel™ tumor invasion chambers, respectively (Becton Dickinson Labware). RPMI-1640 culture media was added to the interior of the bottom chamber as well as to the top chamber of the inserts and allowed to hydrate for 2 hours at 37°C with 5% CO2. Before the addition of PCa cells, 1.0 μg/ml of mouse anti-human CXCR5 (clone 51505.111) or isotype control antibodies (R&D Systems) were added to the top chamber of Matrigel™ or control insert and incubated for 1 hour at 37°C with 5% CO2. Next, 104 PCa cells were added to the top chamber and 100 ng/ml of CXCL13 was added to the bottom chamber. Migration and invasion chamber assays were incubated overnight (i.e., 16 hours) at 37°C with 5% CO2. After incubation, cells that invaded through Matrigel™ or migrated through inserts to the bottom surface of the inserts were fixed with 100% methanol for 2 minutes and stained for 2 minutes in 1% toluidine blue (Sigma) supplemented with 1% borax (Sigma) and rinsed twice with deionized water (dH2O). Cells were counted by microscopy at 20X magnification. Migration and invasion studies were repeated three times.
CXCR5 immunohistochemistry
PCa tissue micro-arrays (TMA) along with associated clinical pathology [i.e, PCa (n = 139), BPH (n = 17) and NM (n = 12) cases] were obtained from the National Cancer Institute (NCI) Cooperative PCa Tissue Resources (CPCTR). Specifically, PCa cases (n = 139) consisted of T1 (n = 1), T2a (n = 18), T2b (n = 72), T3a (n = 25), and T3b (n = 23) staged samples, having Gleason scores of 5 (n = 26), 6 (n = 46), 7 (n = 54), 8 (n = 11), or 9 (n = 2). NM tissues were derived from normal adjacent tissue from tumor patients; however, NM samples were not available for all of the PCa cases used for analysis. TMAs were deparaffinized in xylene and rehydrated through a graded series of ethanol (100%, 95%, and 70%) for 5 minutes in each series and washed in distilled water. Antigen retrieval was achieved by incubating TMAs with 0.01M EDTA (pH 8.0) in a pressure cooker for 5 minutes. Slides were transferred in running water for cooling and then transferred to Tris-buffer (pH 7.6). The endogenous peroxidase activity was blocked, by incubating the slides with 3% H2O2 solution in PBS for 5 minutes. The slides were then rinsed 3 times with deionized water followed by three washes in Tris-buffer and incubated with Fc block (Innovex Bioscience) for 30 minutes at room temperature (25°C; RT) in a humidity chamber. To reduce non-specific binding, the sections were washed with Tris-buffer and incubated with 3% normal goat serum for 1 hour at RT. Unbound goat serum was removed with Tris-buffer, and the sections were incubated with 5.0 μg/ml mouse anti-CXCR5 antibody (R&D Systems) for 90 minutes in humidity chamber at RT. The negative control slide was incubated with 5.0 μg/ml mouse isotype control antibody (R&D Systems). Sections were then washed with Tris buffer and incubated with alkaline phosphatase (AP)-conjugated goat anti-mouse antibody (Zymed) for 20 minutes at RT. The sections were then washed and incubated with the AP New magenta (BioFX Laboratories) for 25 minutes at RT. Next; the slides were washed in deionized H2O and counter-stained with Mayer’s hematoxlin (Sigma) for 1 minute. Subsequently, sections were washed with water, re-hydrated in 70%, 95%, and absolute alcohol for 5 minutes each and passed through xylene three times for 1 minute each; and finally mounted with permount (Sigma).
Evaluation of CXCR5 Immuno-intensity
Digital photograph of each section was taken using Leica DMLB compound microscope with 20× objective, SPOT INSIGHT Color Camera and software by Diagnostic Instruments, Inc. (Sterling Heights, MI). Image analysis on photographs, of individual sections was performed using Image-Pro (Media Cybernetics, Inc, Silver Spring, MD). Color intensity of stain was measured after setting Red and Blue color intensity to channels 0–255 and Green to 50–125. For most samples this collected the upper half of the color intensity range located in mainly in epithelial cells. Measurements = % density/area where area is area of the entire sample
Active MMP protein detection
Briefly, 105 PCa cells or PrECs were seeded in 24-well plates and treated with 0 or 100 ng/ml of CXCL13; cell culture supernatants were collected for subsequent analysis. Levels of active collagenases, gelatinases, and stromelysins were measured by Fluorokine® E assay (R&D systems), according to manufacturer’s protocols. Similarly, an enzyme-linked immuno-sorbent assay (ELISA) method for active stromelysin-3 (MMP-11) detection was developed to quantify active MMP-11 expression in conditioned media. Briefly, 96-well Falcon ELISA plates (Fisher Scientific) were coated with 100 μl of 5 μg/ml rabbit anti-human (hinge region) MMP-11 antibody (Triple Point Biologicals) overnight at 4°C and blocked with 200 μl of 2 % BSA (Sigma) in PBS for 2 hours at RT. Serial dilutions of experimental samples and purified human MMP-11 (Triple Point Biologicals), as the standard, were added and incubated overnight at 4°C. Plates were washed 4 times with PBS containing 0.05% Tween-20 (PBS-T). Mouse anti-human (active domain) MMP-11 antibody (Calbiochem) diluted in PBS (1:3000) was added in each well, and the plates were incubated at RT for 2 hours. After washing the plates 4 times with PBS-T, 100 μl goat anti-mouse IgG-HRP (1:3000, Southern Biotechnology Associates) was added and the plates were incubated at RT for 1 hour. After three washes, tetramethylbenzidine substrate (eBioscience) was added, allowed to react, and stopped with 50 μl of 2% H2SO4. Subsequently, the optical density of plates was read at 450 nm.
Statistics
The mRNA and active MMP levels are expressed as the mean ± SEM (standard error of mean) and compared using a two-tailed student's t-test. The Kolmogorov-Smirnov (K-S) two-sample test using CellQuest Software (BD-Pharmingen) for Macintosh computers was used to compute the statistically significant differences between PrEC and LNCaP or PC3 cell CXCR5-histograms. CXCR5 expression intensity by prostate TMAs were tested for normality assumptions using the Shapiro-Wilk test and were transformed to a logit scale.25, 26 The general linear models (GLM) procedure was used to test the association of CXCR5 expression and disease condition (PCa, BPH, and NM) as well as other covariates (tumor stage and Gleason score) using SAS version 9.1.3 statistical analysis software. Results were declared significant at an alpha level of 0.01.
Results
CXCR5 expression by prostatic tissue and PCa cell lines
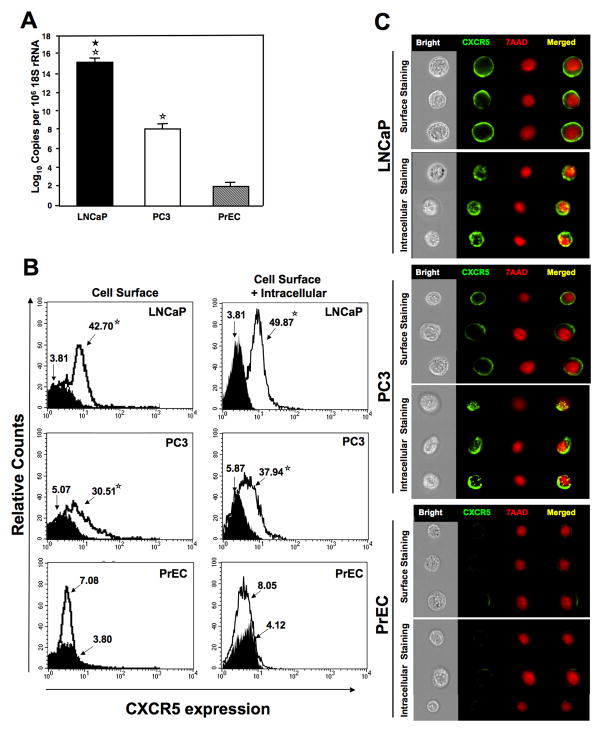
Prostate TMAs consisting of tissue from PCa, BPH, and NM cases were evaluated for CXCR5 expression. The highest CXCR5 expression was observed in PCa cases followed by BPH and NM tissues (Figure 1 and 2). CXCR5 expression by PCa cases was significantly higher (p < 0.001) than NM tissues and correlated with Gleason score (R2 = 0.97). There were no significant differences in CXCR5 immuno-intensities between PCa and BPH (p < 0.099) or BPH and NM (p < 0.290). PCa tissues displayed membrane, cytoplasmic as well as nuclear localization of CXCR5. PCa tissues with Gleason scores ≥ 7 displayed predominantly nuclear and to a lesser degree membrane and cytoplasmic CXCR5 expression patterns. PCa cases with Gleason scores ≤ 6 for the most part displayed membrane and cytoplasmic, but minimal nuclear, CXCR5 expression patterns. BPH and NM tissues had overall lower reactivity and displayed membrane expression patterns. In confirmation with previous studies 17, 24, PCa cell lines (PC3 and LNCaP) expressed significantly higher copies of CXCR5 mRNA than compared to normal prostate cells (Figure 3A). CXCR5 protein expression by PCa cells and PrECs was confirmed by flow cytometry (Figure 3B). Similar to the tissue expression, significantly higher CXCR5 was expressed by LNCaP and PC3 cells than compared to PrEC. Expression pattern of CXCR5 expression patterns (i.e., surface/cytoplasmic versus nuclear localization) was further confirmed in PCa cell lines by multispectral imaging. Representative immuno-fluorescent images show surface expression as well as localization of CXCR5 with the nuclear stain 7AAD stain (Figure 3C).
Figure 1. CXCR5 expression by prostatic tissues.
Prostate tissues from PCa, BPH and NM patients were stained with isotype control (i.e., negative stain control) or anti-CXCR5 antibodies. Magenta color shows CXCR5 staining. A Leica DMLB microscope with a 40X objective took representative digital photographs of NM, BPH and PCa sections. Representative prostate tumor stages (T2 and T3) along with Gleason score (GS) are further indicated. The immuno-intensities of CXCR5 were quantified by image analysis of photographs (40X and 80X) from individual sections using Image-Pro Plus software (Media Cybernetics, Inc.). Open arrows indicate membrane/cytoplasmic CXCR5 expression patterns, while solid arrows indicate nuclear CXCR5 expression patterns.
Figure 2. Clinical significance of CXCR5 expression.
Panel A: CXCR5 expression by PCa, BPH and NM was analyzed and presented in modified Box plot. Lower, middle and upper lines respectively in the box represent the first quartile (Q1), Median (Q2) and third quartile (Q3). Upper (T) and lower (⊥) whiskers are represented by median ± 1.5 (Q3-Q1), which is used to identify potential outliers (●). Significant differences are indicated with an asterisk (★). Panel B: PCa tumor tissue CXCR5 intensity was also correlated with Gleason scores (≤ 5, 6, 7, and ≥ 8). Panel C: The frequency of CXCR5 cellular expression patterns were evaluated in prostate tumor tissues with Gleason scores ≤ 5, 6, 7, and ≥ 8. Solid bars indicated nuclear localization, while open bars illustrated non-nuclear (i.e., cytoplasmic and plasma membrane) expression patterns.
Figure 3. CXCR5 expression by PCa cells and PrECs.
Panel A: Total RNA was isolated from PCa cell lines LNCaP and PC3 and from PrECs. Quantitative RT-PCR analysis of CXCR5 mRNA expression was performed in triplicate. The copies of transcripts are expressed relative to actual copies of 18S rRNA ± SEM. Panel B: LNCaP, PC3, and PrEC cells were stained with FITC-conjugated anti-CXCR5 (open histogram) or FITC-conjugated isotype control antibodies (solid histogram) and quantified, in triplicate, by flow cytometry before (cell surface) or after permeabilization (cell surface + intratracellular). Asterisk(s) indicate statistical significance (P < 0.01) between normal and cancerous cells (☆) and between LNCaP and PC3 cells (★). The mean fluorescent intensities of PE-positive cells are shown. The experiments were repeated 3 times. Panel C: LNCaP, PC3, and PrEC cells were stained with FITC-conjugated anti-CXCR5 or FITC-conjugated isotype control antibodies. Images were acquired by multispectral imaging flow cytometry.
Migration and invasion of PCa cell lines after CXCL13-CXCR5 interaction
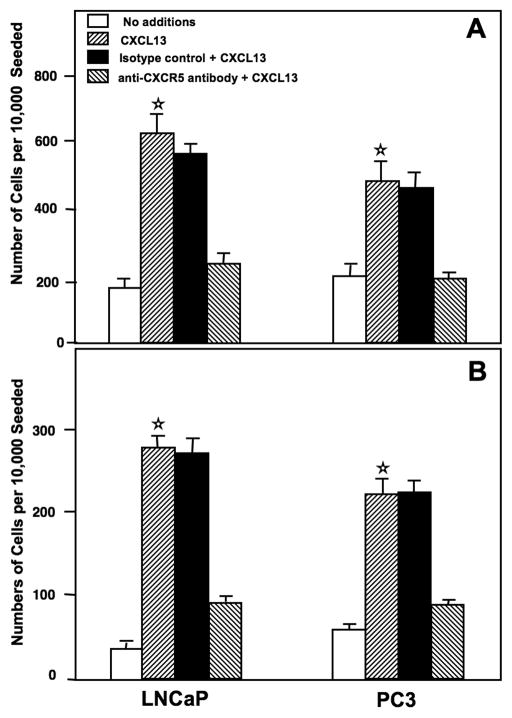
The functional significance of CXCR5 expression by PCa cells was demonstrated by the ability of LNCaP and PC3 cell lines to migrate toward CXCL13. Both PC3 and LNCaP cell lines migrated to CXCL13, but LNCaP cells displayed a higher migration potential than PC3 cells (Figure 4A). The addition of mouse anti-human CXCR5 antibody significantly neutralized this chemokine receptor-dependent motility. The invasive potentials of LNCaP and PC3 cells were accessed using a tumor invasion chamber assay. PCa cell lines significantly invaded Matrigel™ from the top chamber in response to the chemotactic gradient of CXCL13 that was present in the bottom chamber (Figure 4B). This CXCL13-mediated invasion was significantly reduced by CXCR5 blockade.
Figure 4. CXCR5-mediated PCa cell migration and invasion.
Panel A: LNCaP and PC3 cell lines were co-cultured with 1.0 μg/ml of anti-CXCR5 or isotype control monoclonal antibodies during migration assays using CXCL13. Panel B: Similarly, LNCaP and PC3 cells were co-cultured with 1.0 μg/ml of anti-CXCR5 or isotype control monoclonal antibodies during invasion across Matrigel in response to CXCL13. The number of cell (± SEM) that migrated or invaded are shown with asterisk(s) that indicate significant differences (p < 0.01) between no additions and CXCL13-treated cells (☆).
CXCR5-mediated MMP expression by PCa cell lines
PC3 and LNCaP cell lines were able to invade Matrigel by degrading it’s constituents (i.e., laminin, collagen IV, and entactin); therefore, we evaluated CXCL13/CXCR5-mediated MMP expression. Without chemokine stimulation, PC3 cell lines had a higher capacity to generate MMP-1 and -13 (mRNA and active protein) than did the LNCaP cell lines (Figure 5). However, CXCL13 treatment resulted in a dramatic increase in MMP-1 expression by both PCa cells lines compared to untreated cells. Expression of MMP-1 by PC3 cell lines was significantly higher than LNCaP cell lines after CXCL13 treatment. In contrast to MMP-1 expression, CXCL13 treatment caused a decrease in MMP-13 expression by PCa cells. These data suggest that PC3 cells are predisposed to collagenase(s) production without CXCL13 induction, but can be induced to significantly increase their production of MMP-1, but not MMP-13, after CXCL13 treatment.
Figure 5. CXCL13-induced collagenase-1 (MMP-1), collagenase-3 (MMP-13), gelatinase A (MMP-2), and gelatinase B (MMP-9) expression by PCa cells.
LNCaP, PC3 and PrEC cells were cultured for 16 hours with and without CXCL13 (100 ng/ml) or anti-CXCR5 antibody (1 μg/ml). Total RNA was isolated, and quantitative RT-PCR analysis was performed in triplicate to quantify collagenase and gelatinase mRNA expression; copies of mRNA transcripts are expressed relative to actual copies of 18S rRNA (upper panel). Active MMP ELISA was performed in conditioned media to quantify active collagenase and gelatinase proteins (lower panel). Asterisk(s) indicated significant differences (p < 0.01) between similarly treated PrECs and PCa cells (☆), similarly treated cancer cells (★), or untreated and CXCL13-treated cells (+).
PCa cell lines and PrECs expressed MMP-2 mRNA with or without CXCL13 induction. There was a significant increase in active MMP-2 released by PCa cell lines after CXCL13 treatment than compared to PrECs (Figure 5). In contrast to MMP-2 expression, MMP-9 mRNA expression by PCa cells was significantly higher than PrECs. LNCaP cell lines significantly produced active MMP-9 protein following CXCL13-treatment compared to untreated PCa cells. These findings indicated that CXCL13-CXCR5 interactions modestly increased gelatinase mRNA expression by PCa cells, but can significantly enhanced active MMP-2 and -9 production by these malignant cells.
Neither untreated PCa cell lines (LNCaP and PC3) nor PrECs expressed MMP-3. However, CXCL13 treatment of LNCaP cells induced significant increases in MMP-3 mRNA and active protein (Figure 6). Untreated PC3 cells expressed higher levels of MMP-10 mRNA and active protein than did untreated LNCaP cells. There was a significant increase in MMP-10 mRNA expression and active protein after CXCL13 treatment by LNCaP ≫ PC3 cells. MMP-11 mRNA was detected only in LNCaP cells, but CXCL13 treatment did not significantly change MMP-11 expression. Taken together, these data show that CXCL13 modulates stromelysin expression (and activation) in a CXCR5-dependent fashion.
Figure 6. CXCL13-induced stromalysin-1 (MMP-3), stromalysin-2 (MMP-10), and stromalysin-3 (MMP-11) expression.
LNCaP, PC3 and PrEC cells were cultured for 16 hours with and without CXCL13 (100 ng/ml) or anti-CXCR5 antibody (1 μg/ml). Total RNA was isolated and quantitative RT-PCR analysis was performed in triplicate to quantify stromalysin mRNA expression; copies of mRNA transcripts are expressed relative to actual copies of 18S rRNA (upper panel). Asterisk(s) indicated significant differences (p < 0.01) between PrECs and PCa cells (☆), similarly treated PCa cells (★), or untreated and CXCL13-treated cells (+).
Discussion
Mounting evidences suggests that multiple chemokines and their corresponding receptors are involved in PCa progression and play significant roles in metastatic process.15, 16, 18, 19, 22 In this study, we show the chemokines receptor CXCR5 is expressed by the primary PCa tissue. While previously known to be expressed by hematopoietic cells 27, recent studies show CXCR5 is also expressed by the colon carcinoma23 and neuroblastoma28 cells. PCa tissues exhibit significantly higher levels of CXCR5 expression than compared to NM tissues. NM and BPH samples predominately showed membrane and/or cytoplasmic CXCR5 expression patterns. In contrast, PCa cases with advanced Gleason scores showed higher nuclear CXCR5 expression patterns. Chemokine receptors are classically expressed on cell membranes. However, in confirmation to our current observations, CXCR4 expression has also been shown to be localized in the nucleus of advanced breast and lung cancer tissues.29, 30
Recently, CXCR5 mRNA was shown to be elevated in PCa cell lines.17 In contrast, another study suggested that while CXCL12 and CXCR4 mRNAs are increased in PCa cell lines, CXCL13 and CXCR5 mRNAs might be decreased during PCa progression.24 While this supports earlier reports of high CXCR4 mRNA expression patterns by prostate tumors.31, it is important to note that mRNA expression patterns do not always mirror those of their encoded proteins, especially in the case of chemokines.32 Our data show increased protein expression of CXCR5 by PCa tissue. In addition, we show that CXCR5 is functionally expressed by PCa cell lines (LNCaP and PC3). The interaction of CXCR5 with its ligand, CXCL13, has been widely studied in leukocytes, and has been shown to play a significant role in B cell homing to secondary lymphoid tissues as well as in the development of lymph nodes and Peyer’s patches.27 Similarly, CXCR5 expressed by PCa cells might support metastasis to lymph nodes.
CXCL13 induced PCa cells to migrate and invade through Matrigel™, which is a gelatinous protein mixture comprised of ECM components. Its major constituents include: laminin, collagen IV, heparan sulfate proteoglycans, and entactin. Thus, PCa cells must “degrade” and “move” through these ECM components to reach the other side of invasion chambers.33 Hence, the ability of PCa cells to express active collagenases, gelatinases, and stromelysins in response to CXCL13 highlights an important mechanism of CXCR5-mediated PCa cell invasion. MMP-1 over-expression by tumors is correlated with poor patient prognosis.10 CXCL13 treatment resulted in a dramatic increase in MMP-1 expression by PCa cells lines than compared to untreated cells. In contrast to MMP-1 expression, CXCL13 treatment led to a decrease in MMP-13 expression by PCa cell lines. These data suggest that PC3 cells are predisposed to collagenase(s) production, but can be induced to significantly increase their production of active MMP-1, but not MMP-13, after CXCL13 treatment. Interestingly, differential collagenase production has been previously observed in PCa cells following CXCL12-CXCR4 15, 16, but not CCL25-CCR9 22 interactions.
We also demonstrated an increase in MMP-2 and MMP-9 active protein secretion, but not mRNA expression, by PCa cell lines following CXCR5 stimulation. Over expression of MMP-2 has been shown to be associated with PCa progression.34 Expression of MMP-9 has been shown to affect metastasis, angiogenesis, and tumor progression.35 Inhibition of MMP-9 expression by a ribozyme was shown to inhibit metastasis by PCa cell lines without affecting tumorigenesis.36 No doubt, gelatinases contribute significantly to PCa progression and our results show CXCR5-CXCL13 interactions increase gelatinase mRNA and active protein expression by PCa cell lines. Stromelysins (MMP-3, -10, and -11) are expressed by normal epithelial cells, but they are also produced by carcinomas and degrade a broad range of substrates, including type IV, V, IX, and X collagens, fibronectins, laminin, elastin, gelatin, and proteoglycan core proteins.37 MMP-11 expression by epithelial cells is unusual and may partially explain the lymph node metastasis phenotype of LNCaP cell lines.38 MMP-3 and MMP-10 expression have also been reported to be elevated in head and neck carcinomas compared to NM tissues 39. In this study, we show that MMP-3 is expressed by LNCaP cells, but not by PC3 cells. While only untreated PC3 cell lines expressed MMP-10, the mRNA expression and active protein secretion of these important stromelysins were increased following CXCR5-CXCL13 interaction, further supporting the notion that stromelysin expression and activity is induced by CXCL13-CXCR5 interactions.
The current study suggests that the differential expression of CXCR5 and its interactions with CXCL13 partially determine the migration and invasion potentials of PCa cells. Importantly, human bone marrow endothelial cells do not generally secrete CXCL13. However, it has been shown that follicular high endothelial venules express CXCL13 after stimulation.40 We have recently shown that IL-6, which is highly elevated in PCa patients41, can induced CXCL13 production by the human bone marrow endothelial cells and osteoblasts.42 In same study we showed that serum CXCL13 levels were significantly higher in PCa patients than compared to BPH, HGPIN, and normal healthy donors. To this end, CXCL13 is expressed by osteoclasts, osteoblasts, and vascular endothelial cells, following stimulation with inflammatory factors. Taken together with bone and lymphoid expression patterns of CXCL13 shown by others and its effect on MMP expression by PCa cells, our data suggest that CXCL13-CXCR5 interactions support PCa cell invasion. Future studies will focus on discovering the precise tumor-associated or lymphoid cells that express CXCL13 during PCa progression.
In conclusion, we show that PCa cells and tissues significantly express CXCR5. While increased CXCR5 expression by PCa cases suggests it plays a role in invasion, it is also plausible that the reduction in CXCR5 surface expression in advanced cases promotes less adhesion. This could potentially allow tumor cells to escape primary sites and spread. To explain, CXCR5 activation leads to integrin co-aggregation to induce adhesion to (bone marrow) endothelial cells, since integrins co-aggregate with CXCR5 and other chemokine receptors following activation to promote firm cell adhesion.43–45 Indeed, CXCL13 is an arrest chemokine for B cells in high endothelial venules.46 Alternatively, the observation of CXCR5 expression transitioning from the surface to nuclei during PCa progression might also be due to receptor internalization and/or nuclear translocation following CXCL13 stimulation.
It is important to mention that it is not certain whether CXCR5 is involved in nuclear signaling or if we are merely visualizing newly synthesized protein in endoplasmic reticulum that is closely associated with nuclear membranes. The precise mechanisms responsible for the regulation of CXCR5 surface expression, internalization, degradation, internalization, de novo synthesis, and/or nuclear translocation will be the subject of future studies. Nonetheless, CXCR5 might be used as a PCa biomarker; however, this will also require extensive studies using larger sample sizes to confirm our observations.
Acknowledgments
The content of this manuscript benefited from many fruitful conversations with members of the Morehouse School of Medicine, University of Alabama at Birmingham, Tulane University, Emory University, and the University of Louisville. This work benefited from the cooperation between investigators from the Morehouse School of Medicine and the Wallace Tumor Institute at the University of Alabama at Birmingham via the National Cancer Institute sponsored “Comprehensive Minority Institution / Cancer Center Partnership”. This study was supported by funds from the Smith & Lucille Gibson Endowment, Department of Defense Prostate Cancer Research Program Award W81XWH-06-1-0562 and National Institute of Health Grants AI057808, CA092078, CA086359, DK58967, GM08248, GM09248, MD00525, and RR03034.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun M. Cancer Statistics. CA : CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. [comment] CA: CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Nicolson GL. Paracrine and autocrine growth mechanisms in tumor metastasis to specific sites with particular emphasis on brain and lung metastasis. Cancer Metastasis Rev. 1993;12:325–43. doi: 10.1007/BF00665961. [DOI] [PubMed] [Google Scholar]
- 4.Roh KJ, Kim DJ, Kim DW, Wang JM. Chemokines and their role in tumor growth and metastasis. J Bone Joint Surg Br. 1998;80:1005–8. doi: 10.1302/0301-620x.80b6.8966. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmark of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Sternlicht MD, Bergers G. Matrix metalloproteinases as emerging tragets in anti-cancer therapy: status and prospect. Emerging Ther Targets. 2000:609–33. [Google Scholar]
- 7.Liotta LA, Tryggnason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–8. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 8.Mignatti P, Robbins E, Rifkin DB. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986;47:487–98. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- 9.Stetler-Stevenson WG, Hewitt R, Corcoran M. Matrix metalloproteinases and tumor invasion: from correlation and causality to the clinic. Sem Cancer Biol. 1996;7:147–54. doi: 10.1006/scbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- 10.Murray GI, Duncan ME, O'Neil P, McKay JA, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185:256–61. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.Lampert K, Machein U, Machein RL, Conca W, Peter HH, Volk B. Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors. Am J Pathol. 1998;153:429–37. doi: 10.1016/S0002-9440(10)65586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 14.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 15.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 16.Singh S, Singh UP, Grizzle WE, Lillard JW. CXCL12-CXCR4 interactions modulates prostate cancer cell migration, metalloproteinase expression and invasion. Lab Invest. 2004;84:1666–76. doi: 10.1038/labinvest.3700181. [DOI] [PubMed] [Google Scholar]
- 17.Engl T, Relja B, Blumenberg C, Muller I, Ringel EM, Beecken W-D, Jonas D, Blaheta RA. Prostate tumor CXC-chemokine profile correlates with cell adhesion to endothelium and extracellular matrix. Life Sci. 2006;78:1784–93. doi: 10.1016/j.lfs.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 19.Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004;64:4693–8. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 20.Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–34. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- 21.Heresi GA, Wang J, Taichman R, Chirinos JA, Regalado JJ, Lichtstein DM, Rosenblatt JD. Expression of the chemokine receptor CCR7 in prostate cancer presenting with generalized lymphadenopathy: report of a case, review of the literature, and analysis of chemokine receptor expression. Urol. 2005;23:261–7. doi: 10.1016/j.urolonc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Singh UP, Stiles JK, Grizzle WE, Lillard JW. Expression and Functional Role of CCR9 in Prostate Cancer Cell Migration and Invasion. Clin Cancer Res. 2004;10:8743–50. doi: 10.1158/1078-0432.CCR-04-0266. [DOI] [PubMed] [Google Scholar]
- 23.Meijer J, Zeelenberg IS, Sipos B, Roos E. The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in the liver. Cancer Res. 2006;66:9576–82. doi: 10.1158/0008-5472.CAN-06-1507. [DOI] [PubMed] [Google Scholar]
- 24.Wedel SA, Raditchev IN, Jones J, Juengel E, Engl T, Jonas D, Blaheta RA. CXC chemokine mRNA expression as a potential diagnostic tool in prostate cancer. Molecular Medicine Reports. 2008;1:257–62. [PubMed] [Google Scholar]
- 25.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 26.Rai SN, Lensing S, Boyett J, Phipps S. On the Analysis of Messy Longitudinal Data from a Study of Pediatric Cancer Patients. Biometrics Section; Proc Joint Statistical Meetings; 2005; 2004. pp. 427–433. [Google Scholar]
- 27.Ebisuno Y, Tanaka T, Kanemitsu N, Kanda H, Yamaguchi K, Kaisho T, Akira S, Miyasaka M. Cutting edge: The B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J Immunol. 2003;171:1654–546. doi: 10.4049/jimmunol.171.4.1642. [DOI] [PubMed] [Google Scholar]
- 28.Airoldi I, Cocco C, Morandi F, Prigione I, Pistoia V. CXCR5 may be involved in the attraction of human metastatic neuroblastoma cells to the bone marrow. Cancer Immunol Immunother. 2008;57:541–8. doi: 10.1007/s00262-007-0392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo SU, Bae JW, Kim HG, Choi SH, Kang DH, Lee JB, Koo BW. Correlation between the in vitro ATP-based chemosensitivity assay and HER2/neu expression in women with breast cancer. J Intl Med Res. 2007;35:753–61. doi: 10.1177/147323000703500603. [DOI] [PubMed] [Google Scholar]
- 30.Spano JP, Andre F, Morat L, Sabatier L, Besse B, Combadiere C, Deterre P, Martin A, Azorin J, Valeyre D, Khayat D, Le Chevalier T, et al. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004;15:613–7. doi: 10.1093/annonc/mdh136. [DOI] [PubMed] [Google Scholar]
- 31.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, Peled A. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18:1240–2. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 32.Petrek M, Kolek V, Szotkowska J, du Bois RM. CC and C chemokine expression in pulmonary sarcoidosis. Eur Respir J. 2002;20:1206–12. doi: 10.1183/09031936.02.00289902. [DOI] [PubMed] [Google Scholar]
- 33.Kahari VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31:34–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- 34.Still K, Robson CN, Autzen P, Robinson MC, Hamdy FC. Localization and quantification of mRNA for matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) in human benign and malignant prostatic tissue. Prostate. 2000;42:18–25. doi: 10.1002/(sici)1097-0045(20000101)42:1<18::aid-pros3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 35.Arai J, Yasukawa M, Yakushijin Y, Miyazaki T, Fujita S. Stromal cells in lymph nodes attract B-lymphoma cells via production of stromal cell-derived factor-1. Eur J Haematol. 2000;64:323–32. doi: 10.1034/j.1600-0609.2000.90147.x. [DOI] [PubMed] [Google Scholar]
- 36.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–54. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Brikedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 38.Woessner JFJ. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. [PubMed] [Google Scholar]
- 39.Birkedal-Hansen B, Pavelic ZP, Gulckman JL, Stombrook P, Li YQ, Stetler-Stevenson WG. MMP and TIMP gene expression in head and neck squamous cell carcinomas and adjacent tissues. Oral Dis. 2000;6:376–82. doi: 10.1111/j.1601-0825.2000.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 40.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-a correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312–6. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh S, Singh R, Sharma P, Singh UP, Rai SN, Chung LWK, Cooper CR, Novakovic KR, Grizzle WE, Lillard JW., Jr Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell migration, integrin clustering and cell adhesion. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.03.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 44.Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl J. CD4+CD3− cells induce Peyer's patch development: role of alpha4beta1 integrin activation by CXCR5. Immunity. 2002;17:363–73. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 45.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–12. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 46.Kanemitsu N, Ebisuno Y, Tanaka T, Otani K, Hayasaka H, Kaisho T, Akira S, Katagiri K, Kinashi T, Fujita N, Tsuruo T, Miyasaka M. CXCL13 is an arrest chemokine for B cells in high endothelial venules. Blood. 2005;106:2613–8. doi: 10.1182/blood-2005-01-0133. [DOI] [PubMed] [Google Scholar]